Abstract
Background:
Dexmedetomidine is a highly selective α2 agonist with properties of sedation, analgesia and anxiolysis, making it an ideal anesthetic adjuvant. Using an anesthetic adjuvant that decreases requirement of anesthetics and analgesics may predispose the patient to awareness. We monitored the depth of anesthesia (DOA) using entropy to avoid unwanted awareness under anesthesia.
Materials and Methods:
30 patients, American Society of Anesthesiologists grade I and II, aged between 18 to 50 years of either gender undergoing laparoscopic surgeries under general anesthesia were studied. Loading dose infusion of dexmedetomidine was started 1 mcg/kg for 15 minutes and patients were premedicated. Routine induction with propofol and fentanyl was carried out, and maintenance infusion of dexmedetomidine 0.2 mcg/kg/hr was given. Patients were monitored with standard monitoring, and in addition, the DOA was monitored with entropy.
Results:
A 62.5% reduction (0.75 mg/kg) in the induction dose of propofol was observed, with a 30% less end-tidal concentration of isoflurane requirement for maintenance of anesthesia, while maintaining the adequate DOA.
Conclusion:
Dexmedetomidine is an effective anesthetic adjuvant that can be safely used in laparoscopy without the fear of awareness under anesthesia.
Keywords: Anesthetic adjuvant, depth of anesthesia, Dexmedetomidine, entropy, laparoscopic surgery
Introduction
Dexmedetomidine (Dex) has recently been added to the anesthesia armamentarium. It belongs to the class of α2 agonists and possesses the properties of sedation, analgesia and opioid sparing effect. It differs from clonidine in being 16 times more specific for α2 receptors.[1] Laparoscopic surgeries under general anesthesia are associated with unique hemodynamic changes in the form of increased systemic vascular resistance, leading to hypertension, forcing the anesthesiologist to increase the depth of anesthesia (DOA), and at times even requires the use of vasodilators to tackle the rising blood pressure.[2] Dex due to its distinct properties can be used as an anesthetic adjuvant in the form of intravenous infusion.[3] We studied the use of Dex in laparoscopic surgeries and evaluated its effects on hemodynamics, anesthetic and analgesic requirement. Administration and study of a drug known to decrease anesthetic requirement without monitoring the DOA can lead to under-dosing of the anesthetic drugs, causing awareness under anesthesia. We included entropy as a monitoring tool to study the DOA.
Materials and Methods
After the approval of the Institutional Ethics Committee, thirty patients posted for laparoscopic surgery under general anesthesia were enrolled for the study. Inclusion criteria were: patients belonging to American Society of Anesthesiologists (ASA) grade I and II; aged between 18 to 50 years; of either sex; scheduled for laparoscopic surgeries like laparoscopic cholecystectomy, laparoscopic fundoplication, total laparoscopic hysterectomy, laparoscopic pancreaticojejunostomy and laparoscopic adhesiolysis. Exclusion criteria were: patients with ASA grade III/IV; and contraindication to the use of Dex e.g. liver, renal or cardiac disorder. After pre-anesthetic checkup, written, valid and informed consent was taken from patients posted for laparoscopic surgery under general anesthesia. Two intravenous (IV) lines were secured, one for routine fluids and the other exclusively for Dex. Dex infusion was prepared in normal saline in the concentration of 4 mcg/ml.
Baseline monitors like electrocardiogram (ECG), pulse oximetry, noninvasive blood pressure (NIBP) including entropy were attached. Baseline values of heart rate (HR), saturation (SpO2), blood pressure (BP), response entropy (RE) and state entropy (SE) were noted. Loading dose of Dex infusion 1 mcg/kg was started and continued for 15 minutes. Patients were premedicated with glycopyrrolate 4 mcg/kg, midazolam 0.03 mg/kg and ondansetron 4 mg intravenously (IV). After 15 minutes, the rate of Dex infusion was changed to a maintenance infusion of 0.2 mcg/kg/h. All the patients received fentanyl in dose of 1.5 mcg/kg IV. Anesthesia was induced with propofol 5 mg IV incremental doses to reach the entropy value 40-60, and at the same time the eye lash reflex was checked. The dose of propofol needed to achieve entropy of 40-60 was considered as the induction dose.
