Abstract
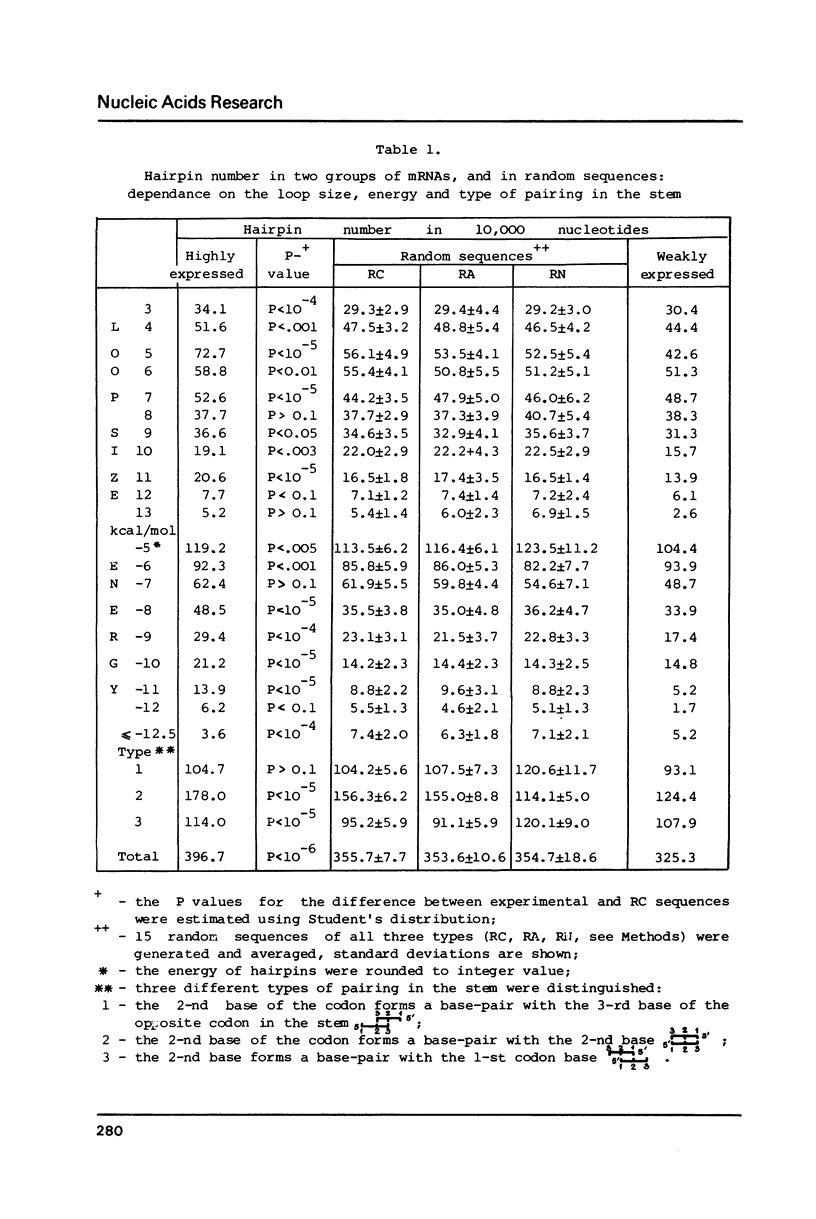
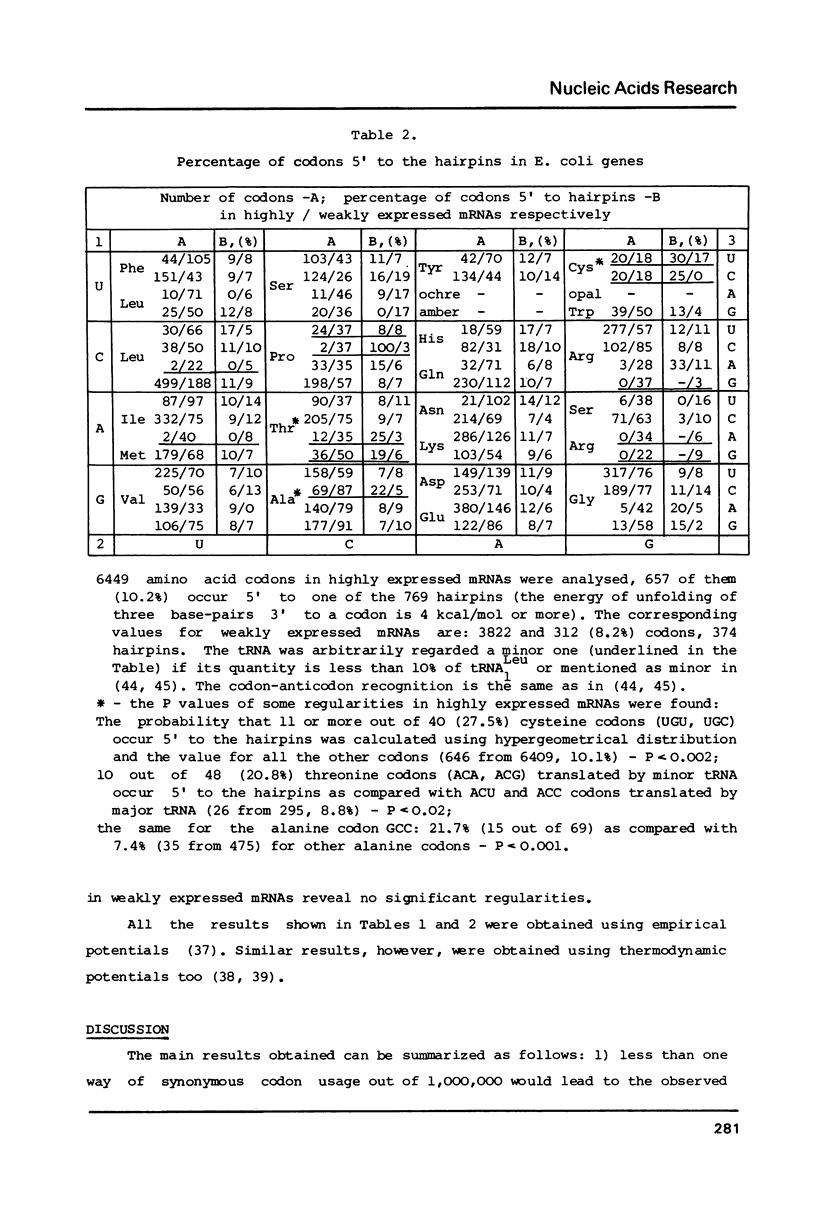
A secondary structure model was proposed for mRNAs during translation (in a polysome) where the secondary structure is described by a set of small unbranched hairpins. Computer simulation experiments reveal that the number of hairpins is much greater (P less than 10(-6) in highly expressed mRNAs from E. coli as compared with the random sequences coding for the same amino acid sequence, i.e. certain synonymous codons are used in definite mRNA positions to increase the number of hairpins. No constraints on the amino acid sequence, which would affect the secondary structure of mRNAs, were found. The codons UGU, UGC (Cys), GCC (Ala), ACA, ACG (Thr), CCU, CCC (Pro), etc. translated by minor tRNAs were found to occur significantly more frequently in the position 5' to the hairpins than the other codons translated by major tRNAs (P less than 5.10(-6). This correlation leads to the hypothesis that the process of hairpin unfolding can increase the time of translocation from the A to P ribosome site of the codon 5' to the hairpin, thus decreasing the probability of translational error (the latter would likely occur more frequently in the codons translated by minor tRNAs).
