Abstract
AIMS:
This study was carried out to investigate the effect of Allium cepa crude extract on cadmium-induced testicular toxicity in rats.
MATERIALS AND METHODS:
Adult male Sprague-Dawley rats were randomized into 4 groups (n = 6). Group 1 was used as control, group 2 was administered 0.3 mg/kgBW of cadmium sulfate (CdSO4) intraperitoneally for 3 days, group 3 was pretreated with 1 ml/100 g BW of Allium cepa (AcE) for 8 weeks followed by intraperitoneal administration of 0.3 mg/kgBW of CdSO4 in the last 3 days of experiment, and group 4 was administered 1 ml/100 g BW of AcE throughout the experiment. Testicular weight and semen analysis revealing the sperm count, sperm motility, and sperm morphology was carried out. Superoxide dismutase (SOD), catalase activities, and lipid peroxidation status were also carried out in testes.
RESULTS:
The study demonstrated that Allium cepa ameliorated CdSO4–induced alteration in testicular weight, sperm count, sperm motility, and sperm morphology. It also showed that Allium cepa attenuated the derangement of lipid peroxidation profile in testicular tissues caused by CdSO4 exposure.
CONCLUSIONS:
The findings in the study showed that pre-treatment of rat model with Allium cepa extract prevented CdSO4–induced reproductive toxicity by improving sperm quality and enhancing testicular lipid peroxidation status.
KEY WORDS: Allium cepa, cadmium sulfate, oxidative stress, testes
INTRODUCTION
Cadmium (Cd), a heavy metal, is toxic to both humans and animals. Cadmium in its elemental form occurs naturally in the earth's crust and it is unusual to find it in its pure form. It is commonly found in combination with other element such as oxygen (cadmium oxide), sulfur (cadmium sulfate), chloride (cadmium chloride), and carbon (cadmium carbonate).
It has been well established that excess cadmium exposure produces adverse health effects on human beings. At sufficiently high exposure, virtually all chemicals have adverse health effects. For certain elements such as copper and zinc, which are essential to human life, a deficiency, as well as an excess can cause adverse health effects.[1] However, cadmium is not regarded as being essential to human life.2 Nevertheless, humans one way or the other get exposed to cadmium through their environment and diet. The major sources of cadmium to the environment include battery industry, nickel-cadmium battery manufacturing[3] plastic industries, smelting and refining of metals (e.g. Zinc refining/Cadmium smelting and production[4] lead smelting and refining, iron and steel production, cadmium-containing pigment production[5] dry color formulating, cadmium-based stabilizer production, coal-fired electrical utilities and garbage incineration[6] fertilizers[7–9] metal plating with cadmium-containing materials[5] production of cadmium alloys. Cigarettes are also a significant source of cadmium exposure.
Cadmium exposure has been reported to be a risk factor for infertility. Studies have shown that exposure to cadmium causes lipid peroxidation, which is associated with cadmium toxicity in testes.[10] Cadmium can directly damage the testis. Its effects on the testis appear to be manifested mainly in the sertoli cells, which present more morphological changes under scanning electron microscopy. It also causes derangement in spermatogenesis and spermiogenesis.[11]
Allium cepa (AcE), popularly known as onion, has been reported to have protective properties such as reduction of the risk of rectal carcinoma[11] antiplatelet activity[12,13] and antioxidant properties.[14–17]
Studies have shown that since humans are inseparable from their environment, and almost entirely not free from cadmium exposure, there is a risk of cadmium toxicity associated with potential testicular dysfunction.[10] Several studies have also reported the inhibitory effects of quercetin, and other flavonoids on in vitro lipid peroxidation.[18–22] The effect of AcE on cadmium-induced testicular toxicity was thus investigated in this study.
MATERIALS AND METHODS
Animals
24 adult male Sprague-Dawley rats weighing between 160 and 200 g were used for this research. They were maintained under standard laboratory conditions and were fed with normal rat chow and water ad libitum.
The animals were divided into 4 groups (6 rats per group). Group 1 served as the control group. Group 2 were administered 0.3 mg/kgBW of cadmium sulfate (CdSO4) intraperitoneally for 3 days. Group 3 animals were pretreated with 1 ml/100 gBW of AcE for 8 weeks before intraperitoneal administration of CdSO4 (0.3 mg/ kgBW) for 3 days while group 4 was given 1 ml/100 g of AcE throughout the experiment.
