Abstract
Diagnosing and managing critically ill patients with renal dysfunction is a part of the daily routine of an intensivist. Acute kidney insufficiency substantially contributes to the morbidity and mortality of critically ill patients. Renal replacement therapy (RRT) not only does play a significant role in the treatment of patients with renal failure, acute as well as chronic, but also has spread its domains to the treatment of many other disease conditions such as myaesthenia gravis, septic shock and acute on chronic liver failure. This article briefly outlines the role of renal replacement therapy in ICU.
Keywords: Acute kidney insufficiency, acute renal failure, anticoagulation, CRRT, CVVH, ICU, IHD, peritoneal dialysis, renal replacement therapy, SLEDD
Introduction
“…The kidneys are so beautifully organized; they do their work of regulation with such a miraculous--it's hard to find another word--such a positively divine precision, such knowledge and wisdom, that there is no reason why our archetypal man, whoever he is, or anyone else, for that matter, should be ashamed to own a pair.”
Aldous Huxley, 1923
Renal replacement therapy (RRT) is being widely used in modern intensive care settings. Diagnosing and managing critically ill patients with renal dysfunction is a part of the daily routine of an intensivist. Acute renal failure (ARF) in the intensive care unit (ICU) is frequent, as a part of multiple organ dysfunction syndrome (MODS), in postoperative states and after interventional studies, in already susceptible individuals. These patients have various co-morbid conditions and are on various life-supportive modalities; and their organ systems may be further injured by fluid overload and electrolyte and acid-base disturbances. Being catabolic, they require continuous clearance of waste products due to on-going illnesses, and at the same time, they receive infusions of nutritional and inotropic agents for sustenance of vital parameters. RRT plays a significant role in ICU in the treatment of patients with renal failure, acute as well as chronic, and it has spread its domains to the treatment of many other disease conditions, such as myasthenia gravis, septic shock, and acute-on-chronic liver failure.
The term ‘acute kidney insufficiency’ (AKI) is preferable to ‘acute renal failure,’ and the ‘I’ in AKI refers to ‘insufficiency’ instead of ‘injury.’ Performing dialysis does not achieve the same degree of homeostasis as a normally-functioning kidney. However, the term ‘RRT’ is not accurate as it is not possible for dialysis to replace all functions of the kidney. ‘Renal support therapy’ would be a better term to refer to this modality of treatment.[1]
Definition of AKI in the ICU
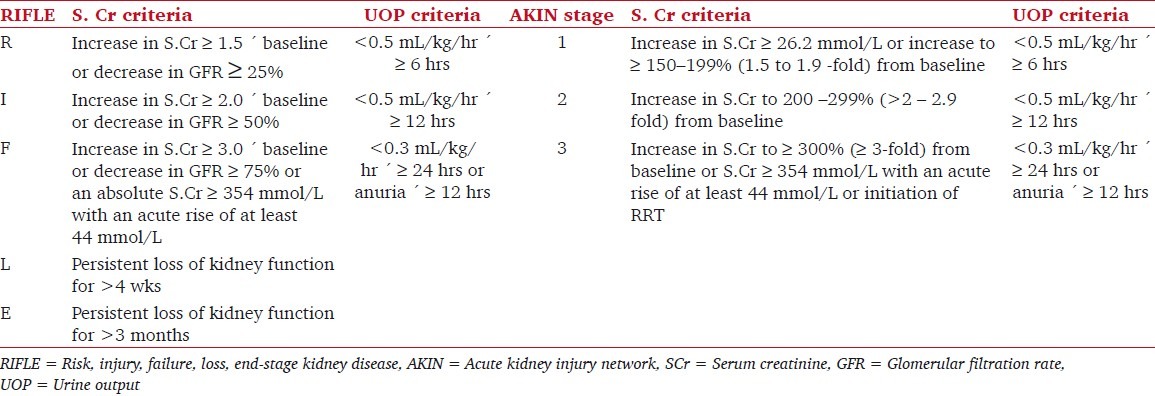
The RIFLE classification[2] (risk, injury, failure, loss, end-stage kidney disease) was proposed by the Acute Dialysis Quality Initiative specifically for AKI in critically ill patients. According to the Acute Kidney Injury Network (AKIN) criteria,[3] which further modified the RIFLE criteria [Table 1], the most current consensus diagnostic criteria for AKI is “an abrupt (within 48 hours) reduction in kidney function, currently defined as an absolute increase in serum creatinine of ≥0.3 mg/dL (≥26.4 μmol/L), a percentage increase in serum creatinine of ≥50% (1.5-fold from baseline), or a reduction in urine output (documented oliguria of <0.5 mL/kg/hr for > 6 hours).”
Table 1.
Classification / staging systems for acute kidney injury
Common causes of AKI in the ICU
The 5 most common causes of AKI in the ICU are sepsis, major surgery, low cardiac output, hypovolemia / renal hypoperfusion, and medications. Other common causes include hepatorenal syndrome, trauma, cardiopulmonary bypass, abdominal compartment syndrome, rhabdomyolysis, and urinary flow obstruction.
Diagnosis of AKI in the ICU
In critically ill patients, the etiology of AKI is multifactorial. Increases in serum creatinine substantially lag behind reductions in glomerular filtration rate (GFR) and thus do not provide a useful real-time assessment of GFR. Urine microscopy and fractional excretion of sodium (FENa) can be valuable tools in determining the cause of AKI, but they are of little value in the early detection or diagnosis of AKI. Various molecules, such as serum cystatin C, urinary neutrophil gelatinase-associated lipocalin (NGAL), urinary kidney injury molecule-1, and urinary interleukin-18 are promising novel biomarkers for the early detection of AKI in ICU.
Prevention of AKI in the ICU
The European society of intensive care medicine provides useful recommendations for the prevention of AKI in critically ill patients.[4]
Volume expansion is recommended for the prevention of AKI in states of true and suspected hypovolemia, but uncontrolled volume expansion and the use of high molecular- weight HES preparations and dextrans should be avoided, especially in sepsis. Prophylactic volume expansion by isotonic crystalloids is recommended in patients at risk of contrast nephropathy. Prevention of nephrotoxicity through prophylactic volume expansion has been shown to be of benefit with amphotericin B, foscarnet, cidofovir, adefovir, indinavir, acyclovir, and sulfadiazine.[5–8]
After adequate volume resuscitation, in hypotensive patients, a vasoconstrictor is recommended, targeting a mean arterial pressure of 60–65 mmHg. In case of vasoplegia as a result of sepsis or SIRS, either norepinephrine or dopamine (along with fluid resuscitation) is recommended as the first choice vasopressor agent to correct hypotension. Low-dose dopamine does not offer protection against AKI.[9] Loop diuretics are not recommended for use to prevent or ameliorate AKI. The prophylactic use of fenoldopam has been suggested in cardiovascular surgery patients at risk of AKI.
