Abstract
OBJECTIVE:
The aim of the present study was to evaluate the prevalence of metabolic syndrome in women with polycystic ovary syndrome (PCOS).
SETTING:
Infertility clinic in a tertiary care hospital.
STUDY DESIGN:
A prospective cross-sectional study.
MATERIALS AND METHODS:
All the women attending the infertility clinic categorized as polycystic ovary syndrome according to Rotterdam criteria (2003) during the study period were included in the study. The women with PCOS underwent screening for metabolic syndrome as defined by the modified American Heart Association/National Heart Lung Blood Institute (AHA/NHLBI) modified ATP 111 (2005) definition. A multivariate logistic regression analysis was applied and significant predictors identified for the prediction of metabolic syndrome.
RESULTS:
The overall prevalence of metabolic syndrome according to the modified AHA/NHLBI ATP III (2005) criteria was 37.5%. A total of 5.8 % cases were detected to have diabetes mellitus, 8.3% had impaired fasting glucose, and 11.7 % had an impaired glucose test. Dyslipidemia was present in 93.3% cases of PCOS. Among all the risk factors, age and waist hip ratio ≥0.85 were strongly associated with the presence of metabolic syndrome.
CONCLUSION:
Infertile women with PCOS, particularly those with age ≥25 years or with central obesity (a waist hip ratio of ≥0.85), are at a higher risk of developing metabolic syndrome and should be offered screening tests.
KEY WORDS: Metabolic syndrome, polycystic ovary syndrome, screening
INTRODUCTION
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders in women, affecting approximately 5% to 8% of premenopausal women.[1] It is characterized by chronic anovulation, oligomenorrhea or amenorrhea, hyperandrogenism, and polycystic ovary morphology on pelvic ultrasound. It also has a metabolic component consisting of hyperinsulinemia and insulin resistance with increased cardiovascular disease risk, which occurs both in lean and obese women with the disorder, and has a strong association with metabolic syndrome.[2–4]
Metabolic syndrome is another cluster of endocrine disturbances, including insulin resistance, dyslipidemia, obesity, and hypertension.[5] It is associated with a two-fold increased risk of cardiovascular disease and a five-fold increased risk of type 2 diabetes.[6] This illustrates the importance of early detection of insulin resistance and metabolic syndrome with subsequent application of preventive measures in women with polycystic ovary syndrome.
The original National Cholesterol Education Programme – Adult Treatment Panel III (NCEP - ATP111) criteria in 2001 defines metabolic syndrome as the co-occurrence of three or more of the following risk factors (i) central obesity with waist circumference ≥88 cm in women, (ii) elevated systolic and/or diastolic blood pressure of ≥130/85 mmHg, (iii) impaired fasting serum glucose ≥110 mg/dL, (iv) elevated fasting serum triglycerides ≥150 mg/dL, and (v) fasting high-density lipoprotein (HDL) cholesterol <50 mg/dL.[7]
The main changes in the modified American Heart Association/National Heart Lung and Blood Institute definition (ATP III 2005) include (i) defining the ethnic-specific difference in central obesity by using the World Health Organization recommendation for waist circumference ≥80 cm in Asian women, and (ii) and reducing the threshold for impaired fasting glucose to 100 mg% in accordance with the American Diabetes Association revised definition.[6]
The prevalence of metabolic syndrome in polycystic ovary syndrome has been studied in different populations. Reported prevalence is 43% in U.S., 28.4% in Brazil, 24.9% in Hong Kong Chinese women, and only 1.6% in Czech women.[8–11]
These varied data indicate the need for evaluation of metabolic syndrome in different populations, as it would help in planning screening strategies to prevent long-term effects.
Hence, the aim of this study was to estimate the prevalence of metabolic syndrome in infertile women with PCOS presenting in our clinic.