Succinylcholine 1.5 mg/kg was administered IV to facilitate intubation. Vasopressor response to laryngoscopy and intubation was documented by noting HR and BP. All patients were intubated with appropriate sized cuffed endotracheal tube passed orally, and the placement was confirmed with auscultation and end-tidal carbon dioxide (EtCO2) reading. Anesthesia was maintained with nitrous oxide and oxygen mixture (60:40), and isoflurane, using a closed circuit. End-tidal concentration of isoflurane was monitored. Vecuronium was used to maintain intraoperative neuroblockade. Intraoperative anesthetic requirement was gauged by hemodynamics (HR and BP, end point for requirement for both was considered a 20% increase from baseline value), and SE value was calculated, while the analgesic requirement was titrated with RE, with an aim to maintain both RE and SE around 40-60. HR and BP response to pneumoperitoneum was documented and requirement of additional anesthetic/analgesic noted. Whenever required, anesthesia was deepened by increasing the isoflurane concentration, followed by propofol top ups of 10 mg, if needed. Analgesia was repeated according to RE in the form of fentanyl top ups of 10 mcg. Any additional requirement of metoprolol or nitroglycerine to control BP was noted. Dex infusion was continued until extubation. Hemodynamic response to extubation was documented by observing the pulse and blood pressure.
Intraoperative monitoring was documented during the pre-induction, after the loading dose of Dex, at the induction of anesthesia, during laryngoscopy and intubation, and at pneumoperitoneum and then every 15 min till the end of surgery and continued during extubation and post operatively of extubation and postoperatively. At the end of surgery, diclofenac sodium 75 mg was added to the IV fluid for postoperative analgesia. Any side effects like hypotension, bradycardia, respiratory depression, postoperative nausea and vomiting were noted. Patients were observed for two hours in the recovery room, and then shifted to the ward.
Our study was observational in nature without a control or placebo group (kind of open label study), the sample size being drawn from EpiInfo software. We have only studied the association of Dex and reported the outcomes in this case series. Results are expressed as mean (Standard Deviation, SD) or median (range) unless otherwise stated. Statistics were performed using Chi Square test and Analysis of Variance (ANOVA) to compare the characteristics, and repeated measures of ANOVA were used to assess the changes in the HR and BP. P value < 0.05 was considered significant. All basic calculations were done using Microsoft excel software.
Results
A total of thirty patients were enrolled in our study. Table 1 depicts the demographic data. Mean HR on starting was 85(17) which fell to lowest mean of 72(13); P = 0.0001 [Figure 1]. There was a transient yet significant fall in HR at beginning of the Dex infusion. HR was however sustained for the entire duration of infusion. Patients had sinus bradycardia (HR < 60) at the start; but none required any therapy for treatment of this bradycardia. This reduction although statistically significant was not clinically relevant as no intervention was required. Mean systolic blood pressure (SBP) to start with was 125(22), and fell to 113(20) with loading dose of Dex (P = 0.009). After that minimal change was observed for entire duration of infusion. Similar observations were made at the time of creation of the pneumoperitoneum. Mean diastolic blood pressure (DBP) fell from 68(12) to 56(10) which was not statistically significant [Figure 2]. There was good control over the vasopressor response during laryngoscopy, with minimal or no change in BP with pneumoperitoneum. None of the 30 patients needed either metoprolol or nitroglycerine to counter the hypertension effect of pneumoperitoneum.
Table 1.
Demographic data of the patients enrolled in the study

Figure 1.
