Abstract
OBJECTIVE:
Appropriate dosage of the long-acting depot gonadotrophin releasing hormone (GnRH) agonist has not been determined in long protocol for IVF, and one-third-dose depot triptorelin was compared with half-dose in a luteal long protocol of in-vitro fertilization/ intra cytoplasmic sperm injection (IVF/ICSI) treatment in this study.
MATERIALS AND METHODS:
This is a prospective, randomized, open clinical trial. 100 patients were randomized into two groups. Group I received one-third-dose (1.25 mg) depot triptorelin. Group II received half-dose (1.87 mg). The clinical and experimental parameters were compared between the two groups.
RESULTS:
There was no premature luteinizing hormone (LH) surge in both groups. On Day 3–5 of menstrual cycle after down-regulation, fewer patients showed low-level LH (<1.0 IU/L) and estradiol (<30 pg/mL) in group I (P <0.05). There were fewer oocytes retrieved (P =0.086), fewer total embryos and available embryos for cryopreservation in Group I (P <0.05), while good-quality embryo rate was higher in group I (P <0.05). The length and dose of ovarian stimulation was lower in Group I, but not significantly. The clinical pregnancy (52% versus 40%), implantation (48% versus 37.5%), delivery (46% versus 32%), or live birth (42% versus 32%) rates and the abortion (8% versus 20%) rates showed no significant differences.
CONCLUSION:
Depot triptorelin 1.25 mg can be successfully used with reduced pituitary suppression and lower cost in a long protocol for in-vitro fertilization.
KEY WORDS: GnRH agonist, in-vitro fertilization, ovarian stimulation, triptorelin
INTRODUCTION
The benefits of GnRH agonist (GnRHa) used for controlled ovarian stimulation (COS) are well known.[1,2] There are two types of GnRHa, the multi-low-dose (short-acting) or the single depot (long-acting). The depo GnRHa has been welcomed because of the single administration.
The currently available depot products were prepared for endometriosis or fibroids, the objective being to achieve a profound pituitary desensitization for a protracted period of time. However, because of oversuppression, the ampoules of gonadotrophin used and the duration of COS cycle were increased without improving clinical outcomes when depot GnRHa was compared with daily-low-dose GnRHa in a recent Cochrane Review.[3]
It might be enough to induce partial pituitary desensitization for IVF.[4–6] Halving the dose of depot triptorelin has been studied against its full dose with similar or better clinical outcomes.[7,8] Following that, two studies compared a half-dose depot GnRHa with short-acting GnRHa in a long protocol.[9,10] They showed similar results, but the length of COS was longer[9,10] and LH and estradiol levels were lower.[9] That indicates further reduced dose of depot GnRHa should be better.
Thus, we originally compared a one-third-dose depot triptorelin with a half-dose depot triptorelin in a long protocol.
MATERIALS AND METHODS
Study design
The study was a prospective, randomized, open (no blind) clinical trial and approved by the ethics committee of Sun Yat-sen Memorial Hospital of Sun Yat-sen University. A signed informed consent was obtained from all patients participating in the study.
Patients
Because of no previous study for calculating the sample size, we decided that 100 patients would be enrolled in this prospective, randomized, and open clinical trial. Inclusion criteria were: (1) patients were ≤38 years old with infertility caused by tubal, idiopathic, male factors or polycystic ovary syndrome (PCOS); (2) they had body mass index (BMI) of no more than 28 kg/m2; (3) serum Follicle stimulating hormone (FSH) level was under 12 IU/L on Day 3 of the previous menstrual cycle; (4) they needed to undergo their first or second IVF/ICSI treatment cycle; and (5) they had no active endometriosis, adenomyoma or uterine fibroid. From January to December in 2005, the eligible patients were randomized by a staff nurse into two treatment groups on the day of initiating down-regulation, using a sealed envelope supplied by the statistician, and then no blindness for patients and clinicians. In group I (50 patients), pituitary desensitization was obtained with a one-third-dose (1.25 mg) depot triptorelin (Dipherelin 3.75 mg, Beaufour Ipsen, Paris, France) in a single i.m. injection in mid-luteal phase (Day 21) of the menstrual cycle preceding treatment or on Day 16 of oral contraceptive pill, and in group II (50 patients) with a half-dose (1.88 mg) depot triptorelin on the same timing.