MATERIALS AND METHODS
A prospective cross-sectional study was planned on women with PCOS who were being evaluated for infertility in an Infertility clinic of a tertiary care hospital between October 2009 and May 2010. All consecutive women with PCOS who presented with infertility were invited to participate in the study. A total of 120 women with PCOS who presented with infertility were enrolled for the study. The diagnosis was based on the 2003 Rotterdam consensus (the Rotterdam European Society for Human Reproduction and Embryology (EHSRE)/American Society for Reproductive Medicine (ASRM) – sponsored PCOS consensus workshop group) with at least two of the following features: (i) oligo-ovulation or chronic anovulation, (ii) clinical and/or biochemical hyperandrogenism, and (iii) ultrasound appearance of polycystic ovaries. Other etiologies that could mimic PCOS were excluded by doing appropriate blood tests depending on the clinical suspicion - 17 hydroxy progesterone for excluding late-onset congenital hyperplasia, dehydroepiandrosterone-sulphate for excluding adrenal tumors, and 24-h urinary free cortisol for excluding Cushing's syndrome. Women with steroid or oral contraceptive drug intake in the preceding 3 months as well as previously diagnosed diabetes were also excluded from the study.
Oligo-ovulation and/or anovulation was characterized by oligomenorrhea (intermenstrual intervals of ≥35 days) and amenorrhea (intervals >3 months). Clinical hyperandrogenism was defined as the presence of hirsutism (Ferriman-Gallwey score of ≥8) and/or acne. Biochemical hyperandrogenism was present if calculated free testosterone level was more than 2.06%. Polycystic ovary on ultrasound was defined as the presence of at least one ovary 10 cm3 or more in volume and/or at least one ovary with 12 or more follicles measuring 2–9 mm in diameter.
Metabolic syndrome was defined according to the modified American Heart Association/National Heart Lung Blood Institute AHA/NHLBI (ATP III 2005) definition. It was diagnosed if at least three of the following five features were present (i) waist circumference of ≥80 cm or more (ii) blood pressure of ≥130/85 mmHg (iii) fasting blood sugar of ≥ 100 mg/dL (iv) triglycerides of ≥150 mg/dL, and (v) HDL of ≤50 mg/dL.
Methods of measurement
A standard questionnaire was used to document length of menstrual cycles; personal, medical, and family history of diabetes; hypertension; obesity; and ischemic heart disease. Signs of androgen excess (hirsutism, acne, and alopecia) and insulin resistance were noted in the physical examination. Anthropometric measurements included a waist circumference in centimeters measured at the narrowest circumference, midway between the upper border of iliac crest and the lower rib margin, whereas the hip circumference was taken as the widest measurement at the level of the greater trochanters. Height was recorded in centimeters and weight in kilograms. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). Overweight was defined as a BMI between 25.0 and 29.9, and obese as 30.0 or higher according to World Health Organization categories. Sitting blood pressure was measured after a 5-min rest using a standard sphygmomanometer.
Overnight fasting blood sample and a 75 g oral glucose tolerance test was obtained in all women. Impaired fasting glucose, impaired glucose tolerance test, and diabetes were defined in accordance with the American Diabetes Association revised definition. Blood tests also included a thyroid stimulating hormone (TSH) level, prolactin, total testosterone, sex hormone-binding globulin (SHBG), and a fasting lipid profile, which included total cholesterol, triglycerides, HDL, and low-density lipoprotein levels.
Laboratory methods
The laboratory analyses used the following assays to measure the endocrine and metabolic parameters: Total testosterone by a solid-phase competitive chemiluminescent enzyme immunoassay (IMMULITE 2000, Siemens, Eschborn, Germany), SHBG by electrochemiluminescence immunoassay (‘ECLIA’ modular analysis E170, Roche Diagnostics, Mannheim, Germany), TSH by two site sandwich immunoassay (ADVIA Centaur, Bayer Corporation, Tarry town, NY, USA), serum prolactin with direct chemiluminometric sandwich method (IMMULITE 2000, Siemens, Eschborn, Germany), and blood glucose levels by glucose GOD POD – glucose oxidase and peroxidase methods. Triglyceride level was measured using the standard lipase, glycerokinase, glycerol-3-phosphate oxidase and peroxidase method and HDL cholesterol was determined by the cholesterol esterase, oxidase, peroxidase method. To calculate free testosterone, initially serum albumin, SHBG, and total testosterone levels were estimated. Using these values, the free testosterone was calculated utilizing the free testosterone calculator formula as described on the website: http://www.issam.ch/freetesto.htm. The inter and intra-assay checks demonstrated coefficient of variation of 2.4 and 5.5% for TSH, 8.3 and 5.9% for total testosterone, 2.1 and 4.3% for SHBG, 1.3 and 2.8% for glucose, 2.3 and 5.3% for prolactin, 2.8 and 4.3% for triglyceride, and 2.1 and 3.4% for HDL cholesterol.
