Abstract
BACKGROUND
Shorter duration of sleep has been associated with risk of a number of medical conditions, including breast cancer. However, no prior study has investigated the relationship of average sleep duration prior to diagnosis and cancer aggressiveness. OncotypeDX is a widely utilized test to guide treatment in early stage hormone receptor positive breast cancer by predicting likelihood of recurrence.
METHODS
We reviewed medical records from ER+ early stage breast cancer patients participating in a case-control study for availability of OncotypeDX scores. All patients in the parent study were recruited at diagnosis and asked about average sleep duration in the two years prior to diagnosis. We analyzed data from 101 breast cancer patients with available OncotypeDX recurrence scores to test the hypothesis that shorter sleep is associated with greater likelihood of recurrence.
RESULTS
We found that OncotypeDX recurrence scores were strongly correlated with average hours of sleep per night prior to breast cancer diagnosis, with fewer hours of sleep associated with a higher (worse) recurrence score (R=−0.30, p=0.0031). This correlation was limited to post-menopausal breast cancer patients only (R=−0.41, p=0.0011, for postmenopausal patients; R=−0.05, p=0.80 for pre-menopausal patients). This association remains statistically significant after adjustment for age, physical activity, smoking status and body mass index in the entire study sample (p=0.0058) as well as in postmenopausal patients (p=0.0021).
CONCLUSION
This is the first study to suggest that women who routinely sleep shorter amounts of time may develop more aggressive breast cancers compared to women who sleep longer.
Keywords: Breast cancer, OncotypeDX, sleep, recurrence
Background
Previous studies have provided strong evidence for the role of sleep in carcinogenesis, with emerging evidence suggesting that disruptions in the circadian rhythm may increase the risk of several types of cancer. Working the night shift has been associated with elevated risk of numerous cancers, including breast cancer [1–5], and we have recently shown that shorter duration of sleep has been associated with an increased risk of colorectal adenomas [1], a precursor of colorectal cancer. Four previous studies investigated the sleep duration and breast cancer risk association with mixed results, one suggesting a decreased risk of breast cancer among women who consistently slept longer hours [2], two other reports suggesting an inverse association between sleep duration and risk of breast cancer [3, 4] and a fourth finding no association [5]. To our knowledge, no study has investigated habitual sleep duration and aggressiveness of tumors, or likelihood of cancer recurrence.
OncotypeDX is a widely used test to predict tumor aggressiveness and direct treatment in early stage estrogen receptor positive breast cancer. The OncotypeDX score is based on the expression of 21 genes from six molecular groups (HER2, ER, proliferation, invasion, CD68, GSTM1 and BAG1) [6] in breast tumors. Higher OncotypeDX scores have been associated with 10 year risk of recurrence [6], as well asbenefit from adjuvant chemotherapy [7]. Clinical application of the OncotypeDX score is indicated for hormone receptor (ER) positive and HER2 negative stage I-IIIA breast cancer patients with resected tumors who will be treated with hormonal therapy. OncotypeDX scores of less than 18 predict a low risk of recurrence, scores 18–30 an intermediate recurrence risk and 31 or higher a high risk [6]. Although this test is now widely used, little is known about lifestyle correlates of OncotypeDX recurrence scores. In this study, we sought to investigate if average hours of sleep per night prior to diagnosis were associated with breast cancer aggressiveness by looking at the correlation of sleep duration and OncotypeDX recurrence scores.
Methods
Medical records were reviewed from 412 ER+ stage I–III female breast cancer patients diagnosed since 2007, when OncotypeDX testing starting becoming routine, participating in a larger breast cancer case-control study. Patients were eligible for inclusion in the parent case-control study if they were diagnosed with any stage breast cancer (including in situ) and were not known carriers of a BRCA1 or BRCA2 mutation. Patients previously diagnosed with any type of cancer were excluded. Breast cancer patients participating in the main study were diagnosed from 2004–2011.
All patients were recruited at diagnosis and responded to a phone survey at the time of recruitment by a research assistant, which included a question asking about their average length of sleep per night in the two years prior to diagnosis. All patients consented to linking their study responses to their medical record data. Body mass index (BMI) was calculated from self-reported height (in inches) and weight (in pounds) prior to diagnosis as: weight *703 /height2. Smoking was coded as current, former and never. Physical activity was coded as average weekly total hours of strenuous and moderate physical activity.