The 3.75-mg triptorelin depot was mixed with 2 mL sterile water for injection, then shaking the vial well after mixing, and used right away with 1 mL sterile syringe; 0.67 mL and 1.0 mL medication contained 1.25 mg and 1.88 mg triptorelin depot, respectively.
COS and IVF/ICSI procedure
After the confirmation of pituitary down-regulation on Day 3–5 of the cycle by sonographic detection of an endometrium with the thickness of ≤5 mm and suppressed ovaries (no antral follicles ≥10 mm) and serum estradiol levels of ≤50 pg/ mL and LH levels of ≤5 IU/L, gonadotrophin stimulation with recombinant FSH (Gonal-F, Serono, Switzerland) was started at the usual dose of 2–3 ampoules (150–225 IU) daily. We adjusted the initial and ongoing dosage according to the patient's age, antral follicle count, BMI, basic FSH level, and follicular growth response. Ovulation was induced with 10,000 IU of hCG (Profasi, Serono, Switzerland) when at least one follicle had reached a diameter of ≥18 mm. Oocytes were retrieved 36–37 h later by guidance of vaginal ultrasound.
Standard laboratory protocols for conventional IVF and ICSI were followed.[11] Embryos were transferred on Day 3. Evaluation of the quality of embryos on Day 3 according to “embryo grading” and embryos of modified Hu's grades 1 or 2 were considered of high quality (6–9 cell; blastomeres of equal size; 0–20% cytoplasmic fragments).[12] As a rule, and when available, two embryos were transferred in young women (<35 years) and three embryos were transferred in older women (35–38 years) or repeated IVF cycles. Embryo transfers were done under ultrasound guidance with a full bladder using a Wallace catheter (Wallace Ltd, Colchester, England). Luteal phase support was sustained with natural progesterone in oil 40 mg i.m. daily from the day of oocyte retrieval.
Blood assays for β-hCG measurement were performed 13 days after embryo transfer, with a result > 25 IU/L being considered biochemical pregnancy. Clinical pregnancy was confirmed with the detection of gestational sacs by ultrasound 4–5 weeks after the embryo transfer. Early pregnancy loss was defined as a biochemical pregnancy without subsequent ultrasound signs of gestational sacs. Implantation rate was calculated as the ratio of the number of gestational sacs over the number of transferred embryos. Early miscarriage was defined as a pregnancy failing to reach the 12th gestational week. Pregnancies were followed to term by obstetricians at the patients’ local hospitals. Every pregnant woman was contacted on pregnancy outcome.
Serum FSH, LH, estradiol, and progesterone determination
Blood samples were obtained on Day 3–5 of menstrual cycle just before gonadotrophin was commenced for FSH, LH, and estradiol activity. Blood sample were obtained on the day of hCG administration and the day of embryo transfer for LH, estradiol, and progesterone activity. All samples were centrifuged at 2000 g for 15 min, and measurements were performed on the same day. FSH, LH, estradiol, and progesterone concentrations were measured by chemiluminescent immunoassay (Beckman Coulter Access® 2 Immunoassay System (Beckman Coulter, Fullerton, USA). The assay's intra- and inter-assay coefficients of variation for FSH, LH, estradiol, and progesterone were 3.1% and 5.4%, 3.6% and 4.3%, <8% and <12%, and 6.1% and 7.51%, respectively.
Assessment
The primary endpoint was LH levels during the procedure of COS. The secondary outcomes were: length of gonadotrophin stimulation, amount of gonadotrophins used, peak estradiol on day of hCG administration, number of oocytes retrieved, clinical pregnancy rate per embryo transfer, implantation rate, first trimester abortion rate, and live birth rate.
Statistical analysis
Independent samples t-test or Mann–Whitney test were used for continuous variables in both groups. Dichotomous variables were analyzed by chi-square test or Fisher's exact test, as appropriate. SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) was used for data analysis. P <0.05 was considered statistically significant.