Full text
PDF


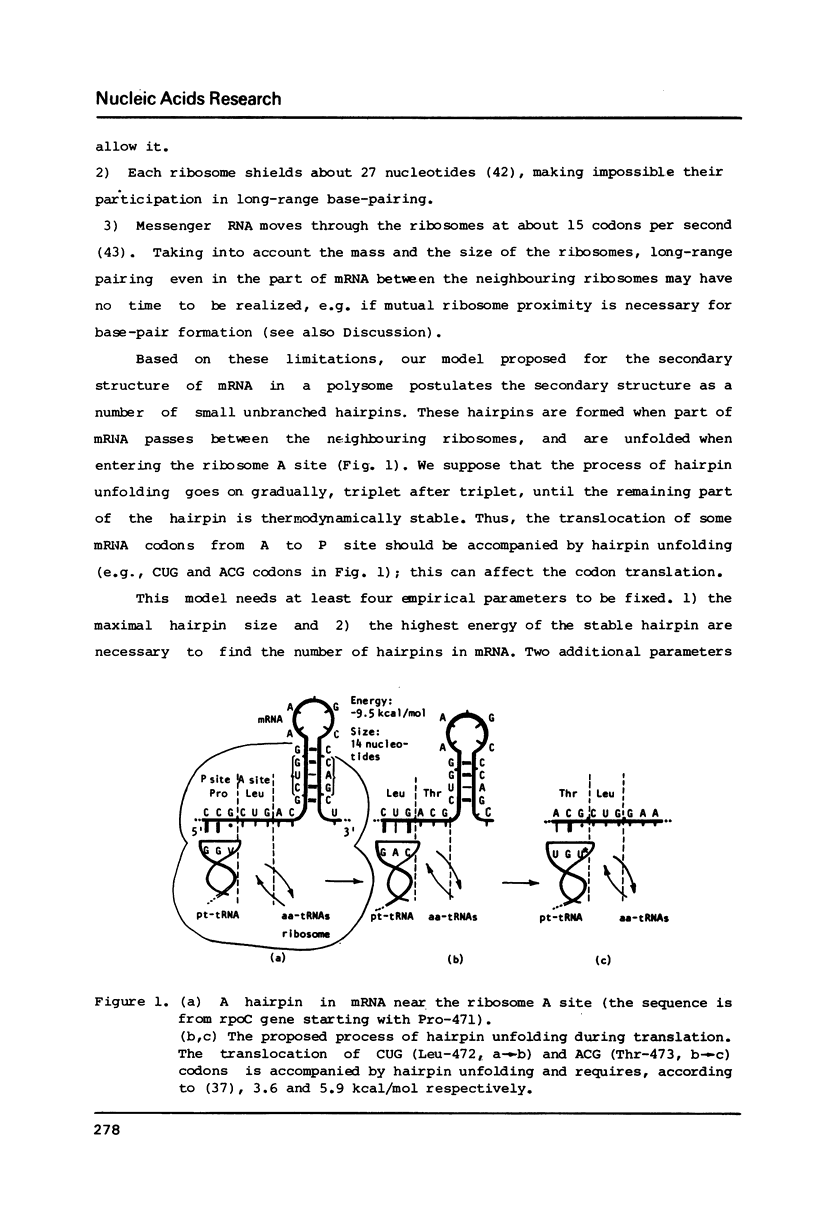








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Jeppesen P. G., Sanger F., Barrell B. G. Nucleotide sequence from the coat protein cistron of R17 bacteriophage RNA. Nature. 1969 Sep 6;223(5210):1009–1014. doi: 10.1038/2231009a0. [DOI] [PubMed] [Google Scholar]
- An G., Bendiak D. S., Mamelak L. A., Friesen J. D. Organization and nucleotide sequence of a new ribosomal operon in Escherichia coli containing the genes for ribosomal protein S2 and elongation factor Ts. Nucleic Acids Res. 1981 Aug 25;9(16):4163–4172. doi: 10.1093/nar/9.16.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins J. F., Gesteland R. F., Reid B. R., Anderson C. W. Normal tRNAs promote ribosomal frameshifting. Cell. 1979 Dec;18(4):1119–1131. doi: 10.1016/0092-8674(79)90225-3. [DOI] [PubMed] [Google Scholar]
- Ball L. A. Secondary structure and coding potential of the coat protein gene of bacteriophage MS2. Nat New Biol. 1973 Mar 14;242(115):44–45. doi: 10.1038/newbio242044a0. [DOI] [PubMed] [Google Scholar]
- Beck E., Bremer E. Nucleotide sequence of the gene ompA coding the outer membrane protein II of Escherichia coli K-12. Nucleic Acids Res. 1980 Jul 11;8(13):3011–3027. doi: 10.1093/nar/8.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton Z. F., Gross C. A., Watanabe K. K., Burgess R. R. The operon that encodes the sigma subunit of RNA polymerase also encodes ribosomal protein S21 and DNA primase in E. coli K12. Cell. 1983 Feb;32(2):335–349. doi: 10.1016/0092-8674(83)90453-1. [DOI] [PubMed] [Google Scholar]
- Burton Z., Burgess R. R., Lin J., Moore D., Holder S., Gross C. A. The nucleotide sequence of the cloned rpoD gene for the RNA polymerase sigma subunit from E coli K12. Nucleic Acids Res. 1981 Jun 25;9(12):2889–2903. doi: 10.1093/nar/9.12.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney W. G., Morris A. J. Nonuniform size distribution of nascent peptides. The effect of messenger RNA structure upon the rate of translation. Arch Biochem Biophys. 1979 Apr 15;194(1):283–291. doi: 10.1016/0003-9861(79)90620-9. [DOI] [PubMed] [Google Scholar]
- Derom C., Gheysen D., Fiers W. High-level synthesis in Escherichia coli of the SV40 small-t antigen under control of the bacteriophage lambda pL promoter. Gene. 1982 Jan;17(1):45–54. doi: 10.1016/0378-1119(82)90099-3. [DOI] [PubMed] [Google Scholar]
- Edelmann P., Gallant J. Mistranslation in E. coli. Cell. 1977 Jan;10(1):131–137. doi: 10.1016/0092-8674(77)90147-7. [DOI] [PubMed] [Google Scholar]
- Farabaugh P. J. Sequence of the lacI gene. Nature. 1978 Aug 24;274(5673):765–769. doi: 10.1038/274765a0. [DOI] [PubMed] [Google Scholar]