Medical records were abstracted for OncotypeDX score, and only those patients with available OncotypeDX scores (n=101) were included in this analysis. Menopausal status was defined as post-menopausal for women self-reporting having no menstrual periods within the previous 6 months, including women who have not had a menstrual period due to surgery, and pre-premenopausal for those reporting a recent menstrual period. For those who were unclear as to their menopausal status (7 of the 101 patients), medical records were reviewed for follicle stimulating hormone (FSH) levels, which is sometime obtained to help decide appropriate treatment. After reviewing medical records, 2 patients had their menopausal status inferred from their medical records. Medical records were reviewed for all patients and patients were recoded as pre or post-menopausal as the records indicated at the time of diagnosis (10 post-menopausal patients recoded as pre-menopausal). If not available, patients 51 and younger were coded as pre-menopausal and those older than 51 as post-menopausal. Of the 5 patients falling into this category, 3 were 51 years or younger and were coded as pre-menopausal and 2 were older than 51 and coded as post-menopausal
This study was approved by the University Hospitals Case Medical Center Institutional Review Board and all participants provided written informed consent.
Statistical Analysis
The correlation of recurrence score with hours of sleep as well as other continuous variables was calculated using a Pearson’s Correlation Coefficient. Since sleep duration is well known to be associated with age, smoking, physical activity and obesity [8], a regression model was used to assess the ability of sleep duration to predict recurrence score with adjustment for these covariates. We also categorized average sleep duration into three categories (less than 6 hours, 6 to less than 7 hours and more than 7 hours). P for trend for this categorical classification was calculated by coding these as an ordinal variable (0, 1 and 2) and adding to the regression model in place of hours of sleep as a continuous measure. All statistics were then repeated stratified on menopausal status to see if there were any differences in patients with pre-menopausal breast cancer vs. post-menopausal breast cancer. All statistics reported are two sided and a p-value <0.05 was considered statistically significant. SAS 9.2 was used to perform all statistical calculations.
Results
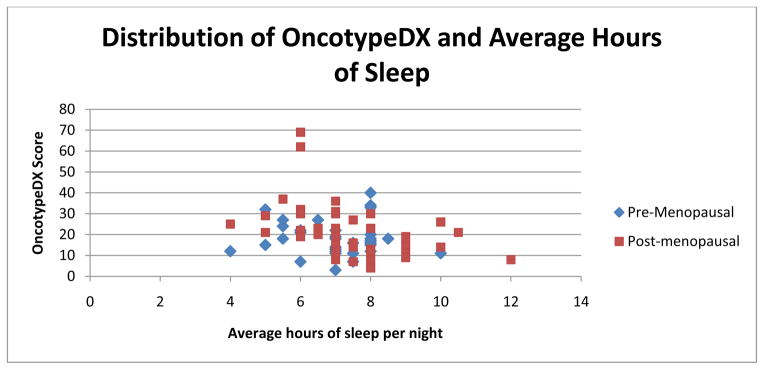
OncotypeDX recurrence scores were available on 101 patients. Table 1 shows the characteristics of the patient population. These patients were of similar age (57.3 years on average) as those for whom OncotypeDX scores were not available (59.1 years), and had similar distributions of stage of disease and race (p>0.1 for all variables). Figure 1 illustrates the distribution of sleep and OncotypeDX scores.
Table 1.
Sample Characteristics
| N (%) or Mean (SD) | |
|---|---|
|
| |
| Race, N (%) | |
| African American | 9 (8.9%) |
| Caucasian | 91 (90.1%) |
| Hispanic | 1 (1.0%) |
|
| |
| Menopausal Status, N (%) | |
| Pre-menopausal | 34 (33.7%) |
| Post-menopausal | 67 (63.3%) |
|
| |
| Smoking status, N (%) | |
| Current | 11 (10.9%) |
| Former | 39 (38.6%) |
| Never | 50 (49.5%) |
| Missing | 1 (1.0%) |
|
| |
| Age (years), mean (SD) | 57.3 (10.0) |
|
| |
| BMI (kg/m2), mean (SD) | 28.6 (6.7) |
|
| |
| Average hours of physical activity per week (SD) | 4.0 (4.2) |
|
| |
| Average hours of sleep per night, mean (SD) | 7.2 (1.3) |
Figure 1.