RESULTS
The clinical characteristics of the patients were comparable between the two groups [Table 1]. All 100 patients completed the treatment cycle and follow-up. LH and progesterone assays performed in each patient on the day of hCG administration showed no evidence of premature LH surge or luteinization in either group. Table 2 shows the hormonal pattern of the two groups of patients during ovarian stimulation. There were no significant differences in FSH, LH, and E2 levels on Day 3–5 of menstrual cycle just before initiating gonadotrophin after down-regulation and LH, Estradiol and Progesterone levels on the days of hCG administration and embryo transfer. However, on Day 3–5 of menstrual cycle just before initiating gonadotrophin after down-regulation, fewer patients showed low-level LH (<1.0 IU/L) and low-level estradiol E2 (<30 pg/mL) in Group I (P <0.05).
Table 1.
Basic characteristics of the two groups
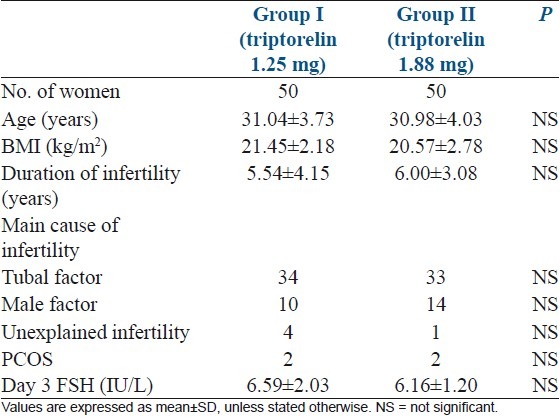
Table 2.
Comparison of hormone levels during ovarian stimulation between two groups
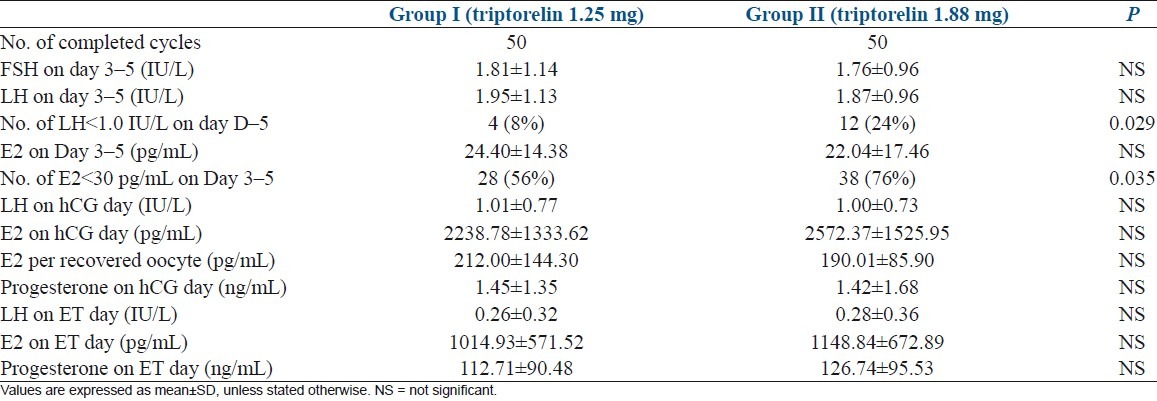
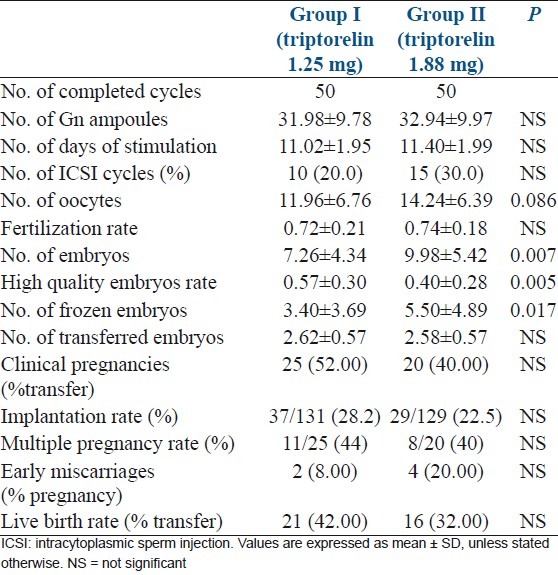
The length of ovarian stimulation—the total dose of gonadotrophin used, and the number of oocytes retrieved showed no significantly differences (P >0.05) [Table 3]. There were fewer total embryos and available embryos for cryopreservation in Group I (P <0.05), while high-quality embryo rate in was higher in group I (P <0.05) [Table 3]. Fertilization rate and number of transferred embryos were not significantly different between two groups [Table 3]. Clinical pregnancy, implantation, live birth, and early miscarriage rates showed no significantly differences between two groups (P >0.05) [Table 3].