Study was approved by the Department Ethical Committee on Experimental Study. All animals received humane care in compliance with the institution's guideline and criteria for humane care as outlined in the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
Extraction of Allium cepa
AcE was prepared following procedures from previous study.[23] Briefly, fresh Allium cepa (common onion) bulbs were rinsed thoroughly in distilled water, air-dried, and 200 grams were then blended. The resulting paste was allowed to stand for 24 hours. Juice was then filtrated and squeezed out of it using a tight sieve. The filtrate/juice was prepared on weekly basis following the same procedure and kept at 4°C. This is to prevent it from losing its potency.
Determination of sperm characteristics
The caudal epididymis was minced in pre-warmed normal saline (37°C). 1 drop of sperm suspension was placed on a glass slide to analyze 200 motile sperm in 4 different fields. The motility of the epididymal sperm was evaluated microscopically within 2 - 4 minute of their isolation from the epididymis, and data were expressed as percentage motility.[24]
Epididymis sperm was obtained by mincing the epididymis in normal saline, and filtering through a nylon mesh (80-μm pore size). The sperm were counted using a hemocytometer. The number of sperm in 5 squares (4 corners and the center) in the center grid of both sides were counted and averaged following the method of Freund and Carol.[25]
Sperm morphology was done using 2 drops of Walls and Ewas stain, air-dried, and examined under the microscope. The normal sperm cells were counted and the percentage calculated.
Determination of testes enzymes activities
Testes were excised and weighed. They were then homogenized in phosphate buffer and then stored in ice for catalase and superoxide dismutase (SOD) activities assay and estimation of lipid peroxidation index, malondialdehyde (MDA). The catalase activity was determined following at 560 nm the consumption of exogenous H2O2 measured according to previous study.[26] The level of SOD activity in supernatant was determined by method of Fridovich.[27] Estimation of lipid peroxidation based on the reaction of MDA with thiobarbituric acid (TBA) forming a MDA-TBA2 that absorbs strong at 532 nm was followed according to the method of Varshney and Kale.[28]
Testicular histology
This was done as described by Akpantah et al.[29] The organs were cut in slab of about 0.5 cm thick transversely and fixed in Bouin's fluid for a day after which it was transferred to 70% alcohol for dehydration. The tissues were passed through 90% alcohol and chloroform for different durations before they were transferred into moisten paraffin wax for 20 minutes each in an oven at 57°C. Serial sections were cut at 5 microns. Slides were prepared from these tissues. The slides were dewaxed and passed through absolute alcohol (2 changes); 70% alcohol and then to water for 5 minutes. The slides were then stained with hematoxylin.
Statistical method
All results are expressed as mean ± SEM. Differences between means were tested for statistical significance using unpaired t-test complemented with one way analysis of variance (ANOVA). P-values <0.05 were considered statistically significant.
RESULTS
Effect of CdSO4 and AcE on sperm quality
Figure 1 shows that CdSO4 caused significant decrease in testicular weight when compared with the control. Administration of AcE prevented testicular weight decrease as observed in rats pre-treated with AcE. Similarly, rats treated with AcE only had comparable testicular weight with those of the control and pre-treated AcE groups.
Figure 1.
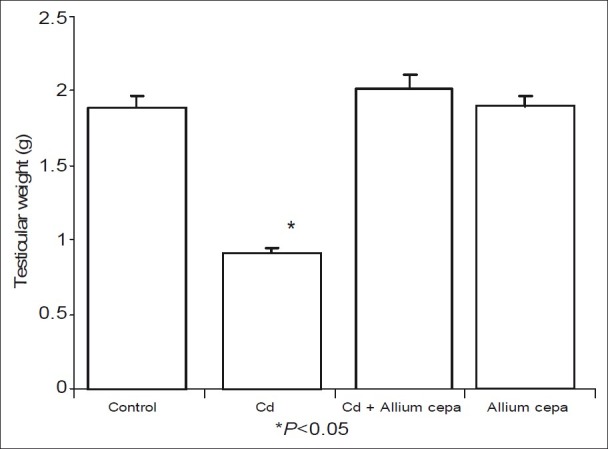
Effect of Allium cepa on testicular weight after testicular toxicity
CdSO4 also induced significant reduction in sperm count when compared to other groups. Rats pre-treated with AcE and AcE only showed a significant increase in sperm count when compared to the control and CdSO4-treated rats [Figure 2].