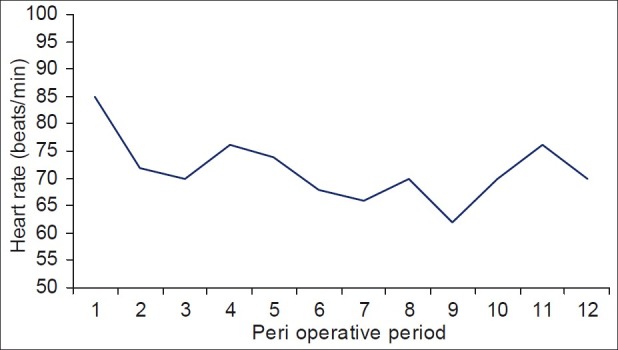
Changes in heart rate of the patients as observed in the study
Figure 2.
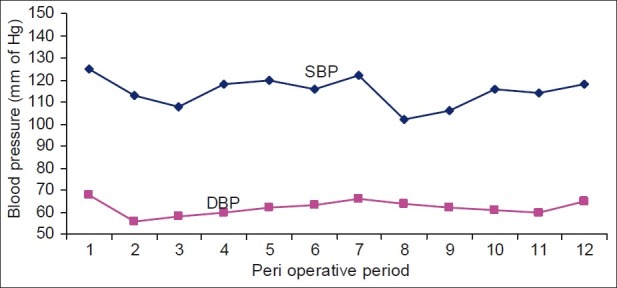
Changes in blood pressure of the patients as observed in the study
The mean dose of propofol required for induction was 37.5(5) mg. Intraoperatively, whenever propofol was required, it was given in 10 mg top ups depending on the SE value, and fentanyl was repeated in 10 mcg top ups according to RE value. We observed that nine patients required 10 mcg top ups of fentanyl (30%) at the time of pneumoperitoneum. The mean total requirement of fentanyl in the entire intraoperative period was 92.5(8) mcg. We also studied the requirement of isoflurane [Figure 3] which was adjusted to maintain stable BP and SE. It was observed that the end-tidal concentration of isoflurane required was between 0.5 to 0.75 all through the surgery; with peak requirement at the beginning immediately after intubation, and then again during creation of pneumoperitoneum. The end-tidal concentration of isoflurane as compared to the traditional requirement was observed to be almost 30% less with Dex.
Figure 3.
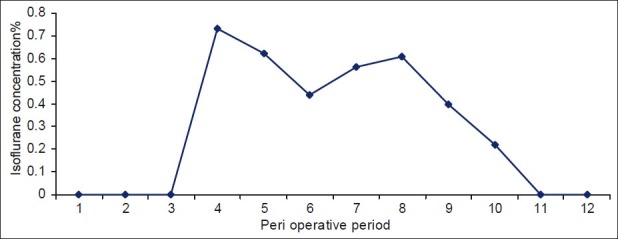
Isoflurane requirement of the patients in the study
Entropy monitoring documented both RE and SE. In the preoperative period, the RE value was 98 to 100, and SE was 90 to 91 in all patients indicating complete consciousness [Figure 4]. With the loading dose of Dex itself, entropy fell by 20% to reach a value 60 to 80, indicating good sedation (sedated but not unconscious, could be awakened by verbal commands). Intraoperatively entropy was maintained 40 - 60 for providing adequate DOA. Increase in RE indicates nociception which was tackled with fentanyl top ups. SE indicates anesthetic depth which was maintained with isoflurane and if required propofol.
Figure 4.