Table 3.
The results of ovarian stimulation between two groups
DISCUSSION
The use of a single-dose depot long-acting GnRHa in a long protocol offers the advantages of better compliance, convenience, and economy of cost for patients with less stress of injections and risk of infection. The recent Cochrane Review showed no significant difference in clinical pregnancy rate between the long protocol using depot or daily GnRHa for IVF cycles.[3] However, because of profound pituitary desensitization, depot formulations require more gonadotrophin ampoules, which are needed for ovarian stimulation, and a significantly longer duration of ovarian stimulation than daily GnRHa.[3]
Previous study demonstrated a direct correlation between the reduction of the daily dosage of short-acting triptorelin and the decrease in the degree of pituitary suppression.[5] To find a lower depot dose with sufficient suppression potential, several studies have shown that a half-dose (1.88 mg) of triptorelin injection was sufficient to prevent LH surges, and similar or better IVF outcomes were obtained comparing full-dose triptorelin with half dose in COS.[7,8,13] A half-dose depot triptorelin in a long protocol has been accepted in IVF treatment. From 2001 to 2004, a half-dose depot triptorelin was routinely used for the long protocol of COS in our IVF centre. However, when comparing half-dose depot GnRHa with daily short-acting GnRHa in a long protocol clinical, outcomes were not significantly different, but the length of gonadotrophin stimulation was significantly longer and estradiol level on Day 3 and LH level on hCG day were lower in the protocol of half-dose depot GnRHa.[9,10] That indicated the half-dose depot GnRHa would still cause profound suppression for ovarian stimulation.
Thus, to investigate the efficacy of the further reduced depot dose in this study, we originally compared a one-third-dose depot triptorelin (1.25 mg) with a half-dose depot triptorelin (1.88 mg) in a long protocol for IVF or ICSI and embryo transfer cycles. Both one-third dose and half-dose group showed adequate suppression and no premature LH surge. There were fewer cases with LH <1.0 IU/L and estradiol <30 pg/mL on Day 3–5 after down-regulation in one-third-dose group (P <0.05). This indicated that reduced depot GnRHa deceased the degree of suppression. There were fewer but still appropriate oocytes retrieved (P =0.086) and embryos obtained (P <0.05) in the one-third-dose group. It seemed that there was a dose-finding effect of GnRHa on the numbers of oocytes and embryos in women with normal ovarian reserve.
Several studies demonstrated the similar dose-finding effect of GnRHa on the degree of pituitary suppression with different doses of daily GnRHa or full-dose and half-dose depot GnRHa.[5,7,8] For the dose-finding effect of GnRHa on the numbers of oocytes and embryos, previous studies show controversial results. Janssens et al. reported firstly a dose effect of short-acting daily triptorelin on the numbers of oocytes and embryos in normal responders.[5] They mentioned the possible mechanisms: A longer duration of stimulation with FSH with higher daily doses of agonist enables more follicles to enter the stage FSH-dependent growth; a greater suppression of endogenous LH than FSH by the higher doses of GnRHa results in an increase in the FSH/LH ratio during the follicular phase.[5] A recently study would suggest another mechanism, which showed that pituitary desensitization with GnRHa results in a significant increase in anti-mullerian hormone (AMH) levels, which may explain the enhanced ovarian response to conventional controlled ovarian stimulation.[14] But it is still unknown whether the effect of GnRHa on AMH levels is associated with the dose of GnRHa. These findings contrast with the work by Dal Prato et al.[8] In their study, the low dose (half-dose) depot triptorelin gave rise to a higher number of oocytes and embryos than the full-dose depot triptorelin. The difference between the two studies might result from the different degree of pituitary suppression. Full-dose depot GnRHa in their study may cause too profound suppression and impair ovarian response to exogenous FSH. Another study by Yim et al.[7] showed no difference in the number of oocytes and embryos with full-dose or half-dose depot triptorelin. The difference lies probably in the kind of gonadotrophin used: rFSH (no LH activity) in the present study and hMG (with LH activity) in that of Yim et al. The administration of hMG may have partially balanced the effects of the different degrees of pituitary suppression on ovarian response.[7]
In the present study, there was a higher rate of high-quality embryos in one-third-dose group than that in half-dose group (P <0.05). Clinical pregnancy, implantation, live birth, and early miscarriage rates showed no significant differences. Previous two studies compared a half-dose and full-dose depot triptorelin in the long protocol of IVF. In the study of Dal Prato et al., a higher number of good-quality embryos and a higher cumulative pregnancy rate were obtained in the half-dose group than the full-dose group.[8] In the present study, similar results showed that decreased suppression improved the quality of embryos, and it is probably due to small sample size that the differences of clinical outcomes were not found significantly. In the study of Yim et al., the data of embryo quality was not mentioned and the clinical outcome showed no differences.[7] The difference lies probably in the kind of gonadotrophin used, just mentioned above.