- Fitch W. M. Calculating the expected frequencies of potential secondary structure in nucleic acids as a function of stem length, loop size, base composition and nearest-neighbor frequencies. Nucleic Acids Res. 1983 Jul 11;11(13):4655–4663. doi: 10.1093/nar/11.13.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. The atp operon: nucleotide sequence of the promoter and the genes for the membrane proteins, and the delta subunit of Escherichia coli ATP-synthase. Nucleic Acids Res. 1981 Aug 25;9(16):3919–3926. doi: 10.1093/nar/9.16.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. The atp operon: nucleotide sequence of the region encoding the alpha-subunit of Escherichia coli ATP-synthase. Nucleic Acids Res. 1981 May 11;9(9):2187–2194. doi: 10.1093/nar/9.9.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheysen D., Iserentant D., Derom C., Fiers W. Systematic alteration of the nucleotide sequence preceding the translation initiation codon and the effects on bacterial expression of the cloned SV40 small-t antigen gene. Gene. 1982 Jan;17(1):55–63. doi: 10.1016/0378-1119(82)90100-7. [DOI] [PubMed] [Google Scholar]
- Goad W. B., Kanehisa M. I. Pattern recognition in nucleic acid sequences. I. A general method for finding local homologies and symmetries. Nucleic Acids Res. 1982 Jan 11;10(1):247–263. doi: 10.1093/nar/10.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M., Grantham R. Polypeptide elongation and tRNA cycling in Escherichia coli: a dynamic approach. FEBS Lett. 1980 Jun 30;115(2):151–155. doi: 10.1016/0014-5793(80)81155-0. [DOI] [PubMed] [Google Scholar]
- Gralla J., DeLisi C. mRNA is expected to form stable secondary structures. Nature. 1974 Mar 22;248(446):330–332. doi: 10.1038/248330a0. [DOI] [PubMed] [Google Scholar]
- Greene P. J., Gupta M., Boyer H. W., Brown W. E., Rosenberg J. M. Sequence analysis of the DNA encoding the Eco RI endonuclease and methylase. J Biol Chem. 1981 Mar 10;256(5):2143–2153. [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Yanofsky C. Nucleotide sequence and expression of Escherichia coli trpR, the structural gene for the trp aporepressor. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7117–7121. doi: 10.1073/pnas.77.12.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M., Yasunaga T., Miyata T. Secondary structure of MS2 phage RNA and bias in code word usage. Nucleic Acids Res. 1979 Dec 11;7(7):2073–2079. doi: 10.1093/nar/7.7.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., McCarthy B. J., Ohtsubo H., Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell. 1979 Dec;18(4):1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes. J Mol Biol. 1981 Feb 15;146(1):1–21. doi: 10.1016/0022-2836(81)90363-6. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Iserentant D., Fiers W. Secondary structure of mRNA and efficiency of translation initiation. Gene. 1980 Apr;9(1-2):1–12. doi: 10.1016/0378-1119(80)90163-8. [DOI] [PubMed] [Google Scholar]