A pelvic ultrasound for status and morphology of ovary was done using a vaginal probe of 6 MHz of a ultrasound machine (TOSHIBA Ultrasound, Osaka, Japan). Ovarian volume measurements were carried out by measuring three perpendicular dimensions (volume for a prolate ellipsoid = 0.5 × length × width × thickness). Follicle number was estimated both in longitudinal and antero-posterior cross-sections of the ovaries. Those with a mean diameter of 2–9 mm were counted for defining polycystic ovary morphology.
Only patients for whom the complete panel of physical examination, ultrasound, and laboratory data for the classification of PCOS and metabolic syndrome was available were considered for the analysis. All data collected was entered on Microsoft Office Excel 2007 for analysis.
The primary outcome measure was to determine the prevalence of metabolic syndrome in women with PCOS and secondary outcome included factors, which may predispose to the risk of development of metabolic syndrome.
Statistical methods used
A pilot study was conducted according to the modified AHA/NHLBI (ATP III 2005) criteria for the metabolic syndrome. The prevalence of metabolic syndrome has been reported to be 46.2% in Indian women with PCOS according to the International Diabetes Federation criteria.[12] A sample size of 120 women with PCOS was needed to estimate the prevalence to within 9% of the true value. Statistical analysis was done using SPSS version 15.0 (SPSS, Inc., Chicago, IL, US). Continuous variables were summarized as mean with standard deviation and analyzed using sample t-test. Categorical variables were expressed as proportions and analyzed by Chi-square test. Univariate analysis was applied to quantify the association between clinical and laboratory variables and the presence of metabolic syndrome. Multivariate logistic regression analysis was used to examine independent predictors of metabolic syndrome and to adjust for confounding factors. A P value of less than 0.05 was considered statistically significant.
The study protocol was approved by the Institutional Review Board, and women were enrolled for the study only after an informed written consent.
RESULTS
A total of 120 consecutive women with PCOS were recruited. We found a prevalence of 37.5% of metabolic syndrome in the study population. Three features were present in 24.2% cases, four features in 10% cases, and all five features were present in 3.3% cases.
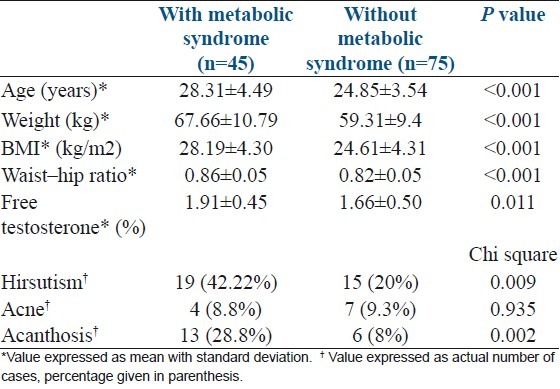
The age of the women in our study ranged from 19 to 38 years with a mean age of 26.15 years SD+4.25. The prevalence of metabolic syndrome increased with age as shown in Table 1. The mean age of women was significantly higher in women with metabolic syndrome than those without metabolic syndrome (28.31+4.49 vs. 24.85+3.54 P value <0.001) [Table 2].
Table 1.
Distribution of metabolic syndrome according to age
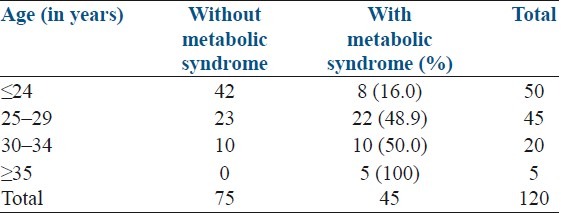
Table 2.