N-acetylcysteine is not indicated for prophylaxis against contrast-induced nephropathy or other forms AKI in critically ill patients. Periprocedural continuous veno-venous hemofiltration (CVVH) is helpful in an ICU environment to limit contrast nephropathy after coronary interventions in high-risk patients with advanced chronic renal insufficiency. The use of theophylline may minimize risk of contrast nephropathy, especially in acute interventions when hydration is not feasible.[10]
A “normal for age” glycemic control with intravenous (IV) insulin therapy is preferable to “tight glycemic control” for preventing AKI in surgical ICU patients. All patients at risk of AKI should have an adequate nutritional support, preferably through the enteral route. A review of all the medications being administered and cessation of the nephrotoxic ones is important along with other preventive measures.
Indications and timing of RRT
Severe AKI results in dysregulation in the homeostasis of fluid, potassium, metabolic acids, and waste products. RRT aids to prevent life-threatening complications and to improve homeostasis. The various techniques of RRT include continuous or intermittent hemodialysis, hemofiltration, hemodiafiltration, peritoneal dialysis etc.
Modern criteria[11] for the initiation of RRT in ICU include
Oliguria (urine output <200 ml/12 h)
Anuria (urine output: 0–50 ml/12 h)
[Blood urea] > 35 mmol/L (>98 mg/dL)
[Serum creatinine] > 400 mmol/L (>4.5 mg/dL)
Uncompensated metabolic acidosis (pH < 7.1)
[Serum K+] > 6.5 mmol/L or rapidly rising values
[Serum Na+] < 110 and >160 mmol/L
Pulmonary edema unresponsive to diuretics
Temperature >40°C
Uremic complications (encephalopathy / myopathy / neuropathy / pericarditis)
Overdose with a dialyzable toxin (e.g. lithium)
Newer indications for RRT include
Cardiac failure
Patients requiring a large amount of fluid, parenteral nutrition or blood products, but at risk of developing pulmonary edema or ARDS
Hyperthermia (core temperature >39.5°C) or hypothermia (core temperature <37°C)
AKI in the ICU often occurs as a part of MODS, and such patients may have less tolerance of metabolic derangements, such as acidosis and electrolyte imbalances. RRT should be initiated in these patients before the development of extreme metabolic derangements. In patients who require renal support because of metabolic derangements, RRT should not be delayed if there is minimal urine production.
It is preferable to commence RRT at either RIFLE injury stage or at AKIN stage II in critically ill patients when both criteria (urine output and serum creatinine) are included. However, so far, no trials have investigated the credibility of RIFLE/AKIN criteria for determining the timing of RRT. Several studies show that an ‘early AKI’ has improved outcomes over ‘late AKI,’ which led to the concept of ‘door-to-dialysis time.’[12,13] Prompt dialysis after admission has been shown to reduce mortality in certain conditions, such as leptospirosis.[14]
The indications for RRT in chronic kidney insufficiency patients with acute decompensation in ICU include[15]
Presence of uremic symptoms
Presence of hyperkalemia unresponsive to conservative measures
Persistent extracellular volume expansion, despite diuretic therapy
Acidosis refractory to medical therapy
A bleeding diathesis
Principles of RRT
Whatever be the technique of RRT, the fundamental principle is the removal of unwanted solutes and water through a semi-permeable membrane.
Water removal occurs through a process named ultrafiltration. This is achieved, 1) by generating a transmembrane pressure, which is greater than the plasma oncotic pressure [as occurs in hemofiltration (HF) or intermittent hemodialysis (IHD)], or 2) by increasing the osmolarity of the dialysate [as in peritoneal dialysis (PD)].
Solute removal occurs by either diffusion or by convection. In diffusion, an electrochemical gradient is created across the membrane using a flow-past system with a toxin-free dialysate solution e.g., IHD and PD. In convection, a transmembrane pressure-driven ‘solvent drag’ is created where solute moves together with the solvent across a porous membrane. The ultrafiltrate is discarded and then replaced with a toxin-free replacement fluid, as in HF. Both diffusion and convection co-exist in hemodiafiltration [Figure 1].
Figure 1.
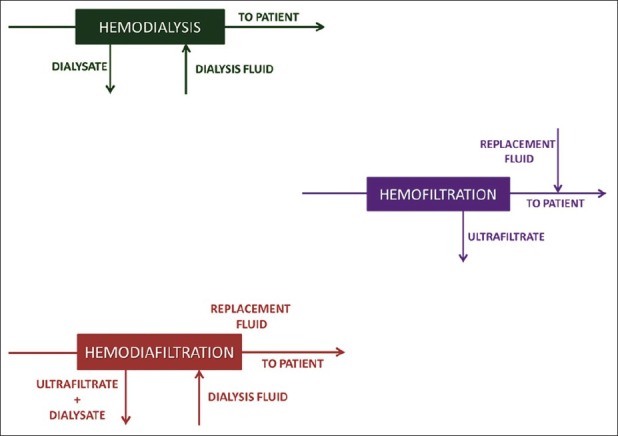
Schematic representation of hemodialysis, hemofiltration and hemodiafiltration
The ultrafiltration rate depends on the coefficient of permeability of the membrane and the transmembrane pressure. Solute transport by diffusion is governed by diffusion coefficient, temperature of the solution, membrane surface area, concentration gradient between the 2 compartments, and thickness of the membrane. The rate of diffusion of a given solute also depends on the molecular weight of the solute, porosity of the membrane, rate of blood flow, rate of dialysate flow, degree of protein-binding, and the concentration gradient across the membrane. Clearance by convection is governed by the ultrafiltration rate and the Sieving Coefficient. The Sieving Coefficient is the ratio of solute concentration in the ultrafiltrate to the solute concentration in plasma water.