Figure 2.
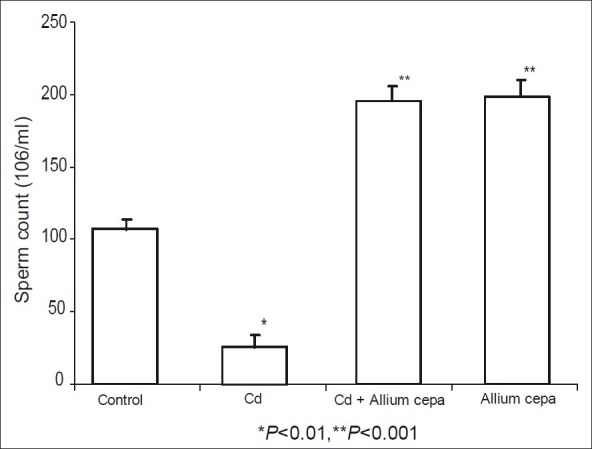
Effect of Allium cepa on sperm count after the induction of testicular toxicity
Furthermore, administration of CdSO4 caused significant reduction in sperm motility when compared with the control and AcE-treated rats. Pre-treated with AcE protected against CdSO4-induced reduced sperm motility [Figure 3].
Figure 3.
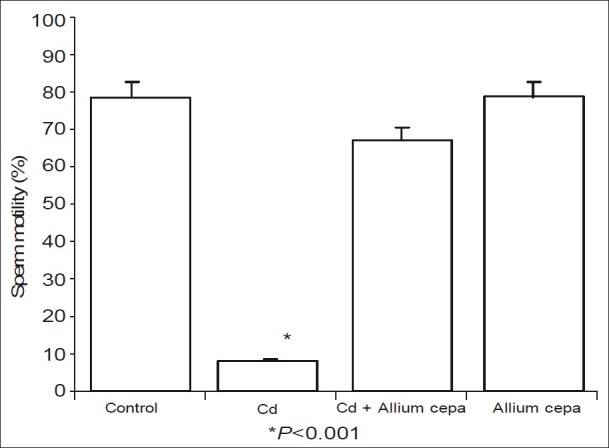
Effect of Allium cepa on sperm motility after testicular toxicity
CdSO4 significantly reduced sperm morphology. Pretreatment with AcE abolished this effect. There was no significant difference in the sperm morphology of animals treated with AcE and the control group [Figure 4].
Figure 4.
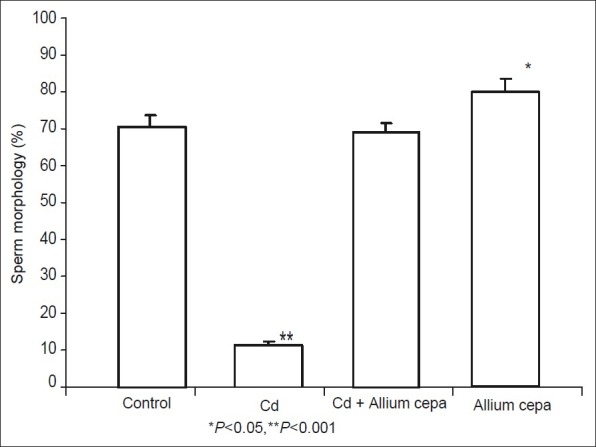
Effect of Allium cepa on sperm morphology after induction of testicular toxicity
Effect of CdSO4 and AcE on sperm oxidative stress markers
Figures 5 and 6 shows the effect of AcE on testicular SOD and catalase activities. CdSO4 significantly reduced testicular SOD and activities while rats treated with AcE-only showed significant rise in testicular SOD and catalase activities when compared with the control. Testicular SOD and catalase activities were comparable in animals pre-treated with AcE and the control.
Figure 5.
