Abstract
Persistent hyperinsulinemic hypoglycemia of infancy (PHHI) is relatively rare but one of the most important causes of severe neonatal hypoglycemia. Recognition of this entity becomes important due to the fact that the hypoglycemia is so severe and frequent that it may lead to severe neurological damage in the infant manifesting as mental or psychomotor retardation or even a life-threatening event if not recognized and treated effectively in time. Near-total pancreatectomy may be required for patients with intractable hypoglycemia despite medical treatment; however, that may result in diabetes mellitus or recurrent postoperative hypoglycemia. This review aims to consolidate the traditional concepts and current information related to the pathogenesis and management of PHHI.
KEY WORDS: Hypoglycemia of infancy, near-total pancreatectomy, nesidioblastosis
INTRODUCTION
Transient, symptomatic hypoglycemia is the most common metabolic abnormality in the neonates and is seen in 4 per 1000 term infants and 6 per 1000 premature infants. However, 1% of these infants have sustained or repeated episodes of hypoglycemia and call for prompt recognition and management. Persistent hyperinsulinemic hypoglycemia of infancy (PHHI), though rare, is the most common cause of neonatal hypoglycemia persisting beyond the first few hours of life.
Laidlaw[1] in 1938 coined the term nesidioblastosis based on his understanding of a diffuse ducto-endocrine β cell proliferative disorder of the pancreas in a patient with a β cell tumor. The term nesidioblastoma was derived from the Greek words nesidion (islet) and blastos (germ) and was proposed for an islet cell adenoma. Brown and Young[2] associated severe infantile hypoglycemia with nesidioblastosis. The term nesidiodysplasia was proposed to signify the overpopulation of the pancreas with the β cells coupled with their maldistribution and malregulated function.[3] The designation PPHI was proposed by Glaser[4] in 1989 and has been accepted in the recent literature replacing all other terms used previously. The term incorporated the focal and diffuse pancreatic involvement associated with hyperinsulinism and hypoglycemia.
INCIDENCE
PHHI occurs sporadically in 1:50,000 live births in a random mating population. The incidence can be as high as 1:2500 in communities with significant inbreeding. The proposed mode of inheritance is autosomal recessive. Familial PHHI was reported for the first time by Woo et al.,[5,6] in a Greek Cypriots family. Usually patients affected with PHHI have healthy parents. The male to female ratio is 1.2:1 for diffuse lesions and 1.8:1 for focal lesions. The most common age for presentation is the neonate and infant. Presentation beyond the age of 2 years is rare.
PATHOLOGY AND PATHOGENESIS
Inappropriate secretion of insulin could be explained by a defect at three levels, viz., defect in the β cells of the pancreas, faulty intracellular signaling responsible for normal islet cell function in the presence of normal β cells,[7] or overpopulation of β cells in the pancreatic tissue.[8] Nesidioblastosis is characterized by the presence of islets in increased numbers throughout the pancreatic lobule in addition to the islets of Langerhans. These islet cells may be present as single cells or in clusters. The histological appearance closely resembles that of third trimester fetal and neonatal pancreas. Resnick and Manivel[9] demonstrated similar histopathology in anterior mediastinal and sacrococcygeal teratoma. De novo formation of intermediate cells (acinar islet cells) has also been associated with nesidioblastosis.
The histopathology of PHHI is heterogeneous and is hallmarked by the presence of ductoendocrine proliferation, supernumerary small endocrine groups, and large endocrine areas.[10] Broadly speaking, two patterns can be distinguished, viz., focal and diffuse. The focal adenomatous hyperplasia, as the name signifies, is characterized by the presence of abnormal β cells in one or more focal areas inside the pancreatic tissue. It is encountered in about 40% of the cases of PHHI. The lesion measures about 2.5–7.5 mm in diameter. The diffuse form of the disease shows the presence of abnormal β cells in all sections of the pancreas.