Clinical and biochemical parameters of polycystic ovarian syndrome women with and without metabolic syndrome
Majority of them (81.7%) presented with primary infertility while the rest had secondary infertility. Mean duration of infertility was 4.68 years SD+3.23 (range of 6 months to 16 years). Except for 2.5 % cases who had regular menstrual periods, most of the study cases had menstrual irregularity with 84.2% having oligomenorrhea and 13.3% with amenorrhea (progesterone withdrawal bleeding).
A family history of diabetes mellitus was present in 34.2% cases, hypertensive disorders in 30.8%, family members with obesity in 20%, and a history of ischemic heart disease in the family in 11.7%.
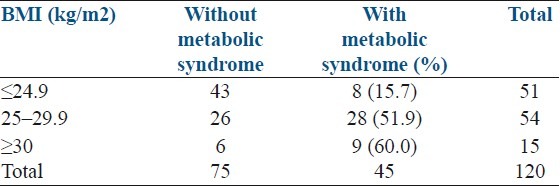
The mean BMI in the study cases was 25.95 kg/m2 (SD+/-4.63) with a range of 17.6 to 41 kg/m2. We found that the prevalence of metabolic syndrome increased with increasing BMI [Table 3]. The mean BMI in those with metabolic syndrome was significantly higher than those without metabolic syndrome (28.19+4.30 vs 24.61+4.31 P value <0.001) [Table 2]. A waist-hip ratio of ≥0.85 was found in 45.8% cases. The mean waist-hip ratio was significantly higher in those found to have metabolic syndrome than in those without metabolic syndrome (0.86+0.05 vs 0.81+0.05) [Table 2].
Table 3.
Distribution of metabolic syndrome according to body mass index
Clinical features of hyperandrogenism such as hirsutism with a Ferriman and Gallwey score of 8 or more was present in 28.3%, and acne in 9.2%. Acanthosis nigricans was seen in 15.8% cases. A significantly greater number of patients had hirsutism (42.22% vs 20%) and acanthosis (28.8% vs 8%) in those with metabolic syndrome than in whom metabolic syndrome was not present. The calculated free testosterone level was also higher in those with metabolic syndrome than those without the syndrome (1.91+0.45 vs 1.66+0.50, P value 0.01) [Table 2]. As hirsutism and free testosterone levels are markers of hyperandrogenism and acanthosis nigricans for insulin resistance, it was found that both hyperandrogenism and insulin resistance were strongly associated to metabolic syndrome.
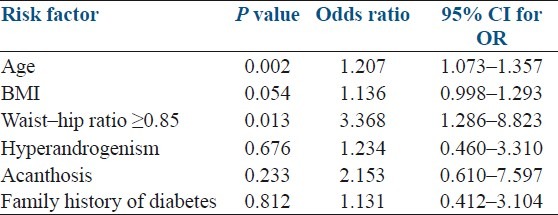
A total of 5.8 % cases were detected to have diabetes mellitus, 8.3% had impaired fasting glucose, and 11.7 % had an impaired glucose test. High blood pressure of ≥130/85 mmHg was recorded in 20% cases. Dyslipidemia was present in 93.3% cases of PCOS with a low HDL (<50 mg/dL) being the commonest feature seen in 91.7 % cases. Among all the risk factors, age and waist-hip ratio ≥0.85 were strongly associated with the presence of metabolic syndrome [Table 4].
Table 4.