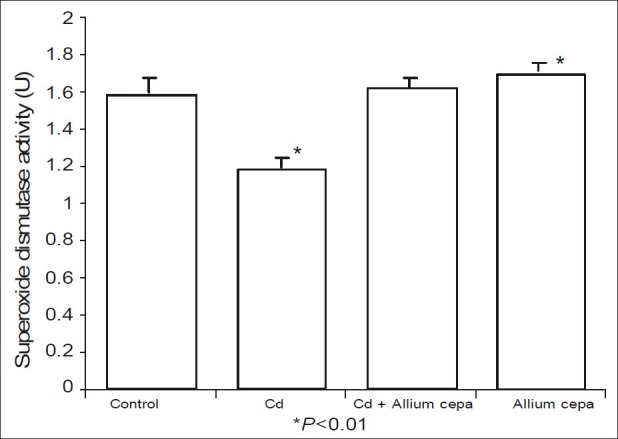
Effect of Allium cepa on superoxide dismutase activity after induction of testicular toxicity
Figure 6.
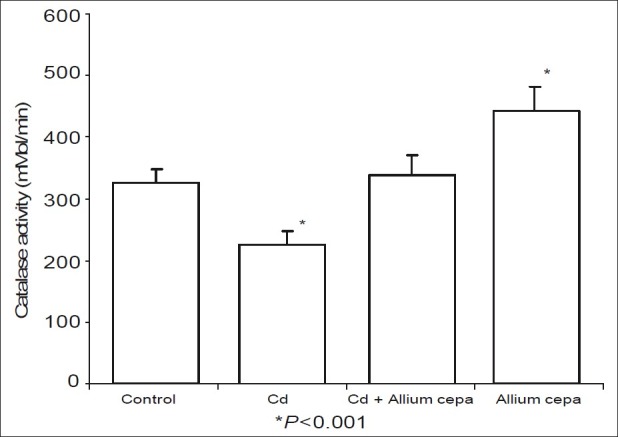
Effect of Allium cepa on catalase activity after induction testicular toxicity
Correspondingly, CdSO4 significantly increased testicular MDA concentration while AcE led to a significant fall in MDA. There was no significant difference in testicular MDA concentrations of animals pre-treated with AcE and control [Figure 7].
Figure 7.
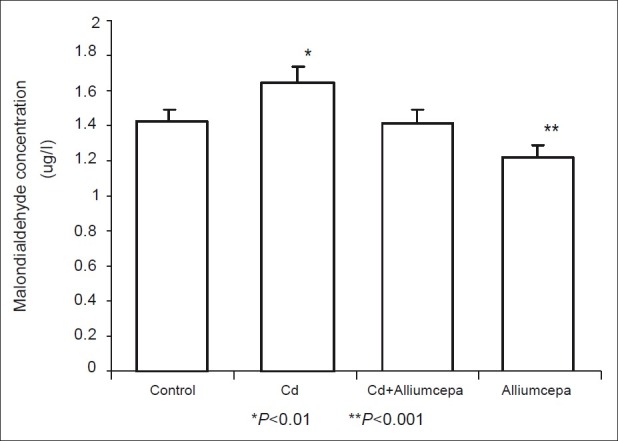
Effect of Allium cepa on malondialdehyde concentration on testicular tissue after testicular toxicity
Effect of CdSO4 and AcE on sperm histomorphology
Figure 8 shows the histomorphology of the testes of the animals. Control rat shows the seminiferous tubules lined with stratified epithelium, composed of 2 major cells, which are the supporting cells (sertoli cells) and spermatogenic cells. The spermatozoa are arranged in rows between and around the cells of sertoli. Seminiferous tubules of the CdSO4 treated shows degeneration of the spermatogenic cells, occlusion of the lumen and hypertrophied seminiferous tubules. There was increase in the spermatogenic cells of the AcE pre-treated rats compared with the control. There were matured spermatozoa in the seminiferous tubules of animals treated with AcE only.
Figure 8.
