Abstract
Objectives:
To compare propofol and thiopental as anesthetic agents for electroconvulsive therapy (ECT) with respect to seizure duration, stimulus charge, and clinical effects.
Materials and Methods:
Randomized, blinded study of 28 patients of depression treated with bilateral ECT. In group P (n = 14), sedation was achieved with propofol 1.5 mg/kg, whereas in group T (n = 14), it was achieved with thiopentone 3 mg/ kg IV. Succinylcholine 0.4 mg/kg intravenous was given in all patients as for neuromuscular blockade.
Results:
The mean seizure duration of the patients in the thiopental group was 83 ± 34.43 seconds vs. 94.45 ± 21.37 seconds in the propofol group (P < 0.01). The energy delivered per treatment was 10.88 ± 4.78 J in the thiopental group vs. 12.20 ± 4.53 J in the propofol group (P < 0.05). Number of ECTs required were significantly higher in propofol group (9.71 ± 2.87) as compared to thiopental group (5.86 ± 0.36) P < 0.0001. No significant difference in duration of hospitalization was seen in both groups. The mean score on Mini-Mental State Examination (MMSE) was 29.14 in the thiopental group vs. 29.57 in the propofol group (P > 0.05). The mean score on Beck Depression Inventory (BDI) was 7.14 in the thiopental group vs. 3.29 in the propofol group (P < 0.05).
Conclusions:
Propofol significantly increases number of ECT required to treat although the patients received higher electrical charge and had longer seizure duration. BDI scores suggest this resulted in better outcome. Results, however, might be confounded by the differences in pharmacological treatment in the groups.
Keywords: Electroconvulsive therapy, major depression, propofol, seizure duration, thiopental
Introduction
The use of electroconvulsive therapy (ECT) to provoke a generalized epileptic seizure was first described in 1938 and was performed without anesthesia for almost 30 years.[1] The efficacy of ECT in alleviating an acute depression is dependent on the duration of the induced seizure.[1,2] EEG seizure activity lasting from 25 to 75 seconds is alleged to produce the optimal anti-depressant response. Patients experiencing initial seizure duration of 15 seconds (very short) or 120 seconds (very long) achieve a less favorable response to ECT.[2,3] Many anesthetic drugs used for ECT have anti-convulsant properties and may decrease the duration of ECT-induced seizure activity in a dose-dependent manner. Use of larger than necessary dosages results in shorting of the duration of ECT-induced seizure activity and could adversely affect the efficacy of the ECT treatments. A delicate balance needs to be maintained to achieve an adequate anesthetic state along with an optimal duration of EEG seizure activity. The essential elements of anesthesia for ECT include rapid loss of consciousness, effective attenuation of the hemodynamic response to the electrical stimulus, avoidance of gross movements, minimal interference with seizure activity, and prompt recovery of spontaneous ventilation, and consciousness. Use of general anesthetic techniques with a rapid onset and recovery is essential to facilitate fast tracking.
Propofol is associated with less nausea and vomiting,[4] faster emergence, better early psychomotor recovery, and better early cognitive recovery.[5,6] Initial concerns that shorter seizures produced with propofol administration may compromise efficacy have not been empirically supported in the period immediately after ECT and have been offset by its demonstrated advantages.[7,8]
This study aimed to compare thiopentone and propofol for use during ECT, the primary outcome measure being the clinical response as assessed by BDI (Beck Depression Inventory) and MMSE (Mini Mental state Examination) scores after the scheduled ECTs.
Materials and Methods
A double-blind, randomized trial was performed following clearance from Institutional Ethical Committee and an informed written consent from all subjects. Adult patients, aged 18 years or older with a major depressive episode as part of a diagnosis of either major depressive disorder or bipolar disorder (International Classification of Diseases, 10th edition Code 296), were included in the study. Patients were excluded from the study if they fulfilled criteria for DSM-IV 8 (Diagnostic and Statistical Manual of Mental Disorders) substance abuse disorder in the last 12 months; had received ECT within the previous 6 months, or were in ASA III/ IV.
Over a period of 18 months, a total of 28 patients were randomly divided into 2 groups, based on the choice of anesthetic agent (thiopentone or propofol). 5 of the patients were referred for ECT again during the study period, but were only not included the second time.
All patients received glycopyrrolate 0.1 mg/kg IV. In group P (n = 14), sedation induction was achieved with propofol 1.5 mg/kg intravenous (IV), whereas in group T (n = 14), sedation was achieved with thiopentone 3 mg/kg IV. Succinylcholine 0.4 mg/kg IV was given for neuromuscular blockade to reduce the muscle contractions associated with ECT-induced seizure activity. Ventilation is assisted with help of a face mask using a standard Mapleson D breathing system.