Both FSH and LH are required for a normal follicular growth and maturation according to the two-cell, two-gonadotrophin theory. Previous study in GnRH antagonist down-regulated primates shows that an intrafollicular environment depleted of LH and estradiol impairs oocyte maturation, embryo development, and implantation.[15] Clinical studies in women with hypogonadal hypogonadism have shown that while follicular development can be achieved by stimulation with pure FSH preparations, concentrations of circulating estradiol and fertilization rates of retrieved oocytes are severely compromised compared with stimulation with preparations containing LH activity.[16,17]
For IVF treatment with GnRHa down-regulation and FSH stimulation, profound pituitary gland suppression with LH levels of <0.5 IU/L led to significantly lower E2 production,[18–20] fewer oocytes, lower fertilization rate, and fewer embryos that were available for cryopreservation.[18,20] An increased risk of early pregnancy loss has also been suggested.[19]
Moreover, the study by Tesarik et al. has suggested that endometrial maturation is disturbed in women with low endogenous LH, and a direct action of LH on uterus may be required to support endometrial growth and uterine receptivity.[21] Another study shows that the expression of endometrial estrogens and progesterone receptors is altered in COS cycles with GnRH agonist and low LH levels.[22] GnRHa has been shown to cause extra-pituitary side effects, including direct inhibition of ovarian steroidogenesis[23] and effects on the differentiation of granulose cells.[24] These findings should furthermore suggest the use of the possibly lower dose of GnRH agonist.
Finally, lower dose of depot GnRHa might reduce the cost for patients undergoing assisted reproduction treatment. In our IVF centre, three patients can share one ampoule of depot triptorelin. Decreased pituitary suppression by lowering the dose of depot GnRHa in our trial suggests that one-third-dose of depot GnRHa may also reduce the LH requirement in patients undergoing IVF treatment.
There were some limitations in the present study. Although sample size was enough for evaluating the degree of pituitary suppression, it may be not adequate for comparing the clinical outcome after IVF. Further reduced dose of triptorelin depot and dose-finding effect had not been studied. Optimal dose of long-acting GnRH agonist for COS should be investigated.