Components of hemodialysis
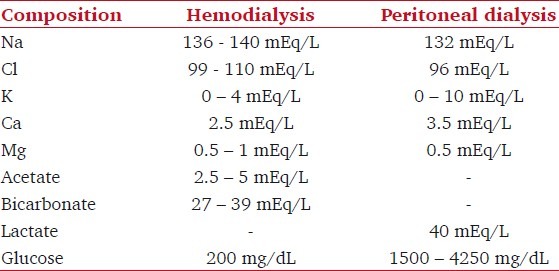
The 3 essential components to hemodialysis include the dialyzer, the composition [Table 2] and delivery of the dialyzate, and the blood delivery system.
Table 2.
Composition of dialysate fluids used in hemodialysis and peritoneal dialysis
Dialyzer
Hollow-fiber dialyzer, the most commonly used type of dialyzer, is composed of bundles of capillary tubes through which blood circulates and the dialyzate travels on the outside of the fiber bundles. The blood and the dialysate may circulate in the same or in opposite directions in co-current and counter-current techniques, respectively.
Synthetic dialysis membranes are more biocompatible than other types of membranes (cellulose, substituted cellulose, and cellulosynthetic). Bioincompatibile membranes are those which activate the complement cascade. Unsubstituted cellulose membranes have been associated with delayed renal recovery in AKI.
Blood delivery system
The blood delivery system consists of the extracorporeal circuit in the dialysis machine and the dialysis access. The dialysis machine consists of a blood pump, dialysis solution delivery system, and various safety monitors. The fistula, graft, or catheter through which blood is obtained for hemodialysis is often referred to as a dialysis access [Figure 2].
Figure 2.
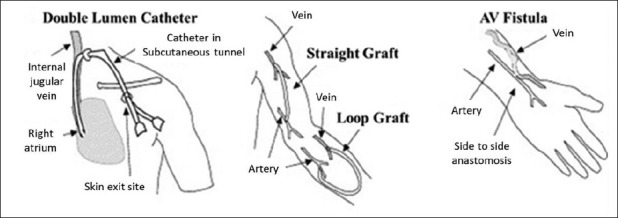
Different types of dialysis access
Goals of dialysis
The dose of dialysis, which is currently defined as a derivation of the fractional urea clearance during a single dialysis treatment, is governed by patient size, residual kidney function, dietary protein intake, degree of anabolism or catabolism, and presence of comorbid conditions.[15]
Efficiency of renal replacement therapy = Kt/Vd
where K is clearance of urea in mL/min, t is time of treatment in minutes, Vd is urea distribution volume in liters. A Kt/Vd equal to 1 indicates that the total body water has been cleared of urea once.
A large multi-center randomized clinical trial[16] failed to show a difference in mortality associated with a large difference in urea clearance, but data are emerging that better uremic control may translate into better survival.
Current targets include[15]
A urea reduction ratio of >65–70%
Kt/Vd above 1.3 or 1.05
Urea levels between 10 and 20 mmol/L throughout the treatment period despite adequate nutrition support with a protein intake around 1.5 g/kg/day
Continuous renal replacement therapy at urea clearances of 35 – 45 L/day
Modes of RRT
The common modes include
Intermittent Hemodialysis (IHD)
Continuous RRT (CRRT) – Slow Continuous Ultrafiltration (SCUF), Continuous Venovenous Hemodialysis, Hemofiltration, Hemodiafiltration (CVVHD, CVVHF, CVVHDF), Continuous Arterio-Venous Hemofiltration (CAVHF), Slow low-efficiency daily dialysis (SLEDD)
Peritoneal dialysis (PD)
Plasmapheresis or plasma exchange
Intermittent Hemodialysis (IHD)
This technique uses high dialyzate flows (300 – 400 ml/min). Treatment is applied for short periods of time (3 – 4 hours), usually every 2nd day.
Disadvantages of IHD
A reasonably large volume has to be removed over a short period of time, which can result in hypotension. Repeated hypotensive episodes may delay renal recovery. The use of CRRT is preferred for its improved cardiovascular tolerance over daily intermittent hemodialysis.[17] Solute removal in IHD is episodic; this results in inferior uremic control and acid-base control [Figure 3]. Limited fluid and uremic control imposes unnecessary limitations on nutritional support. Rapid solute shifts may increase brain water content and raise intracranial pressure. Standard low-flux dialyzing membranes trigger the activation of several inflammatory pathways when compared to high-flux synthetic membranes (also used for continuous HF). This pro-inflammatory effect contributes to further renal damage and delays recovery or even affects mortality.
Figure 3.
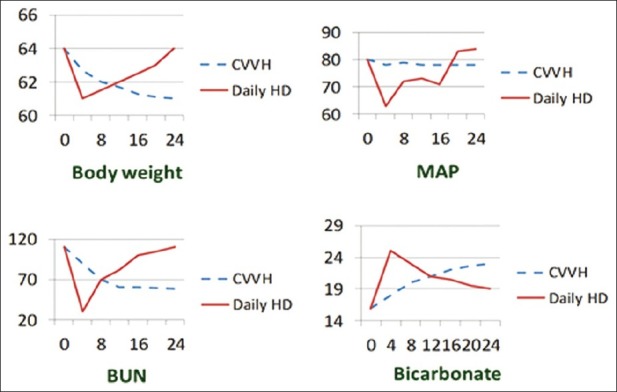
Comparisons of body weight, mean arterial pressure (MAP), blood urea nitrogen (BUN) and bicarbonate control with continuous and intermittent modalities of treatment; demonstrating smoother and better control of all these parameters with the continuous modality. CVVH - continuous venovenous hemofiltration; HD – hemodialysis
Continuous renal replacement therapy
The hemodynamic instability that is often associated with IHD, along with the risk of injury to the recovering kidney, led to the invention of Continuous renal replacement therapy (CRRT) techniques. CRRT is usually initiated with a blood flow of 100 mL/min and gradually increased up to 200 mL/min. CRRT is inherently complex with the requirement for anti-coagulation and the use of high volumes of fluid and is much costlier compared to IHD.
Advantages of CRRT
The advantages of CRRT include continuous control of fluid status, hemodynamic stability, control of acid–base status, ability to provide protein-rich nutrition while achieving uremic control, control of electrolyte balance including phosphate and calcium balance, prevention of swings in intracerebral water, minimal risk of infection, and high level of biocompatibility [Figure 3]. CRRT has to be considered for patients with cerebral edema, severe hemodynamic instability, persistent on-going metabolic acidosis, and large fluid removal requirements.