MOLECULAR BASIS OF THE DISEASE
The most widely accepted hypothesis for the development of PHHI is the presence of dysfunction of the ATP-dependent potassium channels in the β cells of the pancreas.[11,12] Focal PHHI is associated with hemizygosity or homozygosity of a paternally inherited mutation of the SUR gene or the inwardly rectifying potassium channel genes and loss of the maternal allele in the hyperplastic islets.[13,14] The SUR gene belongs to the ATP binding cassette super family and the SUR protein is a putative subunit of the β cell ATP-sensitive potassium channel. The gene has been mapped to 11p15.1 by fluorescence in situ hybridization.[15] The presence of a diffuse lesion involves the genes encoding the sulfonylurea receptor or the inwardly rectifying potassium in recessively inherited hyperinsulinism.[16,17] The transmission could infrequently be dominant[6] and involves the glucokinase gene[18] or other loci[19] such as the glutamate dehydrogenase gene.[20]
Autoantibodies[21] with a doubtful significance have also been demonstrated in the serum of patients with PHHI. It is yet not clear whether these antibodies are the result of the release of islet antigens in response to the proliferative process or they have a pathogenic role by acting as growth factors. A new pituitary protein 7B2 has been reported to be elevated in patients with PHHI and may have a marker role in facilitating the diagnosis.[22]
CLINICAL SPECTRUM
Majority of the babies are macrosomic at birth with a mean birth weight of 3.7 kg. Mild to moderate hepatomegaly is usually present. Symptoms of hypoglycemia appear within 72 h of birth. The presentation includes hunger, jitteriness, lethargy, apnea, and seizures. Seizures are generalized tonic-clonic and present in one half of the cases.[23] Tremors, hypotonia, cyanosis, and hypothermia are not infrequent. Older children may also present with diaphoresis, confusion, and unusual mood or behavioral changes. Facies typical of hyperinsulinism include high forehead, large and bullous nose with a short columella, smooth philtrum, and a thin upper lip.
The hypoglycemia is severe and permanent (both fasting and postprandial). Usually, the blood glucose levels at the time of first presentation are very low (<18 mg/dL). Frequent or continuous glucose infusion or feeding is required to maintain adequate blood glucose levels. These children are highly susceptible to develop neurological damage, psychomotor retardation, and even death.
DIAGNOSIS OF PERSISTENT HYPERINSULINEMIC HYPOGLYCEMIA OF INFANCY
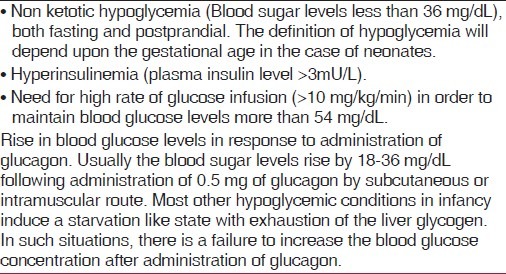
The diagnostic criteria for PHHI have been enumerated in Table 1. The insulin to glucose ratio is elevated (0.4–2.7) in patients with PHHI. In case of diagnostic dilemma, the patient may be further evaluated for levels of ketone bodies, free fatty acids, and branched chain amino acids after a fasting period of 4–6 h.
Table 1.
Diagnostic criteria for persistent hyperinsulinemic hypoglycemia of infancy
MANAGEMENT OF PERSISTENT HYPERINSULINEMIC HYPOGLYCEMIA OF INFANCY
Nearly all patients require high rates of glucose infusion. Continuous feeding with a glucose polymer orally or through a nasogastric drip is recommended to maintain the blood glucose levels. Feeding should continue even during sleep. A good venous access must always be secured with a wide bore intravenous canula. A central venous catheter is desirable to support the high rates of glucose infusion. The medical management comprises of three drug categories [Table 2].
Table 2.
Drugs used for Persistent hyperinsulinemic hypoglycemia of infancy

Glucagon or somatostatin may be added to the glucose drip if the blood glucose is unstable despite high rates of glucose infusion. Glucagon has a transient effect but is useful in emergency situations such as the time while looking out for a fresh intravenous access. Somatostatin is the only hormone in the normal islet of Langerhans which has an inhibitory effect on the glucose-mediated release of insulin. Concurrent administration of somatostatin abolishes the insulin-stimulating effect of glucose being administered to address the hypoglycemia.