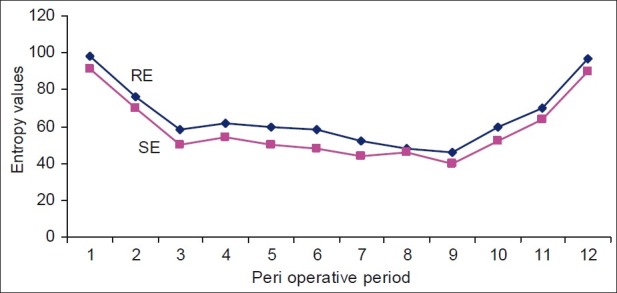
Entropy values of the patients as observed in the study Peri operative period: 1- preop, 2- with loading dose of dex, 3- at induction, 4- during laryngoscopy and intubation, 5- during pneumoperitoneum, 6,7,8,9- every 15 minutes monitoring, 10- at closure, 11- extubation, 12- post op
We observed that entropy fell with induction to < 40 and rose to 60 with intubation. As can be seen from Figure 4, when the gases were started (nitrous, oxygen and isoflurane) after intubation, the entropy dropped to < 60 and remained so till the creation of pneumoperitoneum. With pneumoperitoneum, however, 30% patients showed increase in RE, indicating pain and required fentanyl top ups. For rest of the period, entropy was well maintained. Extubation response was studied which appeared to be smooth in all patients with minimal change in hemodynamics. Patients showed immediate eye opening and were responsive to verbal commands indicating no residual effects of Dex. There was no difference in vecuronium requirement with the use of Dex. Patients were pain free in postoperative period. All patients were hemodynamically stable and comfortable in the recovery room. None of patients had postoperative nausea and vomiting (PONV), hypotension, bradycardia or episodes of respiratory depression and were shifted to the ward after two hours.
Discussion
Laparoscopic surgery offers intraoperative stress during pneumoperitoneum by increasing the systemic vascular resistance and blood pressure at the same time producing nociception.[2] Dex, an imidazole compound, displays specific and selective α2 adrenergic receptor agonism.[4] In the past, xylazine and detomidine have been employed to induce analgesia and sedation in animals.[5] The unique properties of Dex render it suitable for analgesia during the perioperative period.[6] The major sedative and antinociceptive effects of Dex are attributable to its stimulation of α2 A subtype located in locus coeruleus.[7] It is the specificity of dex for α2 receptor that makes it a more effective sedative and analgesic agent than clonidine. Dex is eight times more specific for α2 receptors than clonidine (α2: α1 ratio for dex is 1620:1 and that for clonidine is 220:1).
Dex potentiates anesthetic effects of all intraoperative anesthetics, regardless of the method of administration. The profound reduction in anesthetic requirement was shown to be mediated through central α2 adrenergic receptors. Possible anesthetic effects also have been suggested in humans. IV and intramuscular administration has shown to reduce requirement of thiopentone by 17% in a group that receiving low dose Dex, and up to 30% in a group receiving high dose Dex.[8] We observed that Dex significantly reduces induction dose of propofol. When compare to the traditional induction dose of propofol (2 mg/kg), we observed a 62.5% reduction (0.75 mg/ kg) with the use of Dex. Dex also decreases the requirement of inhalational agents. Routine end tidal concentration required for maintenance of anesthesia is 1.5 to1.8%.[3] First report of reduced requirement of isoflurane with Dex in humans was published in 1991,[9] which showed a 25% reduction of maintenance and concentration of isoflurane in patients who received Dex. A 35-50% reduction of isoflurane requirement in patients treated with either low or high doses of isoflurane without premedication has been reported.[10] Similar observations regarding isoflurane requirement were made in our study.
Dex has been shown to attenuate the sympathoadrenal stimulation during intubation effectively.[11] We observed that Dex effectively attenuates the vasopressor response of laryngoscopy, and intubation and the sympathoadrenal response occurring with pneumoperitoneum. Analgesic properties have been demonstrated in studies that used Dex as a sole analgesic after minor surgeries.[12] Opioid requirements in the intra and post-operative period are reduced by Dex. The α2 mechanism of action is involved in modulation of nociception at the level of spinal noradrenergic systems.[13] There is a clear evidence that α2 receptors located in dorsal horn neurons of spinal cord might release endogenous opiate compounds.[14] We observed that fentanyl in the dose of 1.5 mcg/kg was sufficient to provide analgesia, at least till the time of pneumoperitoneum. However, 30% of patients required fentanyl top up during pneumoperitoneum. Dex has been shown to provide good hemodynamic stability. Dex maintained HR and BP during perioperative period including during laryngoscopy and pneumoperitoneum.