Anti-Coagulation during CRRT
The flow of blood into the extracorporeal circuit causes activation of the coagulation cascade and clotting of the filter and the circuit. Anti-coagulants are indicated to delay clotting and to achieve acceptable operational lives (approximately 24 hours) for the circuit. The timing of RRT (intermittent, continuous), use of convection or diffusion, membrane choice, treatment dose, blood flow, co-morbidities, hematocrit, and pre-dilution may all affect anti-coagulant requirements.
Strategies for Circuit Anti-Coagulation during CRRT[11]
No anti-coagulation - in patients under systemic anti-coagulation for an associated clinical condition,[1] and in those with pre-existing coagulopathy or recent surgery.
Unfractionated heparin
Regional anti-coagulation (pre-filter heparin and post-filter protamine, usually at 100 IU:1 mg ratio)
Low-molecular-weight heparin
Regional citrate anti-coagulation (pre-filter citrate and post-filter calcium) - in patients without liver failure with an increased bleeding risk in whom anti-coagulation of the circuit is necessary.
Heparinoids
Argatroban - for patients with heparin-induced thrombocytopenia (HIT)
Prostacyclin
Serine proteinase inhibitors (nafamostat mesylate)
Use of dialysis membranes coated with anti-coagulants, such as heparin-bound hemophan, heparin-binding to surface-treated AN69 membranes, and complete covalent coating of the whole extracorporeal system with LMWH may allow successful IHD completely without or with reduced systemic anti-coagulation.[18,19]
Adequate filter life can be attained if blood flow is kept at about 200 ml/min and if the vascular access is reliable. Responding to frequent filter clotting by just increasing anti-coagulation without correcting mechanical factors, such as inadequate access, kinking of catheter will expose the patient to unnecessary risk of hemorrhage.
Which is the ‘Best’ Mode of RRT?
There is a great deal of controversy as to which mode of RRT is ‘best’ in the ICU, due to the lack of randomized controlled trials comparing different techniques. The techniques of RRT may be judged on the basis of criteria, such as hemodynamic side-effects, ability to control fluid status, biocompatibility, risk of infection, uremic control, avoidance of cerebral edema, ability to allow full nutritional support, ability to control acidosis, absence of specific side-effects, and cost. The choice of RRT modality is currently influenced by various factors, such as availability, expertise, resources, and cost. In case of patients who are candidates for renal transplantation, the intensivist should take an active part in discussing with the patient as well as with the relatives about cost concerns, prognosis, and the options available.
With the wide availability of CRRT machines and the increasing complexity of critically ill patients, it is likely to remain one of the preferred modalities of renal replacement in the ICU. Hybrid therapies like SLEDD and extended daily dialysis have been shown to be safe and effective in critically ill patients.[20–22] Slow low efficiency diafiltration (SLEDD-f) by combining SLEDD with ultrafiltration has been shown to provide stable renal replacement therapy.[17]
Dose and Intensity of RRT
The optimal dose (expressed as effective effluent/kg/hour) of CRRT in ICU has not been firmly established. The largest and most recent trials suggest that there is no additional benefit to using effluent flow rates in excess of 20 ml/kg/hour.
In the ARF Trial Network study, intensive renal support involving IHD and SLEDD 6 times per week and CVVHDF at 35 ml/kg/hour in critically ill patients with AKI did not decrease mortality, improve recovery of kidney function, or reduce the rate of non-renal organ failure as compared with less-intensive therapy involving a defined dose of IHD 3 times per week and CRRT at 20 ml/kg/hour.[23] In a multicenter randomized trial, conducted by The RENAL Replacement Therapy Study Investigators in critically ill patients with AKI, treatment with higher-intensity post-dilution CVVHDF with an effluent flow of either 40 ml/kg/hour did not reduce mortality at 90 days compared to lower intensity treatment with 25 ml/kg/hour flow.[24]
Nutritional Support during RRT
RRT can cause additional protein losses of 10 to 15 g/day for CVVH and 6 to 8 g/day for an intermittent dialysis. The recommended protein supplementation is between 1.1 to 2.5 g/kg/day of protein for patients on CVVH and between 1.1 and 1.2 g/kg/day for patients on intermittent HD.[1] At present, the available nutritional formulae with a chemical composition approaching the theoretical ideal for patients with AKI are hepatic formulations.
Patients who are critically ill are usually in a catabolic state. Excessive protein supplementation results in increased accumulation of end-products of protein metabolism. The fluid infusion that is required to provide nutrients may predispose these patients to volume overload. Aggressive nutrition with parenteral nutrition may also predispose patients to metabolic and electrolyte derangements, such as hyperglycemia, hyperlipidemia, hypernatremia, or hyponatremia.
Complications during Hemodialysis
Hypotension
Hypotension is the most common acute complication of hemodialysis, particularly among diabetics. It results from excessive ultrafiltration with inadequate compensatory vascular filling, impaired vasoactive or autonomic responses, osmolar shifts, overzealous use of anti-hypertensive agents, reduced cardiac reserve, high output cardiac failure in patients with arterio-venous fistulae and grafts, and vasodilatory and cardiodepressant effects of acetate buffer in the dialysate. Hypotension can be prevented by careful evaluation of the dry weight, performance of sequential ultrafiltration followed by dialysis, use of midodrine, cooling of the dialysate during dialysis treatment, and avoiding heavy meals during dialysis. Management of hypotension during dialysis includes discontinuing ultrafiltration, administration of 100 – 250 mL of isotonic saline or 10 mL of 23% saturated hypertonic saline and administration of salt-poor albumin.
Complications During transport to dialysis unit
Certain medical centers are equipped with dialysis machines inside the ICU, whereas some others may require the patient to be transported from the ICU to the dialysis unit. The critical issues during transport include accidental extubation, unintentional disconnection from ventilator, unintentional removal of dialysis catheters and vascular catheters, hemodynamic instability, and cardiac arrest. These patients should be accompanied by health-care professionals trained in airway management and emergency management. Meticulous monitoring of the patient during transport as well as during dialysis is of paramount importance.
Infections
The various acquired infections in patients undergoing hemodialysis include Hepatitis C, Hepatitis B, and catheter-related blood stream infections.