Logistic regression analysis showing the predictive association of clinical variables and presence of metabolic syndrome
DISCUSSION
Metabolic syndrome is characterized by three main interrelated abnormalities: elevated plasma glucose, dyslipidemia, and elevated blood pressure, which directly contribute to a pro-thrombotic and pro-inflammatory state, predisposing to the development of atherosclerotic cardiovascular disease and type 2 diabetes mellitus.[6] Hyperinsulinemia and insulin resistance are the common underlying metabolic abnormalities seen in PCOS and metabolic syndrome. Insulin resistance with elevated circulating insulin levels induces unfavorable changes in the lipid metabolism and increased androgen production from the theca cells. Androgen excess may support the presence of an unfavorable metabolic state leading to dyslipidemia and central distribution of fat (android pattern). In obese women, excess insulin and androgens may contribute to the development of the PCOS and metabolic syndrome.[12] The android pattern of fat distribution may be the result as well as the cause of hyperandrogenism, setting up a vicious circle of hyperinsulinism, hyperandrogenism, central adiposity, and metabolic abnormalities.[13]
The present study shows that the overall prevalence of metabolic syndrome in women with PCOS presenting with infertility is 37.5%. In comparison, a study done on Indian women, which included both adolescent as well as adult women with PCOS, reported a prevalence of 46.2% by the International Diabetes Federation criteria.[14] A recent consensus definition incorporating IDF and AHA/NHLB (2004) risk factors has been introduced.[15] The main modification in the consensus definition is the incorporation of elevated waist circumference according to population and country-specific cut-offs (for Asian women, waist circumference >80 cm). The main finding of our study (prevalence of metabolic syndrome in PCOS women with infertility - 37.5%) remains unchanged even after applying the new consensus definition.
Earlier studies have suggested that certain phenotypes of PCOS women have a higher risk of developing metabolic syndrome and consequently long-term risk of cardiovascular disease/type 2 diabetes mellitus.[16] The prevalence of metabolic syndrome has been found to be higher in weight-matched PCOS women compared to non-PCOS women.[17]
Hahn et al. established a prevalence of metabolic syndrome of 33.8% in German women with PCOS (International Diabetes Federation criteria) and found that the prevalence rate increased with obesity and age.[18] In a study on Brazilian women with PCOS, the prevalence of metabolic syndrome was found to increase with BMI: 3.2%, 19.2%, and 52.3% for normal, overweight, and obese women, respectively.[9] In our study, the prevalence of metabolic syndrome also increased with both age and BMI as shown in Tables 1 and 3.
In the present study, 5.8% women were found to have diabetes while the prevalence of dyslipidemia was 93%, stressing the need for screening women with PCOS for these derangements.
A Dutch study on anovulatory PCOS women has reported that a waist circumference of >83.5 cm along with biochemical evidence of hyperandrogenism was a powerful predictor of the presence of metabolic syndrome and insulin resistance.[19]
A multivariate logistic regression analysis showed that age and central obesity (waist-hip ratio/waist circumference) were better predictors of metabolic syndrome in women with PCOS compared to other parameters including BMI. Our finding of central obesity correlating with presence of metabolic syndrome in women is in agreement with earlier study by Janssen et al., who concluded that waist circumference is closely related with obesity-related risk factors as compared with the BMI.[20]
Screening all infertile women with PCOS would be ideal but is not always practical, especially in a low-resource scenario. Identifying risk factors for screening would be an alternate strategy. Our results suggest that women having any of the following risk factors: age more than 25 or with central obesity waist-hip ratio >0.85, are at a greater risk of having the metabolic syndrome.
However, the results need to be cautiously interpreted as the present study has certain limitations. The study was done at a tertiary care centre without use of a control group of non-PCOS women for comparison and the sample size was estimated taking a precision of 9% of the true value. A larger sample size will be required for a more precise estimate of the prevalence of metabolic syndrome. We did not find any published data from the Indian subcontinent using the modified AHA/NHLBI ATP III (2005) criteria; therefore, comparison was not possible.