Diazoxide has been used as a first-line drug. Diazoxide causes hyperglycemia by stimulating the secretion of catecholamines and mobilization of glycogen. This inhibits the release of insulin with resultant hyperglycemia. The efficacy of diazoxide has been described in terms of normalization of the blood glucose levels (>54 gm/dL), both fasting and postprandial, in patients who are on normal diet, physiological overnight fast, and off intravenous supplementation for at least 5 days. Any patient who demonstrated two episodes of hypoglycemia in any 24 h would be labeled as diazoxide unresponsive. Unfortunately, 90% of the neonatal forms of PHHI are resistant to diazoxide.
Octreotide is used in patients with diazoxide unresponsiveness. High doses could worsen hypoglycemia paradoxically by suppressing the secretion of glucagon and growth hormone.
Calcium-channel blockers like nifedipine have also been reported to be of some use.[24–26] Cornblath and Schwartz[27] suggested the role of long-acting adrenaline in the initial diagnostic-therapeutic evaluation of severe neonatal hypoglycemia based on its direct inhibitory effect on the β cells of islets of Langerhans. Streptozotocin has been known to selectively destroy the insulin-secreting cells and induces diabetes mellitus in experimental animals. However, due to its toxicity, its use is precluded in the neonates.
DIFFERENTIATION BETWEEN FOCAL AND DIFFUSE FORMS OF PERSISTENT HYPERINSULINEMIC HYPOGLYCEMIA OF INFANCY
The clinical presentation, biochemical profile of the patient, and conventional imaging modalities do not show whether the hyperinsulinism is due to a discrete adenoma or to a more diffuse form of nesidioblastosis.
Genetic analysis[28] has shown that focal PHHI is associated with loss of heterozygosity for paternally inherited mutations in the Katp genes.[29] On the other hand, children with diffuse PHHI have homozygous recessive or compound heterozygote mutations in the ABCC8 and KCNJ11 genes. Percutaneous transhepatic pancreatic venous catheterization with sampling and pancreatic arteriography[30] has been currently replaced by [18-F]-L-DOPA Positron Emission Tomography (PET) scan.
In diffuse PHHI, the PET scan shows uniform uptake of [18-F]-L-DOPA throughout the pancreas. In focal PHHI, the uptake of [18-F]-L-DOPA is concentrated within the foci of the disease. Intraoperative ultrasonic real-time navigation permits imaging of the focal lesion. It is also possible to delineate important anatomic landmarks such as the blood vessels and the relationship of the main pancreatic duct and the intrapancreatic common bile duct to the pancreatic foci. It also helps the surgeon to enucleate the entire focus while preserving the healthy pancreatic tissue.[31]
Intraoperative biopsy followed by a frozen section also helps differentiate between the focal and diffuse forms of the disease and defines the limits of surgical resection in cases with focal pathology.
SURGICAL MANAGEMENT
Indications of surgery include:
failure of medical management;
presence of a focal lesion which is amenable to surgical resection;
poor compliance to medical management.
The optimal extent of resection of pancreas has been a matter of controversy. The first report on major pancreatic resection for intractable hypoglycemia was by Graham and Hartman[32] as early as 1934. Gross[33] in 1953 reported on 65% pancreatic resection (lateral to the superior mesenteric vessels). However, the procedure was inherent with the problem of recurrent hypoglycemia requiring reoperation or continued medical therapy. Harken et al.,[34] suggested duodenum preserving total extirpation of the pancreas to address this issue. Localized resection of discretely palpable pancreatic nodules was suggested by Ravitch and Thomas.[35,36] There was a gradual shift in opinion towards 80–90% pancreatic resection,[36–38] which involved removal of all pancreatic tissue distal to the right side of the superior mesenteric vessels. This is associated with a 25–50% incidence of recurrent hypoglycemia. Schiller et al.,[39] added resection of the medial third of the pancreatic head and the uncinate process.