Cardiovascular diseases and coagulopathy
Cardiovascular diseases constitute the major cause of death in patients with ESRD. The compounding factors include chronic inflammation, shared risk factors (e.g., DM), dystrophic vascular calcification, hyperhomocysteinemia, massive changes in extracellular volume and cardiovascular dynamics, inadequate treatment of hypertension, dyslipidemia, and anemia. Coagulopathy results from excessive anti-coagulation, drug interactions, DIC, and MODS.
Other common problems with hemodialysis include thrombosis of veins, blockade of dialysis access, failure of A-V fistula, air embolism, hypothermia, dyselectrolytemia, cardiac arrhythmias, metabolic alkalosis, heparin-induced thrombocytopenia, inadvertent removal of therapeutic drugs, anaphylactoid reactions to the dialyzer, and muscle cramps due to changes in muscle perfusion. Cramps can be prevented by reducing volume removal during dialysis, ultrafiltration modeling and sodium modeling.[15] Type A anaphylactoid reaction, which is an IgE -mediated immediate hypersensitivity reaction, is treated with steroids and if severe, with epinephrine. Type B reaction, which results from complement activation and cytokine release, is usually self-limiting with continued dialysis.
Peritoneal dialysis
In peritoneal dialysis, 1.5 – 3 L of peritoneal dialyzate solution is infused into the peritoneal cavity and allowed to dwell for a set period of time, usually 2 to 4 hours. A combination of convective clearance and diffusive clearance occurs. The clearance of solutes and water depends on the balance between the movement of solutes and water into the peritoneal cavity versus absorption from the peritoneal cavity into the peritoneal capillary circulation across the peritoneal membrane. The rate of diffusion diminishes with time and eventually stops when equilibration between plasma and dialyzate is reached. PD in ICU is indicated when the patient is so hemodynamically unstable that transport to the dialysis unit situated away from the ICU is forbidden. PD is found to be more effective in AKI in pediatric patients compared to adult patients.
The rate of peritoneal solute transport varies from patient to patient and may be altered by the presence of infection (peritonitis), drugs, and physical factors, such as position and exercise. Lactate is the preferred buffer in peritoneal dialysis solutions [Table 2]. The most common additives to peritoneal dialysis solutions are heparin to prevent obstruction of the dialysis catheter lumen with fibrin, and antibiotics during an episode of acute peritonitis. Insulin may also be added in patients with diabetes mellitus.
A non-absorbable carbohydrate osmotic agent, icodextrin, has been found to be associated with more efficient ultrafiltration than dextrose-containing solutions.[25] However, in a recent abstract presented at the congress of the American Society of Nephrology, the duration of icodextrin treatment was independently associated with the complication of encapsulating peritoneal sclerosis.[26]
Complications during peritoneal dialysis
These include peritonitis, catheter-associated non-peritonitis infections, weight gain, unpredictable hyperglycemia, fluid leaks, protein loss and other metabolic disturbances, interference with diaphragm function, residual uremia, and peritoneal membrane failure.
The most consistent change observed in the peritoneal tissues of a patient who is on PD is an increase in the submesothelial thickness associated with peritoneal fibrosis and angiogenesis.[27] High lactate concentrations contribute to the glucose-induced neoangiogenesis by pseudo-hypoxia. Glucose degradation products are more important in the induction of peritoneal fibrosis. The combination of glycerol, amino acids, and dextrose, dissolved in a bicarbonate/lactate buffer (GLAD), may be an option for a new generation of dialysis fluids as it causes only minimal peritoneal damage, even after long-term exposure.[28]
Drug dosage during dialytic therapy
RRT contributes to the elimination of many critically important drugs, including the active ones, thereby leading to under-dosages. Conversely, RRT is usually much less efficient in drug clearance than the native kidney, and thus drug over dosage is of equal concern.
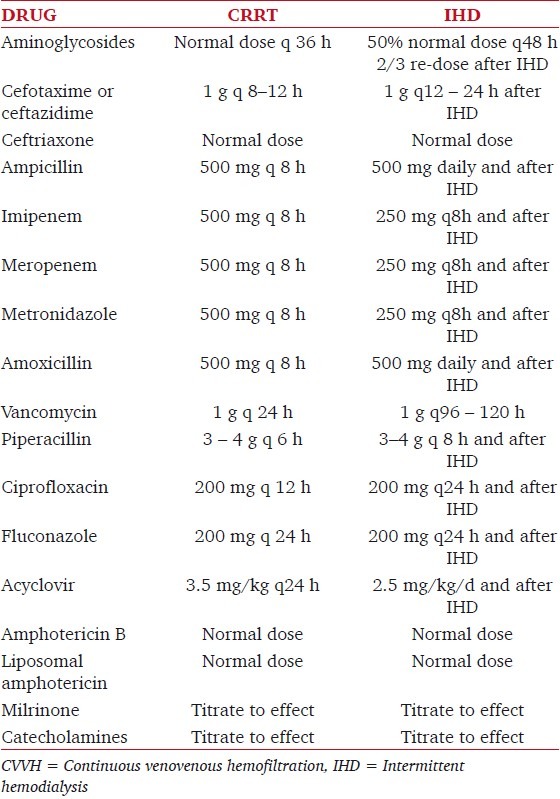
In a critically ill patient, different organ failures are responsible for some important pharmacokinetic modifications of distribution volume, protein-binding, clearance, and elimination half-life.[29] During RRT, different factors influence the elimination of drugs and other compounds, such as solubility, protein-binding, interaction with the dialyzer/hemofilter membrane, size, and charge. Therefore, altered pharmacokinetics of many drugs must be taken into account, and a modification of dosages is usually necessary. A complete discussion is beyond the scope of this review. Table 3 provides guidelines for the prescription of drugs that are commonly used in the ICU.
Table 3.
Drug dosage during dialytic therapy
Prevention and Treatment of AKI in Specific Disease States
Liver Failure
Hepatorenal syndrome (HRS) and acute tubular necrosis (ATN) account for more than 98% of AKI in patients with cirrhosis. Early recognition and treatment of sepsis, hypotension, bleeding, elevated abdominal compartment pressures, and avoidance of nephrotoxins are of primary importance. Albumin volume expansion reduces the incidence of AKI in patients with spontaneous bacterial peritonitis and during therapeutic paracentesis for tense ascites. Prompt intervention for HRS with vasopressors and albumin may reverse AKI.[1]
In patients with liver failure and AKI who are not candidates for liver transplant, RRT is better avoided. Molecular Adsorption Recirculating System (MARS), an albumin-based dialysis, has been proposed as a bridging option in patients awaiting living donor liver transplantation. MARS has also shown benefits in patients with elevated intracranial pressure and/or acute-on-chronic liver failure.[30]
Lung Injury
In patients with ALI/ARDS, ventilation using lung protective ventilatory strategies avoiding high VT and airway plateau pressure higher than 30 cm H2O are recommended[1] to avoid AKI as well as to promote renal recovery in those who develop AKI.