CONCLUSION
In conclusion, it has been shown in our study that one-third-dose (1.25 mg) depot triptorelin can be successfully used in ovarian stimulation for IVF or ICSI. LH and estradiol levels confirm the reduced pituitary suppression caused by one-third-dose than half-dose depot triptorelin. Higher good-quality embryo rate could be explained better quality oocytes, while clinical outcome were similar between two groups. Whether a further reduced dose of depot GnRHa still results in a similar or better outcome remains to be determined.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Caspi E, Ron-El R, Golan A, Nachunv A, Herman A, Soffer Y, et al. Results of in vitro fertilization and embryo transfer by combined long-acting gonadotrophin-releasing hormone analog D-Trp-6-luteinizing hormone-releasing hormone and gonadotrophins. Fertil Steril. 1989;51:95–9. doi: 10.1016/s0015-0282(16)60435-1. [DOI] [PubMed] [Google Scholar]
- 2.Liu HC, Lai YM, Davis O, Berkeley AS, Graf M, Grifo J, et al. Improved pregnancy outcome with gonadotrophin releasing hormone agonist stimulation is due to the improvement in oocytes quantity rather than quality. J Assist Reprod Genet. 1992;9:338–42. doi: 10.1007/BF01203956. [DOI] [PubMed] [Google Scholar]
- 3.Albuquerque LE, Saconato H, Maciel MC. Depot versus daily administration of gonadotrophin releasing hormone agonist protocols for pituitary desensitization in assisted reproduction cycles. Cochrane Database Syst Rev. 2005;25:CD002808. doi: 10.1002/14651858.CD002808.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Olivennes F, Righini C, Fanchin R, Torrisi C, Hazout A, Glissant M, et al. A protocol using a low dose of gonadotrophin-releasing hormone agonist might be the best protocol for patients with high follicle-stimulating hormone concentrations on day 3. Hum Reprod. 1996;11:1169–72. doi: 10.1093/oxfordjournals.humrep.a019348. [DOI] [PubMed] [Google Scholar]
- 5.Janssens RM, Lambalk CB, Vermeiden JP, Schats R, Bernards JM, Rekers-Mombarg LT, et al. Dose-finding study of triptorelin acetate for prevention of a premature LH surge in IVF: A prospective, randomized, double-blind, placebo-controlled study. Hum Reprod. 2000;15:2333–40. doi: 10.1093/humrep/15.11.2333. [DOI] [PubMed] [Google Scholar]
- 6.Dal Prato L, Borini A, Trevisi MR, Bonu MA, Sereni E, Flamigni C. Effect of reduced dose of triptorelin at the start of ovarian stimulation on the outcome of IVF: A randomized study. Hum Reprod. 2001;16:1409–14. doi: 10.1093/humrep/16.7.1409. [DOI] [PubMed] [Google Scholar]
- 7.Yim SF, Lok IH, Cheung LP, Briton-Jones CM, Chiu TT, Haines CJ. Dose-finding study for the use of long-acting gonadotrophin-releasing hormone analogues prior to ovarian stimulation for IVF. Hum Reprod. 2001;16:492–4. doi: 10.1093/humrep/16.3.492. [DOI] [PubMed] [Google Scholar]
- 8.Dal Prato L, Borini A, Coticchio G, Cattoli M, Flamigni C. Half-dose depot triptorelin in pituitary suppression for multiple ovarian stimulation in assisted reproduction technology: A randomized study. Hum Reprod. 2004;19:2200–5. doi: 10.1093/humrep/deh415. [DOI] [PubMed] [Google Scholar]
- 9.Isikoglu M, Ozdem S, Berkkanoglu M, Jamal H, Senturk Z, Ozgur K. Single-dose depot leuprolide is as efficient as daily short-acting leuprolide in ICSI cycles. Hum Reprod. 2007;22:1657–61. doi: 10.1093/humrep/dem054. [DOI] [PubMed] [Google Scholar]
- 10.Safdarian L, Mohammadi FS, Alleyassin A, Aghahosseini M, Meysamie A, Rahimi E. Clinical outcome with half-dose depot triptorelin is the same as reduced-dose daily buserelin in a long protocol of controlled ovarian stimulation for ICSI/embryo transfer: A randomized double-blind clinical trial ( NCT00461916) Hum Reprod. 2007;22:2449–54. doi: 10.1093/humrep/dem223. [DOI] [PubMed] [Google Scholar]