At clinically effective doses, Dex has been shown to cause much less respiratory depression than any other sedatives.[15] The danger of respiratory depression with sedative agents often necessitates their discontinuation during extubation period; however, Dex infusion can be continued safely through extubation.[16] In our study, none of the 30 patients had episodes of respiratory depression in the post-operative period. Dex use permits lower doses of anesthetics to be used and decreases the opioid requirement, thus resulting in a more rapid recovery from anesthesia. Patients are able to return to the baseline level of consciousness when stimulated. This feature was shown by Hall et al. using bispectral index (BIS) monitoring system and other psychometric tests.[1] Studying Dex could invariably result in under-dosing the patient with anesthetics and analgesics, possibly resulting in inadequate DOA. Hence, we included entropy monitoring in our study. Electroencephalogram (EEG) was employed before arrival of BIS, which based on EEG and Fourier analysis for measuring DOA.[17] BIS and EEG evaluate only the DOA, and the depth of nociception remains ignored, and the patient may be free from awareness; however, may not be free from pain.
Entropy is an innovative monitoring based on the acquisition and processing of raw EEG and facial electromyogram (FEMG) signals by using entropy algorithm.[18–20] There are two entropy parameters: fast reacting response entropy (RE) and more steady and robust state entropy (SE). RE is sensitive to the activation of facial muscles i.e. FEMG. Activation of RE to painful stimulus may be interpreted as sign of inadequate analgesia.[21] SE is indicative of depth of hypnosis. Display range of RE is from 0 to 100 and that of SE is 0 to 91. SE value is always less than or equal to RE. We aimed at keeping both the entropy values between 40-60 to ensure adequate DOA as well as nociception, and observed that with the use of Dex, we can taper down anesthetics as well as opioids while keeping the patients free from awareness at the same time, rendering them pain free.
The only limitation of our study is the lack of a control group. Our intention was to observe the effects of Dex as an anesthetic adjuvant while maintaining the adequate anesthetic depth. We observed that Dex is a good anesthetic adjuvant that decreases the requirement of anesthetics and opioids, attenuates sympathoadrenal response, maintains the stable hemodynamics and adequate DOA, and provides an excellent recovery profile.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 2.Mann C, Boccara G, Pouzeratte Y, Eliet J, Serradel-Le Gal C, Vergnes C, Bichet DG, et al. The relationship among carbon di oxide pneumoperitoneum, vasopressin release and hemodynamic changes. Anesth Analg. 1999;89:278–83. doi: 10.1097/00000539-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Eger El. Isoflurane: a review. Anesthesiology. 1981;55:559–76. doi: 10.1097/00000542-198111000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Maze M, Virtanen R, Daunt D, Banks SJ, Stover EP, Feldman D. Effects of dexmedetomidine, a novel imidazole sedative-anesthetic agent, on adrenal steroidogenesis: in vivo and in vitro studies. Anesth Analg. 1991;73:204–8. doi: 10.1213/00000539-199108000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Clarke KW, Hall LW. “Xylazine”- a new sedative for horses and cattle. Vet Rec. 1969;85:512–7. doi: 10.1136/vr.85.19.512. [DOI] [PubMed] [Google Scholar]
- 6.Jaakola ML, Salonen M, Lehtinen R, Scheinin H. The analgesic action of dexmedetomidine – a novel alpha 2 adrenoceptor agonist- in healthy volunteers. Pain. 1991;46:281–5. doi: 10.1016/0304-3959(91)90111-A. [DOI] [PubMed] [Google Scholar]