Cardiac Surgery
AKI in the post-cardiac surgery period is due to multiple factors, such as intraoperative hypotension, inflammation, microemboli secondary to cardiopulmonary bypass (CPB), medications, oxidative stress, and hemolysis along with risk factors, such as left ventricular dysfunction, emergency surgery, prolonged CPB time, use of intra-aortic counterpulsation, diabetes mellitus, peripheral vascular disease, chronic obstructive pulmonary disease, nephrotoxins (e.g., radiocontrast medium for coronary angiography elevated preoperative serum creatinine value (in chronic kidney disease) and female sex.
Off-pump coronary bypass grafting is associated with a decreased risk of AKI requiring RRT.[31] The potential benefit in reducing the incidence of AKI should be balanced with the risk of inferior graft patency rates.
Tumor lysis syndrome
This results from the release of intracellular purines, phosphate, and potassium from rapidly proliferating tumor cells, which may occur spontaneously or with the initiation of anti-tumor therapy. Tumor lysis leads to hyperuricemia, hyperkalemia, hyperphosphatemia, hypocalcemia, and renal insufficiency (due to deposition of uric acid and calcium phosphate crystals in the renal tubules), which is further worsened by concomitant intravascular volume depletion.
Preventive measures include aggressive hydration and diuretics to maintain a high urine output and allopurinol or recombinant urate oxidase (rasburicase) administration at least 2 days before chemotherapy or radiotherapy in patients at risk. In established tumor lysis syndrome, aggressive hydration, correction of dyselectrolytemia and RRT to remove uric acid, phosphate, and potassium are the main supportive measures.
Rhabdomyolysis
Intensive hydration with isotonic crystalloids after volume restoration to maintain a large urine output is of primary importance. CVVH may be of help in removing some myoglobin, but the clinical efficacy has not been established.[1] As initial serum levels of creatinine greater than 150 mmol/L as well as creatine kinase greater than 5,000 U/L are associated with increased risk of AKI or need for RRT, close monitoring of renal function is warranted.
Intra-abdominal Pressure
Abdominal compartment syndrome (ACS) is defined as a sustained intra-abdominal pressure (IAP) greater than 20 mm Hg that is associated with new organ dysfunction or failure. Renal function depends on the renal filtration gradient (FG); FG = mean arterial pressure minus twice the IAP.[32] In patients with ACS, interventions such as evacuation of intraluminal contents, evacuation of abdominal fluid collections, and correction of positive fluid balance which improve abdominal wall compliance are to be considered. Urgent abdominal decompression surgery is indicated in patients who fail to respond to or who are not candidates for medical management.
Weaning and Termination of RRT
A clearly-defined criterion for the termination of RRT has not been yet established. Observing and monitoring the patient during intervals without RRT and watching for signs of spontaneous recovery is the usual method. Two retrospective trials concluded that urinary output is the best predictive parameter for termination of RRT. The Beginning and Ending Supportive Therapy for the Kidney (BEST kidney) study defined a threshold urinary output of 450 ml/day as predictive for not requiring further RRT.[33] A study conducted to identify the risk factors for early re-dialysis after weaning from postoperative RRT revealed an increased risk of recommencing dialysis with a urinary output <100 ml/8 hours.[34]
Other Blood Purification Techniques
Hemoperfusion
A charcoal cartridge is used instead of a dialysis membrane, which effectively removes molecules of 300 – 500 Da molecular weight, including some lipid-soluble and protein-bound substances. Heparinization is necessary to prevent clotting. Significant changes in intravascular volume can occur at the initiation of therapy because of the large priming volume of the cartridge (260 ml). The common side-effects include hypoglycemia and thrombocytopenia. Hemoperfusion is useful in patients with life-threatening theophylline overdose.
Plasmapheresis or plasma exchange
Plasma is removed from the patient and exchanged with fresh frozen plasma (FFP) and a mixture of colloid and crystalloid solutions. A plasmafilter (a filter that allows the passage of molecules up to 500 kDa) is used instead of a hemofilter. Replacement (postfilter) fluid can also be a 50/50 combination of FFP and albumin. It is effective in the treatment of thrombotic thrombocytopenic purpura, Guillain – Barre’ syndrome, cryoglobulinemia, myasthenia gravis, and Goodpasture's syndrome.
Blood purification technology in sepsis
Blood purification may provide a clinically significant benefit in patients with severe sepsis/septic shock by removing circulating ‘mediators.’ Inflammatory mediators, such as interleukin-1β, interleukin-6, interleukin-8, and other middle molecules that mediate sepsis may be effectively removed by hemofiltration.[35] Plasmapheresis, coupled plasma filtration adsorption, and large-pore HF may be beneficial in this respect. Though high volume hemofiltration for sepsis in the absence of AKI has been proposed as a therapeutic strategy, this approach lacks a strong scientific rationale. A large on-going study of patients with sepsis and acute renal injury in the absence of overt failure (IVOIRE) is comparing hemofiltration at 35 ml/kg/hour to 70 ml/kg/hour and will hopefully provide an additional information.[36]
Future trends
Early identification of AKI with novel candidate biomarkers is an important step in improving outcomes in AKI. These biomarkers help not only in early detection of AKI before the onset of a rise in the serum creatinine, but also aid in the differential diagnosis and prognosis. Serum cystatin C is a sensitive marker of small changes in GFR and has been found to be a useful biomarker for early detection of AKI.[37,38] In a recent study, urinary cystatin C was found to be superior to conventional plasma markers in the early identification of AKI in post-cardiac surgery patients.[39] Several studies support NGAL, kidney injury molecule-1, and interleukin (IL) -18 as promising candidate biomarkers for the early detection of AKI.[40–42] Urinary excretion of enzymes (alkaline phosphatase, γ-glutamyl transaminase, N-acetyl-β-d-glucosamine), transporters (Na+ - H+ exchanger isoform- 3), cytokines (IL-6, IL-8, and IL-18), and protein-like substances (fetuin A) are also being studied for early identification of AKI.[43,44]
Future novel therapies aimed at more optimal and more specific blood purification may prove promising in the management of complex critically ill patients with AKI and other co-morbid conditions. The bioartificial kidney,[45] which incorporates a hemofilter (analogous to a glomerular unit) with tubular cell lines laid on hollow tubes of less immunogenic nature, is of special importance in this regard. The ultrafiltrate produced removes excess of fluid, and circulation through the tubular cell lines causes re-absorption of essential nutrients (glucose, amino acids etc.) and various electrolytes like sodium, calcium. It also serves the endocrinal function by synthesizing an active form of vitamin D.