Distribution of OncotypeDX and Average Hours of Sleep
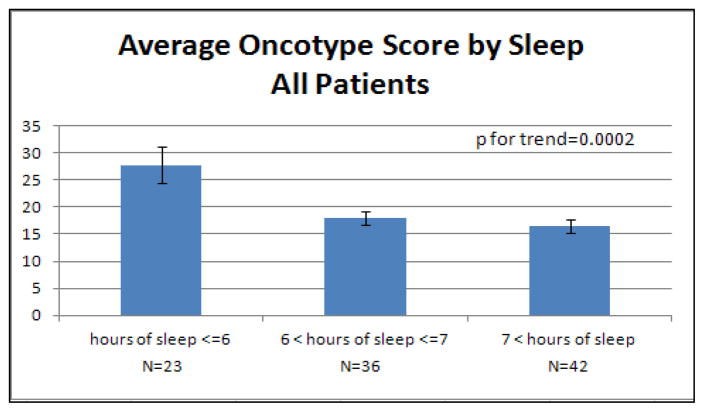
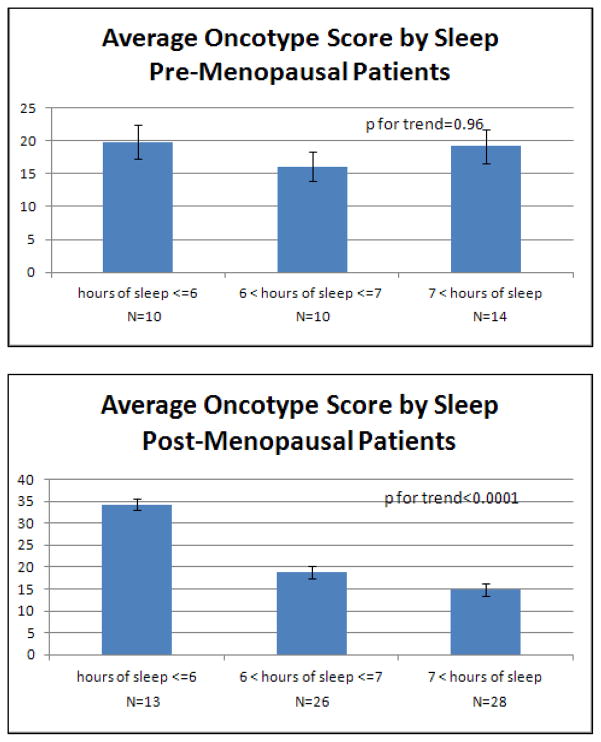
Interestingly, we noted an inverse correlation between average hours of sleep and recurrence score (R=−0.30, p=0.0031, Table 2). Thus, women who reported less sleep prior to breast cancer diagnosis had higher tumor recurrence scores. Figure 2 illustrates the average OncotypeDX recurrence score for different levels of sleep. Those women who reported 6 or less hours of sleep per night on average had higher average recurrence scores (mean=27.8, SD=14.9) compared with women reporting between 6 and 7 hours of sleep per night (mean=18.0, SD=7.5) or more than 7 hours of sleep (mean=16.4, SD=8.0). This correlation with sleep appears to be limited to the post-menopausal patients, where the correlation is even more pronounced (R=−0.41, p=0.0011) than in the combined sample.
Table 2.
Correlations with OncotypeDX Recurrence Scores
| Mean (SD) | Range | R | p | |
|---|---|---|---|---|
| All Breast Cancer Patients (N=101): | ||||
| OncotypeDX score | 19.8 (11.2) | 3–69 | N/A | N/A |
| Age (years) | 57.3 (10.0) | 36–85 | 0.02 | 0.85 |
| BMI (kg/m2) | 28.6 (6.7) | 19.3–45.5 | −0.13 | 0.21 |
| Average Hours of physical activity | 4.0 (4.2) | 0–20 | 0.12 | 0.27 |
| Average Hours of sleep per night | 7.2 (1.3) | 4–12 | −0.30 | 0.0031 |
|
| ||||
| Pre-Menopsausal Breast Cancer Patients: (N=34) | ||||
| OncotypeDX score | 18.4 (8.2) | 3–40 | N/A | N/A |
| Age (years) | 46.5 (4.6) | 36–55 | 0.01 | 0.96 |
| BMI (kg/m2) | 27.5 (6.4) | 19.3–42.8 | −0.01 | 0.97 |
| Average Hours of physical activity | 2.8 (3.0) | 0–10 | −0.12 | 0.54 |
| Average Hours of sleep per night | 7.0 (1.2) | 4–10 | −0.05 | 0.80 |
|
| ||||
| Post-Menopsausal Breast Cancer Patients (N=67): | ||||
| OncotypeDX score | 20.5 (12.4) | 4–69 | N/A | N/A |
| Age (years) | 62.8 (7.0) | 52–85 | −0.10 | 0.43 |
| BMI (kg/m2) | 29.2 (6.8) | 19.5–45.5 | −0.18 | 0.14 |
| Average Hours of physical activity | 4.7 (4.6) | 0–20 | 0.16 | 0.22 |
| Average Hours of sleep per night | 7.4 (1.4) | 4–12 | −0.41 | 0.0011 |
Figure 2. Average Oncotype Score by Hours of Sleep.