- Jacobson A. B., Good L., Simonetti J., Zuker M. Some simple computational methods to improve the folding of large RNAs. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):45–52. doi: 10.1093/nar/12.1part1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaurin B., Grundström T. ampC cephalosporinase of Escherichia coli K-12 has a different evolutionary origin from that of beta-lactamases of the penicillinase type. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4897–4901. doi: 10.1073/pnas.78.8.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou W. M., Haegeman G., Fiers W. Studies on the bacteriophage MS2. Nucleotide fragments from the coat protein cistron. FEBS Lett. 1971 Feb 19;13(2):105–109. doi: 10.1016/0014-5793(71)80210-7. [DOI] [PubMed] [Google Scholar]
- Kanehisa M. I., Goad W. B. Pattern recognition in nucleic acid sequences. II. An efficient method for finding locally stable secondary structures. Nucleic Acids Res. 1982 Jan 11;10(1):265–278. doi: 10.1093/nar/10.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland C. G. Translational accuracy in vitro. Cell. 1982 Feb;28(2):201–202. doi: 10.1016/0092-8674(82)90336-1. [DOI] [PubMed] [Google Scholar]
- Min Jou W., Haegeman G., Ysebaert M., Fiers W. Nucleotide sequence of the gene coding for the bacteriophage MS2 coat protein. Nature. 1972 May 12;237(5350):82–88. doi: 10.1038/237082a0. [DOI] [PubMed] [Google Scholar]
- Miyada C. G., Horwitz A. H., Cass L. G., Timko J., Wilcox G. DNA sequence of the araC regulatory gene from Escherichia coli B/r. Nucleic Acids Res. 1980 Nov 25;8(22):5267–5274. doi: 10.1093/nar/8.22.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninio J. A semi-quantitative treatment of missense and nonsense suppression in the strA and ram ribosomal mutants of Escherichia coli. Evaluation of some molecular parameters of translation in vivo. J Mol Biol. 1974 Apr 5;84(2):297–313. doi: 10.1016/0022-2836(74)90586-5. [DOI] [PubMed] [Google Scholar]
- Nussinov R. RNA folding is unaffected by the nonrandom degenerate codon choice. Biochim Biophys Acta. 1982 Aug 30;698(2):111–115. doi: 10.1016/0167-4781(82)90125-7. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Monastyrskaya G. S., Gubanov V. V., Guryev S. O., Salomatina I. S., Shuvaeva T. M., Lipkin V. M., Sverdlov E. D. The primary structure of E. coli RNA polymerase, Nucleotide sequence of the rpoC gene and amino acid sequence of the beta'-subunit. Nucleic Acids Res. 1982 Jul 10;10(13):4035–4044. doi: 10.1093/nar/10.13.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Monastyrskaya G. S., Gubanov V. V., Guryev S. O., Chertov OYu, Modyanov N. N., Grinkevich V. A., Makarova I. A., Marchenko T. V., Polovnikova I. N. The primary structure of Escherichia coli RNA polymerase. Nucleotide sequence of the rpoB gene and amino-acid sequence of the beta-subunit. Eur J Biochem. 1981 Jun 1;116(3):621–629. doi: 10.1111/j.1432-1033.1981.