Recent literature supports near-total pancreatectomy as the definitive management option for diffuse PHHI. Kramer et al.,[35] and Moazam et al.,[37] have separately shown the efficacy of “near-total” pancreatectomy and shown it to be the procedure of choice in infants and neonates. Near-total pancreatectomy incorporates removal of the tail, body, uncinate process, and part of the pancreatic head. A rim of pancreatic tissue surrounding the common bile duct and along the duodenum is left behind. It has also been suggested that up to 98% of pancreatectomy may be required in refractory cases. Only small islands of pancreatic tissue are left along the pancreaticoduodenal arcade bordering the duodenum.
In patients with focal PHHI, a localized resection of the focal lesion is curative. Lesions of the pancreatic head and neck mandate open resection of the lesion with a small rim of surrounding normal tissue followed by a pancreatico-jejunostomy to drain the distal part of the pancreas. Lesions situated more distally may be managed with distal pancreatectomy.
Laparoscopic pancreatectomy[40] is associated with decreased blood loss, morbidity, and hospital stay. Cryopreservation of the islet cells followed by autotransplantation holds some promise against the subsequent development of diabetes mellitus.
POSTOPERATIVE MANAGEMENT
Surgery for nesidioblastosis represents an acute transition from a hyperinsulinimic state to a diabetic status. The patient should be kept on a continuous intravenous glucose drip accompanied by regular blood glucose monitoring. Most of the patients would need to be started on an insulin drip within the first 3 to 6 h to prevent glucose-induced diuresis. Patients should be started on alimentary feeds as soon the ileus has resolved. Once the oral alimentation is well established, the patients should be shifted to intermittent subcutaneous injections of insulin.
LONG-TERM OUTCOMES
Leibowitz et al., reported that 6 out of 14 patients treated with subtotal pancreatectomy developed diabetes before puberty as against none in the medically managed group.[41] The Children's Hospital of Philadelphia[42] reported experience of more than 250 cases of hyperinsulinism of infancy over the past 10 years. More than 50% of the lesions were found in the head of the pancreas (focal involvement). The extent of pancreatectomy in their series varied from 5% to 98%, most involved more than 50% resections. More than 90% of the focal hyperinsulinism cases were proven to be cured after surgery. However, in cases of diffuse hyperinsulinism, approximately 50% of the cases continued to have hypoglycemia after surgery which was managed medically. Another 25% of the cases were well controlled with no need for added medications and another one-fourth of the cases of diffuse hyperinsulinism managed surgically showed signs of insulin deficiency and required insulin replacement therapy.
CONCLUSION
With the deeper understanding of the disease process and the recent advances in imaging and surgical techniques, it is now possible to cure focal forms of PHHI completely. However, the management of diffuse PHHI is still a matter of great controversy. It is a double edged sword wherein delay in treatment may lead to developmental delay, epilepsy, and mental retardation, while near-total pancreatectomy is associated with the problems of recurrent postoperative hypoglycemia and diabetes mellitus in the long term.[43] In intractable cases, surgery if not curative helps in making the disease more manageable.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Laidlaw GF. Nesidioblastoma: The islet cell tumor of the pancreas. Am J Pathol. 1938;14:125–34. [PMC free article] [PubMed] [Google Scholar]