Mean (with standard error bars) OncotypeDX recurrence score by average reported sleep duration. The first figure shows the entire sample, the second provides average scores for pre-menopausal breast cancer patients only and the third post-menopausal. Reported p for trend values are from logistic regression accounting for age, smoking status, body mass index and physical activity.
In the regression analysis to assess the ability of sleep duration to predict OncotypeDX score, after adjustment for smoking, physical activity, age and BMI, the hours of sleep per night remains statistically significantly associated with OncotypeDX recurrence score in the entire sample (β=−2.38, SE=0.84, p=0.0058) and post-menopausal patients (β=−3.42, SE=1.06, p=0.0021), and there remains no evidence for association in pre-menopausal patients (β=−0.63, SE=1.53, p=0.69). This suggests that the association of sleep with recurrence score is independent of these potential confounders.
Discussion
In this report we present results from the first study to assess the association of sleep duration, a potentially modifiable lifestyle factor, with OncotypeDX recurrence scores in breast cancer patients. We found a strong inverse correlation between average hours of sleep per night and recurrence scores, specifically in post-menopausal breast cancer patients. Our data suggest that lack of sufficient sleep may cause biologically more aggressive tumors, although future work will need to be done to more thoroughly characterize the biology underlying this epidemiological association.
We found that the correlation of sleep duration and recurrence score was strong in post-menopausal women, but not in pre-menopausal women. It is well known that there are different mechanisms underlying pre-menopausal and post-menopausal breast cancer. Our data suggest that sleep may affect carcinogenic pathway(s) specifically involved in the development of post-menopausal breast cancer, but not pre-menopausal cancer.
The breast cancer OncotypeDX test assigns a tumor a recurrence score based on the expression level of a combination of 21 genes. It is not known how the expression levels of these 21 genes are influenced by regular sleep, or lack thereof. However, genes related to sleep and/or sleep regulation have been shown to be deregulated in cancer [9]. In addition, two sleep related genes, PERIOD1 (PER1) and PERIOD3 (PER3) have been shown to influence apoptosis during chemotherapy in cancer cells [10]. Interestingly, just recently it was shown that PER3 deletion was related to tumor recurrence in ER+ breast cancer patients [11]. While these genes are not captured in the OncotypeDX test, it is likely that sleep alters the expression of the genes that are captured in the recurrence score and/or the other genes that are influenced by sleep directly or indirectly regulate the OncotypeDX genes.
Circadian rhythm disruption has known associations with breast cancer risk, including studies showing an association between melatonin, the primary hormone regulating circadian rhythm, levels and risk of breast cancer (reviewed in [12, 13]). Lack of consistent sufficient sleep may mimic disruptions in the circadian rhythm. However, research on the association of circadian rhythm and breast cancer aggressiveness is lacking. While our study provides interesting preliminary findings, future research into circadian rhythm patterns and breast cancer aggressiveness and the mechanisms underlying this association is warranted. One possible mechanism for our observed association may be the increased estrogen and altered estrogen receptor activity in response to reduced melatonin from lack of sleep (reviewed in [13]). Indeed, the estrogen receptor gene is one measured as part of the OncotypeDX test. Another possible mechanism may be the well known role of sleep in DNA repair. Circadian rhythm is closely integrated with DNA repair (reviewed in [14]). The circadian rhythm gene, NPAS2, has been suggested to be a tumor suppressor as it was shown to be involved in DNA damage response [15].
In the analyses presented here we adjusted for several potential confounders, including age, BMI, physical activity and smoking. We were unable to adjust for additional variables related to sleep, such as use of sleep aids or stressors that influence sleep, such as major life changes or depression or anxiety. However, our main hypothesis is that lower levels of sleep results in more aggressive tumors. This is independent of the reason for lower levels of sleep, be it lifestyle, stressors, co-morbid conditions or medication use. Indeed it is extremely important to consider the diversity of reasons for lack of sleep among women when proposing an intervention to help increase sleep duration for the reduction of tumor aggressiveness among high risk women or for overall better health. Nonetheless, because the factors associated with OncotypeDX have not been characterized and remain largely unknown, we feel that these will account for a majority, if not all, of the key confounders.