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Oxender D. L., Zurawski G., Yanofsky C. Attenuation in the Escherichia coli tryptophan operon: role of RNA secondary structure involving the tryptophan codon region. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5524–5528. doi: 10.1073/pnas.76.11.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou C., Gouy M., Ninio J. An energy model that predicts the correct folding of both the tRNA and the 5S RNA molecules. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):31–44. doi: 10.1093/nar/12.1part1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. Termination of transcription and its regulation in the tryptophan operon of E. coli. Cell. 1981 Apr;24(1):10–23. doi: 10.1016/0092-8674(81)90496-7. [DOI] [PubMed] [Google Scholar]
- Post L. E., Strycharz G. D., Nomura M., Lewis H., Dennis P. P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser W. Globin mRNA sequences: analysis of base pairing and evolutionary implications. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):985–1002. doi: 10.1101/sqb.1978.042.01.099. [DOI] [PubMed] [Google Scholar]
- Sancar A., Stachelek C., Konigsberg W., Rupp W. D. Sequences of the recA gene and protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2611–2615. doi: 10.1073/pnas.77.5.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Gay N. J., Eberle A., Runswick M. J., Walker J. E. The atp operon: nucleotide sequence of the genes for the gamma, beta, and epsilon subunits of Escherichia coli ATP synthase. Nucleic Acids Res. 1981 Oct 24;9(20):5287–5296. doi: 10.1093/nar/9.20.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stragier P., Patte J. C. Regulation of diaminopimelate decarboxylase synthesis in Escherichia coli. III. Nucleotide sequence and regulation of the lysR gene. J Mol Biol. 1983 Aug 5;168(2):333–350. doi: 10.1016/s0022-2836(83)80022-9. [DOI] [PubMed] [Google Scholar]
- TAKANAMI M., ZUBAY G. AN ESTIMATE OF THE SIZE OF THE RIBOSOMAL SITE FOR MESSENGER RNA BINDING. Proc Natl Acad Sci U S A. 1964 May;51:834–839. doi: 10.1073/pnas.51.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M. The accuracy of translation. Prog Nucleic Acid Res Mol Biol. 1979;23:195–225. doi: 10.1016/s0079-6603(08)60134-8. [DOI] [PubMed] [Google Scholar]
- Yokota T., Sugisaki H., Takanami M., Kaziro Y. The nucleotide sequence of the cloned tufA gene of Escherichia coli. Gene. 1980 Dec;12(1-2):25–31. doi: 10.1016/0378-1119(80)90012-8. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Nilsson L., Blomberg C. Models for mRNA translation: theory versus experiment. Eur J Biochem. 1978 Dec;92(2):397–402. doi: 10.1111/j.1432-1033.1978.tb12759.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Nilsson L., Blomberg C. Translation and messenger RNA secondary structure. J Theor Biol. 1977 Oct 7;68(3):321–329. doi: 10.1016/0022-5193(77)90063-7. [DOI] [PubMed] [Google Scholar]