- 2.Brown RE, Young RB. A possible role for the exocrine pancreas in the pathogenesis of neonatal leucine-sensitive hypoglycemia. Am J Dig Dis. 1970;15:65–72. doi: 10.1007/BF02239348. [DOI] [PubMed] [Google Scholar]
- 3.Gould VE, Memoli VA, Dardi LE, Gould NS. Nesidiodysplasia and nesidioblastosis of infancy: Ultrastructural and immunohistochemical analysis of islet cell alterations with and without associated hyperinsulinaemic hypoglycaemia. Scand J Gastroenterol. 1981;70 Suppl:S129–42. [PubMed] [Google Scholar]
- 4.Glaser B, Landau H, Smilovic A, Nesher R. Persistent hyperinsulinemic hypoglycemia of infancy: Long-term treatment with the somatostatin analogue Sandostatin. Clin Endocrinol (Oxf) 1989;31:71–80. doi: 10.1111/j.1365-2265.1989.tb00455.x. [DOI] [PubMed] [Google Scholar]
- 5.Woo D, Seppes JW, Polak JM. Idiopathic hypoglycaemia in sibs with morphological evidence of nesidioblastosis of the pancreas. Arch Dis Child. 1976;51:528–31. doi: 10.1136/adc.51.7.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huopio H, Reimann F, Ashfield R, Komulainen J, Lenko HL, Rahier J, et al. Dominantly inherited hyperinsulinism caused by a mutation in the sulfonylurea receptor type 1. J Clin Invest. 2000;106:897–906. doi: 10.1172/JCI9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pipeleers D. The biosociology of pancreatic B cells. Diabetologia. 1987;30:277–91. doi: 10.1007/BF00299019. [DOI] [PubMed] [Google Scholar]
- 8.Sanvito F, Herrera PL, Huarte J, Nichols A, Montesano R, Orci L, et al. TGF-B1 influences the relative development of the exocrine and endocrine pancreas in vitro. Development. 1994;120:3451–62. doi: 10.1242/dev.120.12.3451. [DOI] [PubMed] [Google Scholar]
- 9.Resnick JM, Manivel JC. Immunohistochemical characterization of teratomatous and fetal neuroendocrine pancreas. Arch Pathol Lab Med. 1994;118:155–9. [PubMed] [Google Scholar]
- 10.Goudswaard WB, Houthoff HJ, Koudstaal J, Zwierstra RP. Nesidioblastosis and endocrine hyperplasia of the pancreas: A secondary phenomenon. Hum Pathol. 1986;17:46–54. doi: 10.1016/s0046-8177(86)80154-x. [DOI] [PubMed] [Google Scholar]
- 11.Lindley KJ, Dunne MJ, Kane C, Shepherd RM, Squires PE, James RF, et al. Ionic control of β cell function in nesidioblastosis. A possible therapeutic role for calcium channel blockade. Arch Dis Child. 1996;74:373–8. doi: 10.1136/adc.74.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aynsley-Green A, Dune MJ, James RF, Lindley KJ. Ions and genes in persistent hyperinsulinemic hypoglycemia in infancy: A commentary on the implications for tailoring treatment to disease pathogenesis. J Pediatr Endocrinol Metab. 1998;11:121–9. doi: 10.1515/jpem.1998.11.s1.121. [DOI] [PubMed] [Google Scholar]
- 13.De Lonlay P, Fournet JC, Rahier J, Gross-Morand MS, Poggi-Travert F, Foussier V, et al. Somatic deletion of the imprinted 11p15 region in sporadic persistent hyperinsulinemic hypoglycemia of infancy is specific of focal adenomatous hyperplasia and endorses partial pancreatectomy. J Clin Invest. 1997;100:802–7. doi: 10.1172/JCI119594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verkarre V, Fournet JC, de Lonlay P, Gross-Morand MS, Devillers M, Rahier J, et al. Paternal mutation of the sulfonylurea receptor (SUR1) gene and maternal loss of 11p15 imprinted genes lead to persistent hyperinsulinism in focal adenomatous hyperplasia. J Clin Invest. 