ECT was administered twice a week using a brief-pulse, square-wave, constant-current ECT device [Figure 1] (120 mC, 70 Hz/ 0.1 sec). Bilateral ECT was given using the standard bifrontotemporal placement. During the course of ECT, each patient was given maintenance anti-depressant medication by the psychiatrist to reduce the risk of relapse.[9] Patients are required to fast overnight for solid food, but clear liquids were allowed for taking an oral medication up to 1 h before the procedure. The psychiatrist was blinded to anesthetic agent, and an independent observer, blinded to the type of drug being used, recorded the data.
Figure 1.

MEDICAID, BPE 2000 (Brief Pulse ECT Machine)
Outcome measures recorded included duration of seizure [Figure 2], amount of energy (in Joules) delivered, and duration of recovery from the anesthesia (response to verbal command). Other data recorded included demographic profile such as age, sex, weight, underlying disease, duration of hospitalization, duration of disorder, and number of ECT. Mean blood pressure, heart rate, and pulse oximetry were evaluated and recorded before and at the end of the procedure.
Figure 2.
EEG Seizure detected by the Machine (MEDCAID, BPE 2000)
All patients were evaluated by the psychiatrist on the BDI (Beck Depression Inventory) and MMSE (Mini Mental state Examination) scale the day before the commencement of treatment (baseline), the day after the third ECT (3 ECT), and 1 to 3 days after the final treatment (completion). The MMSE has a score ranging from 0-30, with the mean score for normal individuals being 27.6 and mean score in dementia being as low as 9.7.
In BDI, the total score ranges from 0-62, with 0-9 being normal, rising to 10-18 during mild to moderate depression, 19-29 during moderate to severe depression, and rising to > 30 during an extreme severe depression.
For the comparisons, P < 0.05 was considered to be statistically significant. The power of the study being adequate, data were analyzed using the software package SPSS version 11 by using t-test, Z test, MW test, and Wilcoxon test.
Results
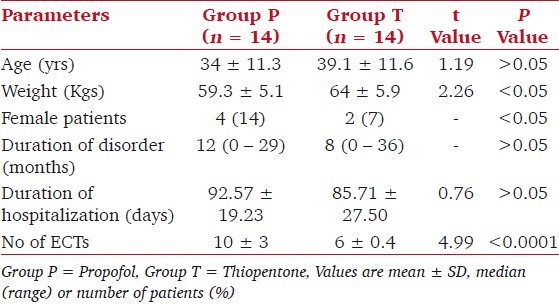
The mean age of patients in both groups was statistically similar, but patients in group P weighed significantly lesser, and there were significantly more number of females [Table 1, P < 0.05]. The mean duration of mental disorder and of hospitalization was statistically similar in both groups [Table 1, P > 0.05]. Almost all the patients were receiving concurrent medical treatment with anti-depressants or anti-psychotics; none received mood stabilizers.
Table 1.
Patient characteristics
The mean number of ECTs received per patient was significantly greater in group P as compared to group T [Table 1, P < 0.05]. ECT data were analyzed for all patients included. In group P, the energy delivered and duration of seizures as measured by EEG was significantly more as compared to group T [Table 2, P < 0.05]. The correlation between duration of disorder and duration of seizure is r = -0.23, which was significant (P < 0.05). The time to recovery from anesthesia was significantly longer in group T [Table 2, P < 0.05].
Table 2.
Outcome measures
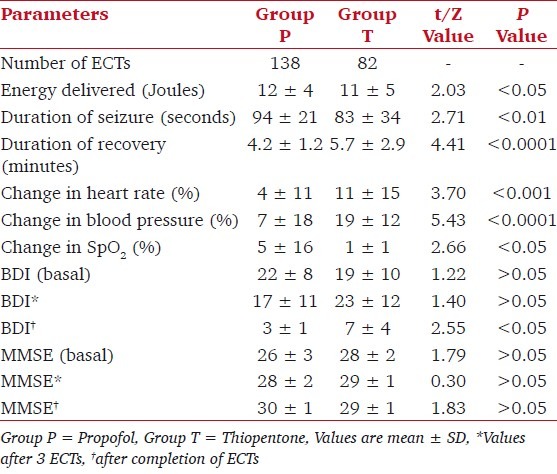
In group P, an increase in all hemodynamic parameters was seen after the procedure, mean heart rate (80 ± 14 bpm vs. 76 ± 14 bpm), systolic blood pressure (98 ± 17 mmHg vs. 91 ± 11 mmHg), and the SpO2 (100 ± 0.7 % vs. 98 ± 2%). In group T too, a similar trend of increase in all hemodynamic parameters was seen after the procedure, mean heart rate (81 ± 12 bpm vs. 70 ± 11 bpm), systolic blood pressure (119 ± 11 mmHg vs. 100 ± 12 mmHg), and the SpO2 (100 ± 0.6% vs. 99 ± 0.9%). However, the percentage increase in each of the variables following the procedure was significantly greater in group T as compared to group P [Table 2, P < 0.05].