Progenitor cell therapies represent an exciting future for treatment of AKI in the critically ill. Phase 1 trials of mesenchymal stem cells for treatment of patients at high risk for cardiac surgery-associated AKI are underway.
Conclusion
The field of RRT has undergone remarkable changes over the last decade and is continuing to evolve rapidly. CRRT is now firmly established throughout the world as perhaps the most commonly used form of RRT. CRRT is clearly superior to IHD with regard to physiological end points. Modifications of IHD, such as SLEDD, are able to combine the advantages of both IHD and CRRT. The use of novel membranes and of different intensities of treatment is being explored in the areas of sepsis management and in liver support. Critical care nephrology is a fast-emerging subspecialty, and critical care physicians are likely to play a paramount role in the management of patients with renal failure.
Acknowledgments
We would like to thank the many excellent researchers in renal replacement therapy around the world whose ideas and insights have been adopted and form the basis for this review.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Brochard L, Abroug F, Brenner M, Broccard AF, Danner RL, Ferrer M, et al. ATS/ERS/ESICM/SCCM/SRLF ad hoc committee on acute renal failure. An official ATS/ERS/ESICM/SCCM/SRLF statement: Prevention and management of acute renal failure in the ICU patient: An international consensus conference in intensive care medicine. Am J Respir Crit Care Med. 2010;181:1128–55. doi: 10.1164/rccm.200711-1664ST. [DOI] [PubMed] [Google Scholar]
- 2.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joannidis M, Druml W, Forni LG, Groeneveld AB, Honore P, Oudemans-van Straaten HM, et al. Prevention of acute kidney injury and protection of renal function in the intensive care unit. expert opinion of the working group for nephrology, ESICM. Intensive Care Med. 2010;36:392–411. doi: 10.1007/s00134-009-1678-y. [DOI] [PubMed] [Google Scholar]
- 5.Corrado G, Giuseppe G, Alessandra M, Pietro M. Nephrotoxicity of amphotericin B Desoxycholate. Clin Infect Dis. 2001;33:915–6. doi: 10.1086/322716. [DOI] [PubMed] [Google Scholar]
- 6.Cheung TW, Jayaweera DT, Pearce D, Benson P, Nahass R, Olson C, et al. Safety of oral versus intravenous hydration during induction therapy with intravenous foscarnet in AIDS patients with cytomegalovirus infections. Int J STD AIDS. 2000;11:640–7. doi: 10.1258/0956462001914995. [DOI] [PubMed] [Google Scholar]
- 7.Hassane I, Vincent LV, Gilbert D. Antiviral drug-induced nephrotoxicity. Am J Kidney Dis. 2005;45:804–17. doi: 10.1053/j.ajkd.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Al-Matter ER, Mona HB, Kadankandy CA. Acyclovir induced reversible renal failure in an immunocompromised child with extensive cutaneous herpes zoster. Kuwait Med J. 2004;36:47–8. [Google Scholar]
- 9.Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Low-dose dopamine in patients with early renal dysfunction: A placebo-controlled randomised trial. Lancet. 2000;356:2139–43. doi: 10.1016/s0140-6736(00)03495-4. [DOI] [PubMed] [Google Scholar]
- 10.Huber W, Eckel F, Hennig M, Rosenbrock H, Wacker A, Saur D, et al. Prophylaxis of contrast material-induced nephropathy in patients in intensive care: Acetylcysteine, theophylline, or both? A randomized study. Radiology. 2006;239:793–804. doi: 10.1148/radiol.2393041456. [DOI] [PubMed] [Google Scholar]
- 11.Bellomo R, D’Intini V, Ronco C. In: Textbook of critical care. 5th ed. Philadelphia: Elsevier Saunders; 2005. Renal replacement therapy in ICU; p. 1152. [Google Scholar]
- 12.Joannidis M, Metnitz PG. Epidemiology and natural history of acute renal failure in the ICU. Crit Care Clin. 2005;21:239–49. doi: 10.1016/j.ccc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Uchino S, Bellomo R, Bagshaw SM, Goldsmith D. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant. 2010;25:1833–9. doi: 10.1093/ndt/gfp624. [DOI] [PubMed] [Google Scholar]
- 14.Andrade L, Cleto S, Seguro AC. Door-to-dialysis time and daily hemodialysis in patients with leptospirosis: Impact on mortality. Clin J Am Soc Nephrol. 2007;2:739–44. doi: 10.2215/CJN.00680207. [DOI] [PubMed] [Google Scholar]
- 15.Liu KD, Chertow GM. In: Harrison's principles of internal medicine. 17th ed. New York: McGraw-Hill; 2008. Dialysis in the treatment of renal failure; p. 1772. [Google Scholar]
- 16.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, et al. Effect of dialysis dose and membra maintenance hemodialysis. N Engl J Med. 2003;348:1491–4. [Google Scholar]
- 17.Marshall MR, Ma T, Galler D, Rankin AP, Williams AB. Sustained low-efficiency daily diafiltration (SLEDD-f) for critically ill patients requiring renal replacement therapy: Towards an adequate therapy. Nephrol Dial Transplant. 2004;19:877–84. doi: 10.1093/ndt/gfg625. [DOI] [PubMed] [Google Scholar]
- 18.Lavaud S, Canivet E, Wuillai A, Maheut H, Randoux C, Bonnet JM, et al. Optimal anticoagulation strategy in haemodialysis with heparin-coated polyacrylonitrile membrane. Nephrol Dial Transplant. 2003;18:2097–104. doi: 10.1093/ndt/gfg272. [DOI] [PubMed] [Google Scholar]
- 19.Lavaud S, Paris B, Maheut H, Randoux C, Renaux JL, Rieu P, et al. Assessment of the heparin-binding AN69 ST hemodialysis membrane: II. Clinical studies without heparin administration. ASAIO J. 2005;51:348–51. doi: 10.1097/01.mat.0000169121.09075.53. [DOI] [PubMed] [Google Scholar]
- 20.Kumar VA, Yeun JY, Depner TA, Don BR. Extended daily dialysis vs.continuous hemodialysis for ICU patients with acute renal failure: A two-year single center report. Int J Artif Organs. 2004;27:371–9. doi: 10.1177/039139880402700505. [DOI] [PubMed] [Google Scholar]