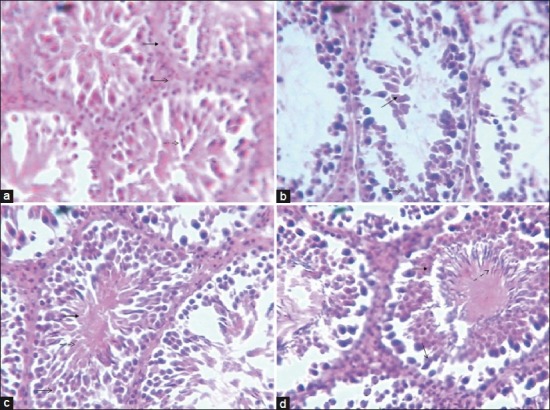
Histologic studies of the effect of AcE and Cadmium on rat testes (a) Histology of testis from control rat showing normal seminiferous tubule lined stratified epithelium, composed of two major cells, which are the supporting cells (sertoli cells) and spermotogenic cells. The spermatozoa are arranged in rows between and around the cells of sertoli. (b)Histology of testis from rat treated with CdSO4 showing degeneration of the spermatogenic cells, occlusion of the lumen and hypertrophied seminiferous tubules (c) Histology of testis from rat treated with Allium extract + CdSO4 showing increase in the spermatogenic cells compared with the control (d) Histology of testis from rat treated with Allium cepa extract only, showing matured spermatozoa in the seminiferous tubules
DISCUSSION
The results of this study revealed that CdSO4 administration significantly decreased sperm count, sperm motility, and sperm morphology of the rats. The changes observed in the above agree with the previous reports, which demonstrated that cadmium impairs testicular function.[30,31] The significant reduction in sperm count, motility and morphology observed in this study following CdSO4 administration may be associated to impairment of spermatogenesis consequent to reduced secretion of testosterone (from testes) caused by administration of CdSO4.[32] This also conforms to the cigarette smoking-induced impairment of steroidogenesis and spermatogenesis documented in previous study.[33]
Studies have described that exposure to cadmium causes lipid peroxidation, which leads to oxidative stress.[10] SOD is an enzymatic antioxidant that converts superoxide radicals into hydrogen peroxide and oxygen, which is further converted into water. This study shows that there was a significant reduction in the activities of both SOD and catalase, but significant rise in MDA levels in testicular tissues of rats administered with CdSO4. The derangement in the lipid peroxidation and anti-oxidant status of testicular tissues might be a contributing factor to the reduction of testosterone secretion with resultant poor sperm quality. AcE significantly increased SOD and catalase activities, thus enhancing the antioxidant system in the testicular tissues. This is in agreement with previous study.[34] This study shows that AcE improved sperm quality in rats. Pre-treated with AcE also prevented CdSO4- induced poor sperm quality by inhibiting lipid peroxidation.
The results of this study show a significant reduction in lipid peroxidation (MDA levels) in testes of AcE-pretreated rats and AcE-only treated rats in consonance with previous study.[35] Several studies have reported the inhibitory effects of catechin, quercetin, and other flavonoids on in vitro lipid peroxidation generally assessed by measuring colorimetrically the formation of thiobarbituric acid-reactive substance.[17–21] This study also agrees with the studies on several flavonoids (flavonoid being one of the constituent of allium cepa) that reported the protective potentials of Allium cepa on lipid peroxidation.[36–38] The improvement of lipid peroxidation profile seen in AcE-treated groups explains the enhanced sperm quality seen in the groups.
The findings in this study showed that AcE enhances testicular oxidative status. The study also shows that AcE pre-treatment prevents cadmium sulfate-induced testicular toxicity by increasing the tissue enzymatic antioxidant activities (SOD and catalase) and reducing lipid peroxidation (MDA).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kido T, Nogawa K, Yamada Y, Honda R, Tsuritani I, Ishizaki M, et al. Osteopenia in inhabitants with renal dysfunction induced by exposure to environmental cadmium. Int Arch Occup Environ Health. 1989;61:271–6. doi: 10.1007/BF00381425. [DOI] [PubMed] [Google Scholar]