This study suggests that women who get fewer than 7 hours of sleep per night may develop more aggressive breast cancers. These results provide novel evidence in support of the promotion of sufficient sleep of all women for breast health. Future studies that address the ability of sleep interventions among healthy, but potentially high risk, women to reduce the overall aggressiveness of breast cancer are still needed.
There are a few limitations in our study. First, our data represents self-reported average sleep duration over the two years prior to diagnosis, which is subjective, and does not capture quality of sleep. Use of a more objective measure of sufficient sleep and/or sleep quality, as well as data on lifetime sleep patterns, may provide a better understanding of this relationship. Other measures of sleep quality using standardized questionnaires may provide additional data on the role of sleep quality in breast cancer aggressiveness. Furthermore, our data is retrospective and may be prone to recall bias, as patients were queried about the two years prior. However, patients were surveyed for sleep duration prior to the availability of their OncotypeDX scores. Therefore it is unlikely that reporting bias of sleep duration if any would be related to the aggressiveness of the patient’s breast tumor. The racial distribution in our sample reflects the distribution in our patient population, which is largely Caucasian. Further research is warranted to verify this association in minority populations. Lastly, the relatively modest sample size of study is a major weakness. However, the positive correlations we reported here are intriguing. Given that this is the first study to report such an association, independent replication in other studies is warranted. Further research will be needed to elucidate the mechanisms underlying this association.
Acknowledgments
This work was supported by the National Cancer Institute (grant numbers K07 CA136758 to CLT and R03 CA143917 to LL) and the Case Comprehensive Cancer Center (P30 CA043703). The authors wish to thank Drew Helmus, Jennifer Zabel and Audrey Lynn for their assistance in recruitment and data collection.
Footnotes
Conflict of Interest: The authors have no conflicts of interest relevant to the presented research.
References
- 1.Thompson CL, et al. Short duration of sleep increases risk of colorectal adenoma. Cancer. 2011;117(4):841–7. doi: 10.1002/cncr.25507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verkasalo PK, et al. Sleep duration and breast cancer: a prospective cohort study. Cancer Res. 2005;65(20):9595–600. doi: 10.1158/0008-5472.CAN-05-2138. [DOI] [PubMed] [Google Scholar]
- 3.Wu AH, et al. Sleep duration, melatonin and breast cancer among Chinese women in Singapore. Carcinogenesis. 2008;29(6):1244–8. doi: 10.1093/carcin/bgn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kakizaki M, et al. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br J Cancer. 2008;99(9):1502–5. doi: 10.1038/sj.bjc.6604684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinheiro SP, et al. A prospective study on habitual duration of sleep and incidence of breast cancer in a large cohort of women. Cancer Res. 2006;66(10):5521–5. doi: 10.1158/0008-5472.CAN-05-4652. [DOI] [PubMed] [Google Scholar]
- 6.Paik S, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–26. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 7.Paik S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–34. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 8.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16(3):643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu CM, et al. Altered expression of circadian clock genes in head and neck squamous cell carcinoma. Tumour Biol. 2012;33(1):149–55. doi: 10.1007/s13277-011-0258-2. [DOI] [PubMed] [Google Scholar]
- 10.Sato F, et al. PERIOD1 (PER1) has anti-apoptotic effects, and PER3 has pro-apoptotic effects during cisplatin (CDDP) treatment in human gingival cancer CA9–22 cells. Eur J Cancer. 2011;47(11):1747–58. doi: 10.1016/j.ejca.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Climent J, et al. Deletion of the PER3 gene on chromosome 1p36 in recurrent ER-positive breast cancer. J Clin Oncol. 2011;28(23):3770–8. doi: 10.1200/JCO.2009.27.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13(4):257–64. doi: 10.1016/j.smrv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16(2):254–8. doi: 10.1097/01.ede.0000152525.21924.54. [DOI] [PubMed] [Google Scholar]
- 14.Collis SJ, Boulton SJ. Emerging links between the biological clock and the DNA damage response. Chromosoma. 2007;116(4):331–9. doi: 10.1007/s00412-007-0108-6. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman AE, et al. The circadian gene NPAS2, a putative tumor suppressor, is involved in DNA damage response. Mol Cancer Res. 2008;6(9):1461–8. doi: 10.1158/1541-7786.MCR-07-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]