- 7.Hunter JC, Fontana DJ, Hedley LR, Jasper JR, Lewis R, Link RE, et al. Assessment of the role of alpha 2 adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br J Pharmacol. 1997;122:1339–44. doi: 10.1038/sj.bjp.0701520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buhrer M, Mappes A, Lauber R, Stanski DR, Maitre PO. Dexmedetomidine decreases thiopental dose requirement and alters distribution pharmacokinetics. Anesthesiology. 1994;80:1216–27. doi: 10.1097/00000542-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Aanta R, Jaakola ML, Kallio A. Reduction of the minimum alveolar concentration of isoflurane by dexmedetomidine. Anesthesiology. 1997;86:1055–60. doi: 10.1097/00000542-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Khan ZP, Munday IT, Jones RM, Thompton C. Effects of dexmedetomidine on isoflurane requirements in healthy volunteers: Pharmacodynamics and pharmacokinetics interactions. Br J Anaesth. 1999;83:372–80. doi: 10.1093/bja/83.3.372. [DOI] [PubMed] [Google Scholar]
- 11.Scheinin B, Lindgren L, Randall T, Sceinin H. Dexmedetomidine attenuates sympathoadrenal responses to tracheal intubation and reduces the need for thiopentone and peri operative fentanyl. Br J Anaesth. 1992;68:126–31. doi: 10.1093/bja/68.2.126. [DOI] [PubMed] [Google Scholar]
- 12.Aho MS, Erkola OA, Scheinin H, Lehtmen AM. Effect of intravenously administered dexmedetomidine on pain after laparoscopic tubal ligation. Anesth Analg. 1991;73:112–8. doi: 10.1213/00000539-199108000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa I, Omote K, Kitahata LM, Collins JG. Serotonergic mediation of spinal analgesia and its interaction with nonadrenergic systems. Anesthesiology. 1990;73:474–8. doi: 10.1097/00000542-199009000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Fleetwood Walker SM, Mitchell R, Hope PJ, Molony V. An alpha 2 receptor mediates the selective inhibition by noradrenaline of nociceptive responses of identified dorsal horn neurons. Brain Res. 1985;334:243–54. doi: 10.1016/0006-8993(85)90216-1. [DOI] [PubMed] [Google Scholar]
- 15.Belleville JP, Ward DS, Bloor BC, Maze M. Effects of intravenous dexmedetomidine in humans. Sedation, ventilation and metabolic rate. Anesthesiology. 1992;77:1125–33. doi: 10.1097/00000542-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Vean RM, Bradshaw CJ, Spencer R, Brealey D. Preliminary UK experience of dexmedetomidine, a novel agent for post-operative sedation in the intensive care unit. Anaesthesia. 1999;54:1136–42. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 17.O’Connor MF, Daves SM, Tung A, Cook RI, Thisted R, Apfelbaum J. BIS monitoring to prevent awareness during general anesthesia. Anesthesiology. 2001;94:520–2. doi: 10.1097/00000542-200103000-00025. [DOI] [PubMed] [Google Scholar]
- 18.Vakkuri A, Yli-Hankala A, Sandin R, Mustola S, Høymork S, Nyblom S, et al. Spectral entropy monitoring is associated with reduced propofol use and faster emergence in propofol–nitrous oxide–alfentanil anesthesia. Anesthesiology. 2005;103:274–9. doi: 10.1097/00000542-200508000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Vanluchene AL, Struys MM, Heyse BE, Mortier EP. Spectral entropy measurement of patient responsiveness during propofol and remifentanil. A comparison with the bispectral index. Br J Anaesth. 2004;93:645–54. doi: 10.1093/bja/aeh251. [DOI] [PubMed] [Google Scholar]
- 20.Vanluchene AL, Vereecke H, Thas O, Mortier EP, Shafer SL, Struys MM. Spectral entropy as an electroencephalographic measure of anesthetic drug effect: a comparison with bispectral index and processed midlatency auditory evoked response. Anesthesiology. 2004;101:34–42. doi: 10.1097/00000542-200407000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler P, Hoffman WE, Baughman VL, Koenig H. Response entropy increases during painful stimulation. J Neurosurg Anesthesiol. 2005;17:86–90. doi: 10.1097/01.ana.0000151408.62650.b5. [DOI] [PubMed] [Google Scholar]