There was no significant difference in MMSE and BDI scores between the groups before treatment. The BDI at completion of ECTs was significantly lower in group P. The MMSE score was statistically similar in both the groups [Table 2].
Discussion
In recent years, ECT has assumed an increasingly important role in the treatment of severe and medication-resistant depression and mania as well as in the treatment of schizophrenic patients with affective disorders, suicidal drive, delusional symptoms, vegetative dysregulation, inanition, and catatonic symptoms.[2] Typically, in the acute phase of the illness, ECT is performed twice in a week for 5 to 12 treatments. In successful cases, an initial clinical improvement is usually evident after 3 to 5 treatments.[2,10] Maintenance therapy can be performed at progressively-increasing intervals from once-a-week to once-a-month to prevent relapses.
Mot or seizure may not be visualized in many patients, and yet ECT produces effective seizure as evident by EEG. We did not record the motor seizure, but quantified the seizure by EEG monitoring. Propofol has been found to have more potent anti-convulsant effects during ECT than other IV anesthetics.[2,8,11] However, the use of a minimally hypnotic dose of propofol (0.75 mg/kg) has been associated with a seizure duration that is comparable to standard hypnotic doses of methohexital.[8] Use of propofol can significantly shorten the duration of seizure activity, and its effect on the anti-depressant action of ECT has been a concern. However, the ECT seizure duration in our study after larger dose of propofol (1.5 mg/kg) was significantly longer than after thiopentone, possibly because higher shock energy was delivered to patients in the propofol group.
2 reports have compared the anti-depressant efficacy of ECT by using the Hamilton Rating Scale for Depression, HRSD (a 17-item scale that evaluates mood, vegetative and cognitive symptoms of depression, and comorbid anxiety symptoms) and the Beck Depression Inventory (a 21-item self-report rating inventory that measures characteristic attitudes and symptoms of depression) when propofol or methohexital was administered as the primary anesthetic. The HRSD scores symptoms of depression were improved to a similar degree in both anesthetic groups after they completed a standard series of ECT treatments.[11] In our study, the mean BDI score was significantly less in propofol group, showing it to have a better anti-depressant effect. Significantly, more patients received an increased charge when propofol was used for ECT, probably due to an elevation in seizure threshold caused by propofol. BDI score at completion was significantly lower in the propofol group. This is in accordance with the findings of Cronholm and Ottosson who found more rapid effect of increase in stimulus intensity (but no change in the final degree of improvement).[12] The increase in cognitive side effect is also a well-known price to be paid for an increased stimulus dose.[13]
Acute hemodynamic response during the ECT procedure is reduced with propofol as compared with thiopental. Emergence from propofol anesthesia was only marginally faster than with thiopentone,[10,14] and recovery of cognitive function is similar to thiopentone during the recovery period as seen with MMSE scores.[15]
Anesthetic agents may have different profiles with regard to cognitive impairment. Crossover studies have explored differences in acute cognitive impairment after propofol and barbiturate anesthesia. A recent crossover study compared thiopental and propofol with regard to cognitive deficits in the acute postictal period after ECT using a comprehensive neuropsychological battery and concluded that propofol reduces acute cognitive impairment as compared with thiopental.[6] We did not find any difference in MMSE score between the propofol group and the thiopental group. The duration of hospitalization was comparable showing no difference in end point of duration of hospitalization.