- 2.Cherian MG, O’Heany J, Kusiak RA. Toronto: Ontario Ministry of Labour; 1985. Health effects of cadmium and its inorganic compounds. [Google Scholar]
- 3.Adams RG. Manufacturing process, resultant risk profiles and their control in the production of nickel-cadmium (alkaline) batteries. Occup Med. 1992;42:101–6. doi: 10.1093/occmed/42.2.101. [DOI] [PubMed] [Google Scholar]
- 4.Ellis KJ, Cohn SH, Smith TJ. Cadmium inhalation exposure estimates their significance with respect to kidney and liver cadmium burden. J Toxicol Environ Health. 1985;15:173–87. doi: 10.1080/15287398509530644. [DOI] [PubMed] [Google Scholar]
- 5.Elinder CG, Edling C, Lindberg E, Kagedal B, Vesterberg O. Assessment of renal function in workers previously exposed to cadmium. Br J Ind Med. 1985;42:754–60. doi: 10.1136/oem.42.11.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zwennis WC, Franssen AC. Assessment of occupational exposure to cadmium in the Netherlands, 1980-1989. Am J Ind Med. 1992;21:793–805. doi: 10.1002/ajim.4700210603. [DOI] [PubMed] [Google Scholar]
- 7.Syers JK, Mackay AD, Brown MW, Currie CD. Chemical and physical characteristics of phosphate rock materials of varying reactivity. J Sci Food Agric. 1986;37:1057–64. [Google Scholar]
- 8.Trueman NA. The phosphate, volcanic and carbonate rocks of Christmas Island (Indian Ocean) J Geol Soc Aust. 1965;12:261–86. [Google Scholar]
- 9.Taylor MD. Accumulation of Cadmium derived from fertilizers in New Zealand soils. Sci Total Environ. 1997;208:123–6. doi: 10.1016/s0048-9697(97)00273-8. [DOI] [PubMed] [Google Scholar]
- 10.Shuenn JY, Chern CL, Jenn YS, Lin TH. Cadmium induced lipid peroxidation in rats testes and protection by selenium. Biometals. 2004;12:353–9. doi: 10.1023/a:1009277121164. [DOI] [PubMed] [Google Scholar]
- 11.Boscolo P, Sacchettoni-Logroscino G, Ranellete FO, Gioia A, Carmigani M. Effects of long term cadmium exposure on the testes of rabbits.Ultrastructural study. Toxicol Lett. 1985;24:145–9. doi: 10.1016/0378-4274(85)90050-5. [DOI] [PubMed] [Google Scholar]
- 12.Dorant E, Van den Brandt PA, Goldbohm AR. Prospective cohort study on the relationship between onion and leek consumption, garlic supplement use and the risk of colorectal carcinoma in The Netherlands. Carcinogenesis. 1996;17:477–84. doi: 10.1093/carcin/17.3.477. [DOI] [PubMed] [Google Scholar]
- 13.Cavagnaro PF, Sance MM, Galmarini CR. Effect of heating on Onion (Allium cepa L.) antiplatelet activity and pungency sensory perception. Food Sci Technol Int. 2007;13:447–53. [Google Scholar]
- 14.De Whalley CV, Rankin SM, Hoult JR, Jussup W, Leake DS. Flavonoids inhibit the oxidative modification of low density lipoproteins by macrophages. Biomed Pharmacol. 1990;39:1743–50. doi: 10.1016/0006-2952(90)90120-a. [DOI] [PubMed] [Google Scholar]
- 15.Helen A, Krishnakumar K, Vijayammal PL, Augusti KT. Antioxidant effect of onion oil (Allium cepa Linn) on the damages induced by nicotine in rat as compared to alpha-tocopherol. Toxicology. 2000;116:61–8. doi: 10.1016/s0378-4274(00)00208-3. [DOI] [PubMed] [Google Scholar]
- 16.Prakash D, Singh BN, Upadhyay G. Antioxidant and free radical scavenging activities of phenols from onion (Allium cepa) Food Chem. 2007;102:1389–93. [Google Scholar]
- 17.Ige SE, Salawu EO, Olaleye SB, Adeeyo OA, Badmus J, Adeteke AA. Onion (Alluim cepa) extract prevents cadmium-induced renal dysfunction. Indian J Nephrol. 2009;19:140–4. doi: 10.4103/0971-4065.59334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Videla LA. Assessment of the scavenging action of reduced glutathione (+) cianidanol-3 and ethanol by the chemiluminescent response of the xanthine oxidase reaction. Experientia. 1983;39:500–2. doi: 10.1007/BF01965175. [DOI] [PubMed] [Google Scholar]
- 19.Videla LA, Fernandez V, Valenzuela A, Ugarte G. Effect of (+) -cianidanol-3 on the changes in liver glutathione content and lipoperoxidation induced by acute ethanol administration in the rat. Pharmacology. 1981;22:343–8. doi: 10.1159/000137514. [DOI] [PubMed] [Google Scholar]