- 11.Magli MC, Van den Abbeel E, Lundin K, Royere D, Van der Elst J, Gianaroli L Committee of the Special Interest Group on Embryology. Revised guidelines for good practice in IVF laboratories. Hum Reprod. 2008;23:1253–62. doi: 10.1093/humrep/den068. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y, Maxon WS, Hoffman DI, Ory SJ, Eager S, Dupre J, et al. Maximizing pregnancy rates and limiting higher-order multiple conceptions by determining the optimal number of embryos to transfer based on quality. Fertil Steril. 1998;69:650–7. doi: 10.1016/s0015-0282(98)00024-7. [DOI] [PubMed] [Google Scholar]
- 13.Balasch J, Gomez F, Casamitjana R, Carmona F, Rivera F, Vanrell JA. Pituitary-ovarian suppression by the standard and half-doses of D-Trp-6-luteinizing hormone-releasing hormone depot. Hum Reprod. 1992;7:1230–4. doi: 10.1093/oxfordjournals.humrep.a137832. [DOI] [PubMed] [Google Scholar]
- 14.Jayaprakasan K, Campbell BK, Hopkisson JF, Clewes JS, Johnson IR, Raine-Fenning NJ. Effect of pituitary desensitization on the early growing follicular cohort estimated using anti-Mullerian hormone. Hum Reprod. 2008;23:2577–83. doi: 10.1093/humrep/den282. [DOI] [PubMed] [Google Scholar]
- 15.Weston AM, Zelinski-Wooten MB, Hutchison JS, Stouffer RL, Wolf DP. Development potential of embryos produced by in-vitro fertilization from gonadotrophin-releasing hormone antagonist-treated macaques stimulated with recombinant human follicle stimulating hormone alone or in combination with luteinizing hormone. Hum Reprod. 1996;11:608–13. doi: 10.1093/humrep/11.3.608. [DOI] [PubMed] [Google Scholar]
- 16.Shoham Z, Balen A, Patel A. Results of ovulation induction using human menopausal gonadotrophin and purified follicle-stimulating hormone in hypogonadotrophic hypogonadism patients. Fertil Steril. 1991;56:1048–53. doi: 10.1016/s0015-0282(16)54715-3. [DOI] [PubMed] [Google Scholar]
- 17.Balasch J, Miro F, Burzaco I. The role of luteinizing hormone in human follicle development and oocyte fertility: Evidence from in vitro fertilization in a woman with long-standing hypogonadotrophic hypogonadism and using recombinant human follicule stimulating hormone. Hum Reprod. 1995;10:1678–83. doi: 10.1093/oxfordjournals.humrep.a136154. [DOI] [PubMed] [Google Scholar]
- 18.Fleming R, Lloyd F, Herbert M, Fenwick J, Griffith T, Murdoch A. Effect of profound suppression of LH during ovarian stimulation on follicular activity, oocyte and embryo function in cycles stimulated with purified FSH. Hum Reprod. 1998;13:1788–92. doi: 10.1093/humrep/13.7.1788. [DOI] [PubMed] [Google Scholar]
- 19.Westergaard LG, Laursen SB, Anderson CY. Increased risk of early pregnancy loss by profound suppression of luteinising hormone during ovarian stimulation in normogonadotrophic women undergoing assisted reproduction. Hum Reprod. 2000;15:1003–8. doi: 10.1093/humrep/15.5.1003. [DOI] [PubMed] [Google Scholar]
- 20.Humaidan P, Bungum L, Bungum M, Andersen CY. Ovarian response and pregnancy outcome related to mid-follicular LH levels in women undergoing assisted reproduction with GnRH agonist down-regulation and recombinant FSH stimulation. Hum Reprod. 2002;17:2016–21. doi: 10.1093/humrep/17.8.2016. [DOI] [PubMed] [Google Scholar]
- 21.Tesarik J, Hazout A, Mendoza C. Luteining hormone affects uterine receptivity independently of ovarian function. RBM Online. 2003;7:59–64. doi: 10.1016/s1472-6483(10)61729-4. [DOI] [PubMed] [Google Scholar]
- 22.Bourgain C, Ubaldi F, Tavaniotou A, Smitz J, Van Steirteghem AC, Devroey P. Endometrial hormone receptors and proliferation index in the periovulatory phase of stimulated embryo transfer cycles in comparison with natural cycles and relation to clinical pregnancy outcome. Fertil Steril. 2002;78:237–44. doi: 10.1016/s0015-0282(02)03228-4. [DOI] [PubMed] [Google Scholar]
- 23.Dor J, Bider D, Shulman A, Levron J, Shine S, Mashiach S, et al. Effects of gonadotrophin-releasing hormone agonists on human ovarian steroid secretion in vivo and in vitro—results of a prospective, randomized in-vitro fertilization study. Hum Reprod. 2000;15:1225–30. doi: 10.1093/humrep/15.6.1225. [DOI] [PubMed] [Google Scholar]
- 24.Gerrero H, Stein P, Asch R, de Fried E, Tesone M. Effect of a gonodotropin-releasing hormone agonist on luteinizing homone receptors and steroidogenesis in ovarian cells. Fertil Steril. 1993;59:803–8. [PubMed] [Google Scholar]