Age, sex, and concurrent medical treatment could influence the duration of seizure, degree of cognitive impairment; for example, lithium is known to have the potential to cause postictal delirium.[16,17]
The patients are evaluated and managed by the psychiatrist. As far as anesthesiologists are concerned is whether we can help in performing a modified ECT in the sense that seizures are not violent and that hemodynamic stability is maintained, the final aim being complete and faster recovery of patients. We found propofol to be a better drug for ECT as it curbs hemodynamic response, has better recovery and better BDI score.[18] Although the incidence of myoclonus after propofol was 80% and number of ECT required was more in propofol group, it did not have any effect on the duration of hospitalization or on recovery after the procedure. More studies are required to evaluate regarding the current treatment strategies for ECT and the need for exploring the mechanism of action and for finding reliable indicators of efficacy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Ding Z, White PF. Anesthesia for electroconvulsive therapy. Anesth Analg. 2002;94:1351–64. doi: 10.1097/00000539-200205000-00057. [DOI] [PubMed] [Google Scholar]
- 2.Geretsegger C, Rochowanski E, Kartnig C, Unterrainer AF. Propofol and methohexital as anesthetic agents for electroconvulsive therapy (ECT): A comparison of seizure-quality measures and vital signs. J ECT. 1998;14:28–35. [PubMed] [Google Scholar]
- 3.Villalonga A, Bernardo M, Gomar C, Fita G, Escobar R, Pacheco M. Cardiovascular response and anesthetic recovery in electroconvulsive therapy with propofol or thiopental. Convuls Ther. 1993;9:108–11. [PubMed] [Google Scholar]
- 4.Boey WK, Lai FO. Comparison of propofol and thiopentone as anaesthetic agents for electroconvulsive therapy. Anaesthesia. 2007;10:1365–2044. doi: 10.1111/j.1365-2044.1990.tb14383.x. [DOI] [PubMed] [Google Scholar]
- 5.Rouse E. Propofol for electroconvulsive therapy. Anaesthesia. 1988;43:61–4. doi: 10.1111/j.1365-2044.1988.tb09073.x. [DOI] [PubMed] [Google Scholar]
- 6.Butterfield NN, Graf P, Macleod BA, Ries CR, Zis AP. Propofol reduces cognitive impairment after electroconvulsive therapy. J ECT. 2004;20:3–9. doi: 10.1097/00124509-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Simpson KH, Halsall PJ, Carr CM, Stewart KG. Propofol reduces seizure duration in patients having anaesthesia for electroconvulsive therapy. Br J Anaesth. 1988;61:343–4. doi: 10.1093/bja/61.3.343. [DOI] [PubMed] [Google Scholar]
- 8.Avramov MN, Husain MM, White PF. The comparative effects of methohexital, propofol and etomidate for electroconvulsive therapy. Anesth Analg. 1995;81:596–602. doi: 10.1097/00000539-199509000-00031. [DOI] [PubMed] [Google Scholar]
- 9.Sackeim HA, Haskett RF, Mulsant BH, Thase ME, Mann JJ, Pettinati HM, et al. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: A randomized controlled trial. JAMA. 2001;285:1299–307. doi: 10.1001/jama.285.10.1299. [DOI] [PubMed] [Google Scholar]
- 10.García García E, Rosa Díaz J, Rodríguez Casas E, Echazabal Martínez, Raola Sánchez ME. Propofol versus thiopental in electroconvulsive therapy. Rev Cubana Med Milit. 2007;36(2) [Google Scholar]
- 11.Fear CF, Littlejohns CS, Rouse E, McQuail P. Propofol anaesthesia in electroconvulsive therapy: Reduced seizure duration may not be relevant. Br J Psychiatry. 1994;165:506–9. doi: 10.1192/bjp.165.4.506. [DOI] [PubMed] [Google Scholar]
- 12.Cronholm B, Ottosson JO. Experimental studies of the therapeutic action of electroconvulsive therapy in endogenous depression. The role of the electrical stimulation and of the seizure studied by variation of stimulus intensity and modification by lidocaine of seizure discharge. Convuls Ther. 1996;12:172–94. [PubMed] [Google Scholar]
- 13.Cronholm, Ottosson JO. Ultrabrief stimulus technique in electroconvulsive therapy, II: Comparative studies of therapeutic effects and memory disturbance in treatment of endogenous depression with the Elther ES electroshock apparatus and Siemens Konvulsator III. J Nerv Ment Dis. 1963;137:268–76. doi: 10.1097/00005053-196309000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Mokriski BK, Nagle SE, Papuchis GC, Cohen SM, Waxman GJ. Electroconvulsive therapy-induced cardiac arrhythmias during anesthesia with methohexital, thiamylal, or thiopental sodium. J Clin Anesth. 1992;4:208–12. doi: 10.1016/0952-8180(92)90067-b. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto A, Hoshino T, Suzuki N, Suzuki H, Kimura M, Ogawa R. Effects of propofol anesthesia on cognitive recovery of patients undergoing electroconvulsive therapy. Psychiatry Clin Neurosci. 1999;53:655–60. doi: 10.1046/j.1440-1819.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- 16.Rasimas JJ, Stevens SR, Rasmussen KG. Seizure length in electroconvulsive therapy as a function of age, sex and treatment number. J ECT. 2007;23:14–6. doi: 10.1097/01.yct.0000263254.21668.f0. [DOI] [PubMed] [Google Scholar]
- 17.Naguib MK. Interactions between psychotropics, anaesthetics and electroconvulsive therapy: Implications for drug choice and patient management. CNS Drugs. 2002;16:229–47. doi: 10.2165/00023210-200216040-00003. [DOI] [PubMed] [Google Scholar]
- 18.Omprakash TM, Ali MI, Anand B, Devi MG, Surender P. Comparision of thiopentone sodium and propofol in ECT anaesthesia. Indian J Psychol Med. 2008;30:48–51. [Google Scholar]