1998;102:1286–91. doi: 10.1172/JCI4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, 4th, Boyd AE, 3rd, González G, et al. Cloning of the B cell high-affinity sulfonylurea receptor: A regulator of insulin secretion. Science. 1995;268:423–6. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 16.Thomas PM, Cote GJ, Wohllk N, Haddad B, Mathew PM, Rabl W, et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995;268:426–9. doi: 10.1126/science.7716548. [DOI] [PubMed] [Google Scholar]
- 17.Thomas P, Ye Y, Lightner E. Mutation of the pancreatic islet inward rectifier Kir6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum Mol Genet. 1996;5:1813–22. doi: 10.1093/hmg/5.11.1809. [DOI] [PubMed] [Google Scholar]
- 18.Glaser B, Kesavan P, Heyman M, Davis E, Cuesta A, Buchs A, et al. Familial hyperinsulinism caused by an activating glucokinase mutation. N Engl J Med. 1998;338:226–30. doi: 10.1056/NEJM199801223380404. [DOI] [PubMed] [Google Scholar]
- 19.Kukuvitis A, Deal C, Arbour L, Polychronakos C. An autosomal dominant form of familial persistent hyperinsulinemic hypoglycemia of infancy, not linked to the sulfonylurea receptor. J Clin Endocrinol Metab. 1997;82:1192–4. doi: 10.1210/jcem.82.4.3904. [DOI] [PubMed] [Google Scholar]
- 20.Stanley CA, Lieu YK, Hsu BY, Burlina AB, Greenberg CR, Hopwood NJ, et al. Hyperinsulinemia and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med. 1998;338:1352–7. doi: 10.1056/NEJM199805073381904. [DOI] [PubMed] [Google Scholar]
- 21.Conget I, Sarri Y, Somoza N, Vives M, Novials A, Ariza A, et al. Betacell function abnormalities in islets from an adult subject with nesidioblastosis and autoantibodies against the islet cells. Pancreas. 1997;14:71–5. doi: 10.1097/00006676-199701000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Rother KI, Carney JA, Couce M, Charlesworth J, Butler PC. Islet amyloid polypeptide in pancreatic tissue of children with persistent hyperinsulinemic hypoglycemia caused by primary islet hyperplasia and nesidioblastosis. J Clin Endocrinol Metab. 1995;80:1956–9. doi: 10.1210/jcem.80.6.7539820. [DOI] [PubMed] [Google Scholar]
- 23.De Lonlay-Debeney P, Poggi-Travert F, Fournet JC, Sempoux C, Vici CD, Brunelle F, et al. Clinical features of 52 neonates with hyperinsulinism. N Engl J Med. 1999;340:1169–75. doi: 10.1056/NEJM199904153401505. [DOI] [PubMed] [Google Scholar]
- 24.Ferry RJ, Jr, Kelly A, Grimberg A, Koo-McCoy S, Shapiro MJ, Fellows KE, et al. Calcium-stimulated insulin secretion in diffuse and focal forms of congenital hyperinsulinism. J Pediatr. 2000;137:239–46. doi: 10.1067/mpd.2000.107386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimberg A, Ferry RJ, Jr, Kelly A, Koo-McCoy S, Polonsky K, Glaser B, et al. Dysregulation of insulin secretion in children with congenital hyperinsulinism due to sulfonylurea receptor mutations. Diabetes. 2001;50:322–8. doi: 10.2337/diabetes.50.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aynsley-Green A, Hussain K, Hall J, Saudubray JM, Nihoul-Fékété C, De Lonlay-Debeney P, et al. Practical management of hyperinsulinism in infancy. Arch Dis Child Fetal Neonatal Ed. 2000;82:F98–107. doi: 10.1136/fn.82.2.F98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cornblath M, Schwartz R. Major Problems in Clinical Pediatrics Series. 2nd ed. Vol. 1. Philadelphia: Saunders; 1976. Disorders of carbohydrate metabolism in infancy; pp. 180–6. [PubMed] [Google Scholar]