CONCLUSION
We found a prevalence of metabolic syndrome of 37.5%, which constitutes more than a third of the PCOS women who presented with infertility in our clinic. The present study highlights the need for comprehensive screening for metabolic syndrome in women with PCOS attending infertility clinic. In our study, age >25 years and presence of central obesity (waist-hip ratio >0.85) were identified as risk factors for metabolic syndrome. The findings can be used to formulate a screening policy for metabolic syndrome, particularly in low resource settings.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Frank S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–61. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 2.Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 3.Legro RS. Polycystic ovary syndrome and cardiovascular disease: A premature association? Endocr Rev. 2003;24:302–12. doi: 10.1210/er.2003-0004. [DOI] [PubMed] [Google Scholar]
- 4.Korhonen S, Hippelainen M, Niskanen L, Vanhala M, Saarikoski S. Relationship of the metabolic syndrome and obesity to polycystic ovary syndrome; a controlled, population-based study. Am J Obstet Gynecol. 2001;184:289–96. doi: 10.1067/mob.2001.109596. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Ferrannini E. Insulin resistance.A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidaemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–94. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Cleeman JL, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association / National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 7.Lepor NE, Vogel RE. National Cholesterol Education Program Adult Treatment Panel III.Summary of the third report of the National Cholesterol Education Program Adult Treatment Panel III. Rev Cardiovas Med. 2001;2:160–5. [PubMed] [Google Scholar]
- 8.Essah PA, Nestler JE. Metabolic syndrome in women with polycystic ovary syndrome. Fertil Steril. 2006;86(Suppl 1):S18–9. doi: 10.1016/j.fertnstert.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Soares EM, Azevedo GD, Gadelha RG, Lemos TM, Maranhao TM. Prevalence of the metabolic syndrome and its components in Brazilian women with polycystic ovary syndrome. Fertil Steril. 2008;89:649–55. doi: 10.1016/j.fertnstert.2007.03.081. [DOI] [PubMed] [Google Scholar]
- 10.Cheung LP, Ma RC, Lam PM, Lok IH, Haines CJ, So WY, et al. Cardiovascular risks and metabolic syndrome in Hong Kong Chinese women with polycystic ovary syndrome. Hum Reprod. 2008;23:1431–8. doi: 10.1093/humrep/den090. [DOI] [PubMed] [Google Scholar]
- 11.Vrbikova J, Vondra K, Cibula D, Dvorakova K, Stanicka S, Sramkova D, et al. Metabolic syndrome in young Czech women with polycystic ovary syndrome. Hum Reprod. 2005;20:3328–32. doi: 10.1093/humrep/dei221. [DOI] [PubMed] [Google Scholar]
- 12.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 13.Barber TM, McCarthy MI, Wass JA, Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol. 2006;65:137–45. doi: 10.1111/j.1365-2265.2006.02587.x. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharya SM. Metabolic Syndrome in females with polycystic ovary syndrome and International Diabetes Federation criteria. J Obstet Gynaecol Res. 2008;34:62–6. doi: 10.1111/j.1447-0756.2007.00685.x. [DOI] [PubMed] [Google Scholar]
- 15.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 16.Shroff R, Syrop CH, Davis W, Van Voorhis BJ, Dokras A. Risk of metabolic complications in the new PCOS phenotypes based on the Rotterdam criteria. Fertil Steril. 2007;88:1389–95. doi: 10.1016/j.fertnstert.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 17.Dokras A, Bochner M, Hollinrake E, Markham S, Van Voorhis BJ, Jagasia DH. Screening women with polycystic ovary syndrome for metabolic syndrome. Obstet Gynecol. 2005;106:131–7. doi: 10.1097/01.AOG.0000167408.30893.6b. [DOI] [PubMed] [Google Scholar]
- 18.Hahn S, Tan S, Sack S, Kimmig R, Quadbeck B, Mann K, et al. Prevalence of the metabolic syndrome in German women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2007;115:130–5. doi: 10.1055/s-2007-967093. [DOI] [PubMed] [Google Scholar]
- 19.Goverde AJ, Van Koert AJ, Eijkemans MJ, Knauff EA, Westerveld HE, Fauser BC, et al. Indicators for metabolic disturbances in anovulatory women with polycystic ovary syndrome diagnosed according to the Rotterdam consensus criteria. Hum Reprod. 2009;24:710–7. doi: 10.1093/humrep/den433. [DOI] [PubMed] [Google Scholar]
- 20.Janssen I, Katzmarzyk PT, Ross P. Waist circumference and not body mass index explains obesity related health risks. Am J Clin Nutr. 2004;74:379–84. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]