- 21.Kielstein JT, Kretschmer U, Ernst T, Hafer C, Bahr MJ, Haller H, et al. Efficacy and cardiovascular tolerability of extended dialysis in critically ill patients: A randomized controlled study. Am J Kidney Dis. 2004;43:342–9. doi: 10.1053/j.ajkd.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Marshall MR, Golper TA, Shaver MJ, Alam MG, Chatoth DK. Sustained low efficiency dialysis for critically ill patients requiring renal replacement therapy. Kidney Int. 2001;60:777–85. doi: 10.1046/j.1523-1755.2001.060002777.x. [DOI] [PubMed] [Google Scholar]
- 23.Intensity of renal support in critically ill patients with acute kidney injury. The VA/NIH acute renal failure trial network. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The RENAL Replacement Therapy Study Investigators. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–38. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 25.Finkelstein F, Healy H, Abu-Alfa A, Ahmad S, Brown F, Gehr T, et al. Superiority of icodextrin compared with 4.25% dextrose for peritoneal ultrafiltration. J Am Soc Nephrol. 2005;16:546–54. doi: 10.1681/ASN.2004090793. [DOI] [PubMed] [Google Scholar]
- 26.Betjes M, Sampimon D, Lingsma H, Korte M. Risk factors associated with increasing incidence of encapsulating peritoneal sclerosis in a controlled multicenter study. J Am Soc Nephrol. 2009 poster:F-PO1506. [Google Scholar]
- 27.Margetts PJ, Churchill DN. Acquired ultrafiltration dysfunction in peritoneal dialysis patients. J Am Soc Nephrol. 2002;13:2787–94. doi: 10.1681/ASN.V13112787. [DOI] [PubMed] [Google Scholar]
- 28.Krediet RT, Zweers MM, van Westrhenen R, Zegwaard A, Struijk DG. Effects of reducing the lactate and glucose content of PD solutions on the peritoneum. Is the future GLAD? NDT Plus. 2008;1(suppl 4):iv56–iv62. doi: 10.1093/ndtplus/sfn126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pea F, Pavan F, Furlanut M. Clinical relevance of pharmacokinetics and pharmacodynamics in cardiac critical care patients. Clin Pharmacokinet. 2008;47:449–62. doi: 10.2165/00003088-200847070-00002. [DOI] [PubMed] [Google Scholar]
- 30.Mitzner SR, Stange J, Klammt S, Peszynski P, Schmidt R, Nöldge-Schomburg G. Extracorporeal detoxification using the molecular adsorbent recirculating system for critically ill patients with liver failure. J Am Soc Nephrol. 2001;12:S75–82. [PubMed] [Google Scholar]
- 31.Hix J, Thakar C, Katz E, Yared J, Sabik J, Paganini EP. Effect of off-pump coronary artery surgery on postoperative acute kidney injury and mortality. Crit Care Med. 2006;34:2979–83. doi: 10.1097/01.CCM.0000248905.67352.BA. [DOI] [PubMed] [Google Scholar]
- 32.Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, et al. Results from the international conference of experts on intraabdominal hypertension and abdominal compartment syndrome. I. Definitions. Intensive Care Med. 2006;32:1722–32. doi: 10.1007/s00134-006-0349-5. [DOI] [PubMed] [Google Scholar]
- 33.Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, et al. Discontinuation of continuous renal replacement therapy: A post hoc analysis of a prospective multi-center observational study. Crit Care Med. 2009;37:2576–82. doi: 10.1097/CCM.0b013e3181a38241. [DOI] [PubMed] [Google Scholar]
- 34.Wu VC, Ko WJ, Chang HW, Chen YW, Lin YF, Shiao CC, et al. Risk factors of early redialysis after weaning from postoperative acute renal replacement therapy. Intensive Care Med. 2008;34:101–8. doi: 10.1007/s00134-007-0813-x. [DOI] [PubMed] [Google Scholar]
- 35.Morgera S, Slowinski T, Melzer C, Sobottke V, Vargas-Hein O, Volk T, et al. Renal replacement therapy with high-cut off hemofilters: Impact of convection and diffusion on cytokine clearances and protein status. Am J Kidney Dis. 2004;43:444–53. doi: 10.1053/j.ajkd.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Honore PM, Joannes-Boyau O, Merson L, Boer W, Piette V, Galloy AC, et al. The big bang of hemofiltration: The beginning of a new era in the third millennium for extra-corporeal blood purification! Int J Artif Organs. 2006;29:649–59. doi: 10.1177/039139880602900702. [DOI] [PubMed] [Google Scholar]
- 37.Herget-Rosenthal S, Marggraf G, Husing J, Göring F, Pietruck F, Janssen O, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–22. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 38.Delanaye P, Lambermont B, Chapelle JP, Gielen J, Gerard P, Rorive G. Plasmatic cystatin C for the estimation of glomerular filtration rate in intensive care units. Intensive Care Med. 2004;30:980–3. doi: 10.1007/s00134-004-2189-5. [DOI] [PubMed] [Google Scholar]
- 39.Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74:1059–69. doi: 10.1038/ki.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 41.Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 42.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–44. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 43.Trof RJ, Di Maggio F, Leemreis J, Groeneveld AB. Biomarkers of acute renal injury and renal failure. Shock. 2006;26:245–53. doi: 10.1097/01.shk.0000225415.5969694.ce. [DOI] [PubMed] [Google Scholar]
- 44.Waikar SS, Bonventre JV. Biomarkers for the diagnosis of acute kidney injury. Curr Opin Nephrol Hypertens. 2007;16:557–64. doi: 10.1097/MNH.0b013e3282f08745. [DOI] [PubMed] [Google Scholar]
- 45.Humes HD, Fissell WH, Weitzel WF. The bioartificial kidney in the treatment of acute renal failure. Kidney Int. 2002;61:S121–5. doi: 10.1046/j.1523-1755.61.s80.22.x. [DOI] [PubMed] [Google Scholar]