- 28.James C, Kapoor RR, Ismail D, Hussain K. The genetic basis of congenital hyperinsulinism. J Med Genet. 2009;46:289–99. doi: 10.1136/jmg.2008.064337. [DOI] [PubMed] [Google Scholar]
- 29.Glaser B, Kesavan P, Heyman M, Davis E, Cuesta A, Buchs A, et al. Familial hyperinsulinism caused by an activating glucokinase mutation. N Engl J Med. 1998;338:226–30. doi: 10.1056/NEJM199801223380404. [DOI] [PubMed] [Google Scholar]
- 30.Dubois J, Brunelle F, Touati G, Sebag G, Nuttin C, Thach T, et al. Hyperinsulinism in children: Diagnostic value of pancreatic venous sampling correlated with clinical, pathological and surgical outcome in 25 cases. Pediatr Radiol. 1995;25:512–6. doi: 10.1007/BF02015782. [DOI] [PubMed] [Google Scholar]
- 31.Von Rohden L, Mohnike K, Mau H, Eberhard T, Mohnike W, Blankenstein O, et al. Visualization of the focus in congenital hyperinsulinism by intraoperative sonography. Semin Pediatr Surg. 2011;20:28–31. doi: 10.1053/j.sempedsurg.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Graham EA, Hartmann AF. Subtotal resection of the pancreas for hypoglycemia. Surg Gynecol Obstet. 1934;59:474–9. [Google Scholar]
- 33.Gross RE. The Surgery of Infancy and Childhood. Philadelphia: WB Saunders; 1953. p. 574. [Google Scholar]
- 34.Harken AH, Filler RM, AvRuskin TW, Crigler JF., Jr The role of “total” pancreatectomy in the treatment of unremitting hypoglycemia of infancy. J Pediatr Surg. 1971;6:284–9. doi: 10.1016/0022-3468(71)90469-6. [DOI] [PubMed] [Google Scholar]
- 35.Kramer JL, Bell MJ, DeSchryver K, Bower RJ, Ternberg JL, White NH. Clinical and histologic indications for extensive pancreatic resection in nesidioblastosis. Am J Surg. 1982;143:116–9. doi: 10.1016/0002-9610(82)90140-4. [DOI] [PubMed] [Google Scholar]
- 36.McFarland JO, Gillett S, Swemer RJ. Total pancreatectomy for hyperinsulinism in infants. Surgery. 1965;57:313–8. [Google Scholar]
- 37.Moazam F, Rodgers BM, Talbert JL, Rosenbloom AL. Near-total pancreatectomy in persistent infantile hypoglycemia. Arch Surg. 1982;117:1151–4. doi: 10.1001/archsurg.1982.01380330019006. [DOI] [PubMed] [Google Scholar]
- 38.Ravitch MM. The pancreas in infants and children. Surg Clin North Am. 1975;55:377–85. doi: 10.1016/s0039-6109(16)40587-6. [DOI] [PubMed] [Google Scholar]
- 39.Schiller M, Krausz M, Meyer S, Lijovetzky G, Landau H. Neonatal hyperinsulinism-Surgical and pathologic considerations. J Pediatr Surg. 1980;15:16–20. doi: 10.1016/s0022-3468(80)80395-2. [DOI] [PubMed] [Google Scholar]
- 40.Tang CN, Tsui KK, Ha JP, Wong DC, Li MK. Laparoscopic distal pancreatectomy: A comparative study. Hepatogastroenterology. 2007;54:265–71. [PubMed] [Google Scholar]
- 41.Leibowitz G, Glaser B, Higazi AA, Salameh M, Cerasi E, Landau H. Hyperinsulinemic hypoglycemia of infancy (nesidioblastosis) in clinical remission: High incidence of diabetes mellitus and persistent beta-cell dysfunction at long term follow-up. J Clin Endocrinol Metab. 1995;80:386–92. doi: 10.1210/jcem.80.2.7852494. [DOI] [PubMed] [Google Scholar]
- 42.Palladino AA, Stanley CA. A specialized team approach to diagnosis and medical versus surgical treatment of infants with congenital hyperinsulinism. Semin Pediatr Surg. 2011;20:32–7. doi: 10.1053/j.sempedsurg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Huopio H, Otonkoski T, Vauhkonen I, Reimann F, Ashcroft FM, Laakso M. A new subtype of autosomal dominant diabetes attributable to a mutation in the gene for sulfonylurea receptor 1. Lancet. 2003;361:301–7. doi: 10.1016/S0140-6736(03)12325-2. [DOI] [PubMed] [Google Scholar]