- 20.Younes M, Siegers CP. Inhibitory action of some flavonoids on enhanced spontaneous lipid peroxidation following glutathione depletion. Planta Med. 1981;43:240–4. doi: 10.1055/s-2007-971503. [DOI] [PubMed] [Google Scholar]
- 21.Muller A, Sies H. Role of alcohol dehydrogenase activity and of acetaldehyde in ethane and pentane production by isolated perfused rat liver. Biochem J. 1982;206:153–6. doi: 10.1042/bj2060153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valenzuela A, Guerra R. Differential effect of silybin on the Fe 2+ -ADP and t-butyl hydroperoxide-induced acrosomal lipid peroxidation. Experientia. 1986;42:139–41. doi: 10.1007/BF01952435. [DOI] [PubMed] [Google Scholar]
- 23.Azu NC, Onyeagba RA, Nworie O, Kalu J. Antibacterial activity of allium cepa (Onions) and zingiber officinale (Ginger) on staphylococcus aureus and Pseudomonas aeruginosa isolated from high vaginal swab. Internet J Trop Med. 2007;3:2. [Google Scholar]
- 24.Morrisey RE, Schwctz BA, James C, Monica DR, Teague JL, Moris RW. Evaluation of rodent sperm, vagina cytology and Reproductive organ weight from National Toxicology Programme-13 weeks studies. Fundam Appl Toxicol. 1988;11:343–58. doi: 10.1016/0272-0590(88)90159-5. [DOI] [PubMed] [Google Scholar]
- 25.Freund M, Carol B. Factors affecting haemocytometer count of sperm concentration in human semen. J Reprod Fertil. 1964;8:149–52. doi: 10.1530/jrf.0.0080149. [DOI] [PubMed] [Google Scholar]
- 26.Jiang ZY, Woolland AC, Wolff SP. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1972;268:69–71. doi: 10.1016/0014-5793(90)80974-n. [DOI] [PubMed] [Google Scholar]
- 27.Fridovich I, Misra HP. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 28.Varshney R, Kale RK. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol. 1990;58:733–43. doi: 10.1080/09553009014552121. [DOI] [PubMed] [Google Scholar]
- 29.Akpantah AO, Oremosu AA, Ajala MO, Noronha CC, Okanlawon A. The effect of crude extract of Garcinia kola seed on the histology hormonal milieu of male Sprague dawley rats’reproductive organs. Nig J Health Biomed Sci. 2003;2:40–6. [Google Scholar]
- 30.Garside DA, Harvey PW. ndocrine toxicology of the male reproductive system. In: Atterwill CK, Flack JD, editors. Endocrine Toxicology. Cambridge: Cambridge University Press; 1992. pp. 285–312. [Google Scholar]
- 31.Li LH, Heindel JJ. Sertoli cell toxicants. In: Korach KS, editor. Reproductive and Developmental Toxicology. New York: Marcel Dekker; 1998. pp. 655–91. [Google Scholar]
- 32.Pasqualotto FF, Locambo CV, Ahayde KS, Arap S. Measuring male infertility:epidemiological aspects. Rev Hosp Clin Fac Med Sao Paulo. 2003;58:173–8. doi: 10.1590/s0041-87812003000300008. [DOI] [PubMed] [Google Scholar]
- 33.Aydos K, Güven MC, Can B, Ergün A. Nicotine toxicity to the ultrastructure of the testis in rats. BJU Int. 2001;88:622–6. doi: 10.1046/j.1464-4096.2001.02384.x. [DOI] [PubMed] [Google Scholar]
- 34.Fraga CG, Motchnik PA, Shigenaga MK, Helbock HJ, Jacob RA, Ames BN. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci U S A. 1991;88:11003–6. doi: 10.1073/pnas.88.24.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suleiman SA, Ali ME, Zaki ZM. Lipid peroxidation and human sperm motility: Protective role of vitamin E. J Androl. 1996;17:530–7. [PubMed] [Google Scholar]
- 36.Letan AJ. The relation of structure to antioxidant activity of quercetin and some of its derivatives. II. Secondary (metal-complexing) activity. J Food Sci. 1966;31:395–9. [Google Scholar]
- 37.Ige SF, Akhigbe RE, Adewale AA, Badmus JA, Olaleye SB, Ajao FO, et al. Effect of Allium cepa (Onion) extract on Cadmium–induced nephrotoxicity in rats. Kidney Res J. 2011;1:41–7. [Google Scholar]
- 38.Ige SF, Akhigbe RE, Edeogho O, Ajao FO, Owolabi OQ, Oyekunle OS, et al. Hepatoprotective activities of Allium cepa in cadmium-treated rats. Int J Pharm Pharm Sci. 3:60–3. [Google Scholar]


