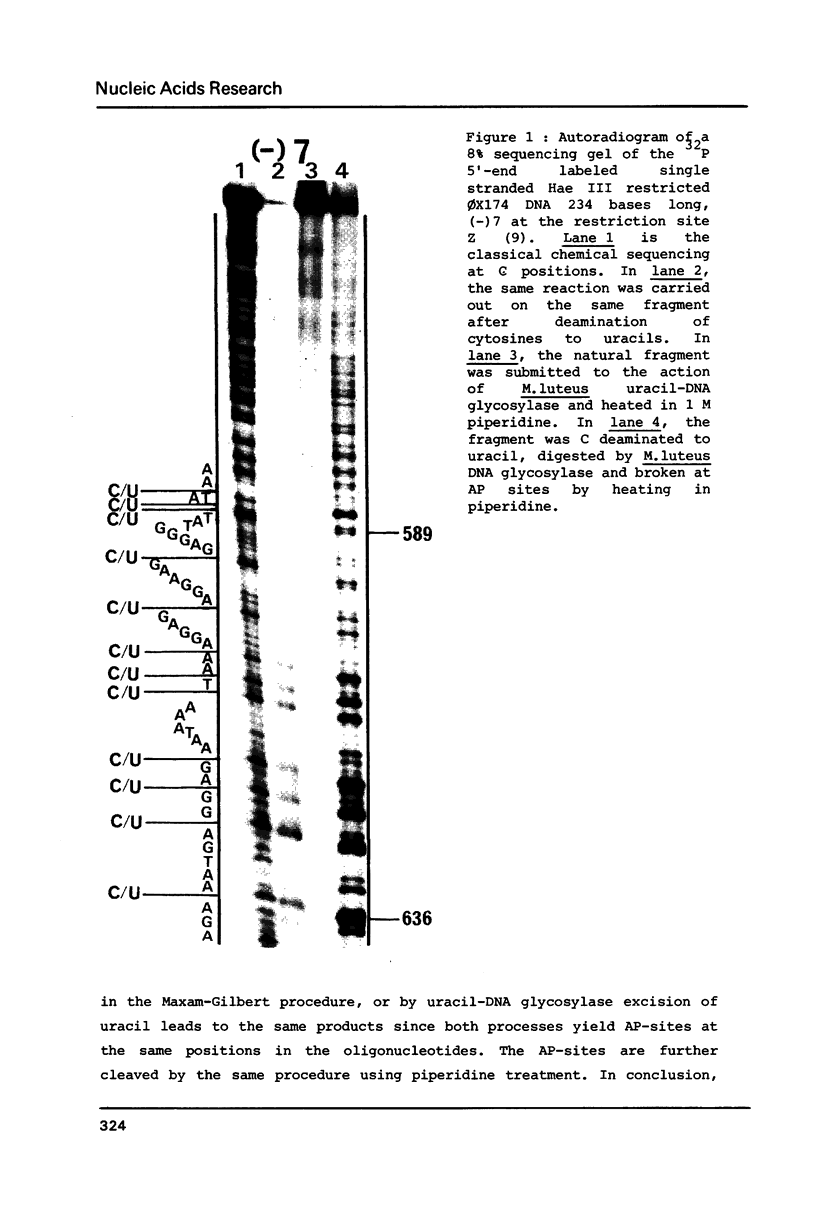
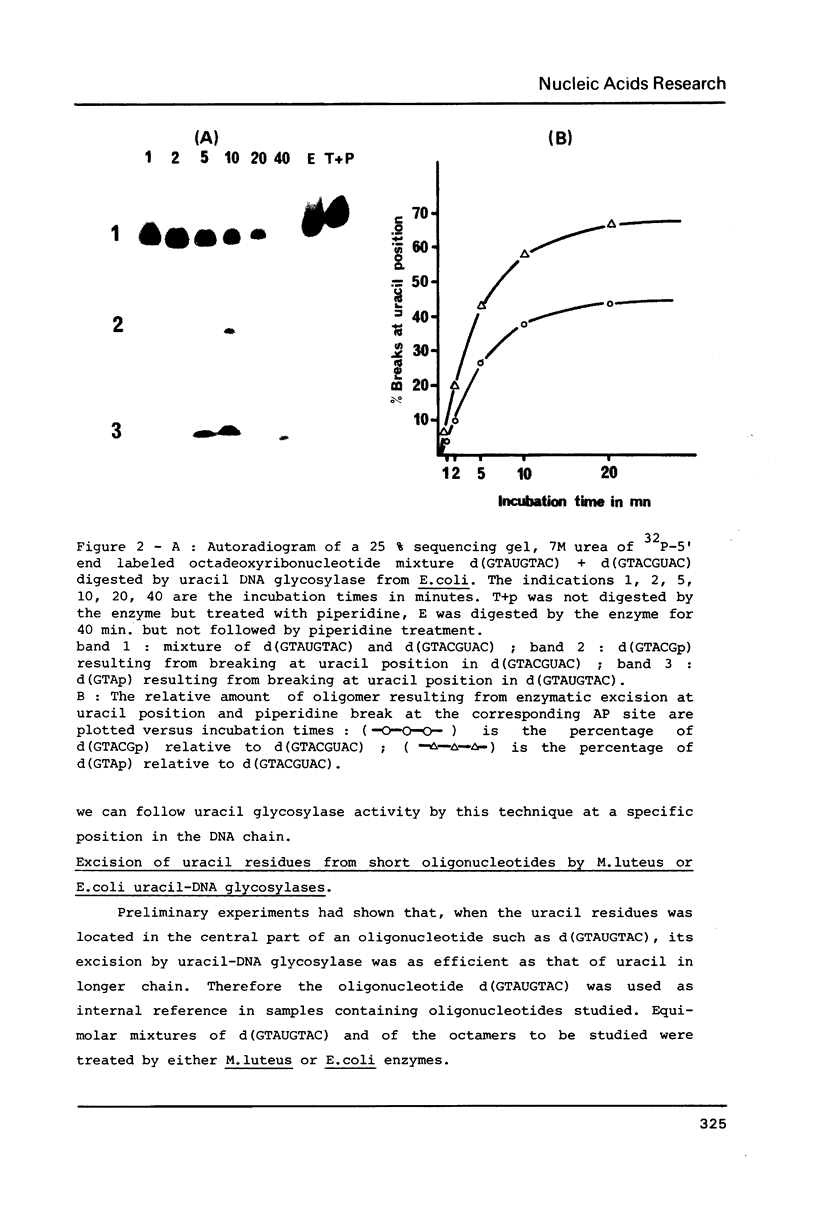
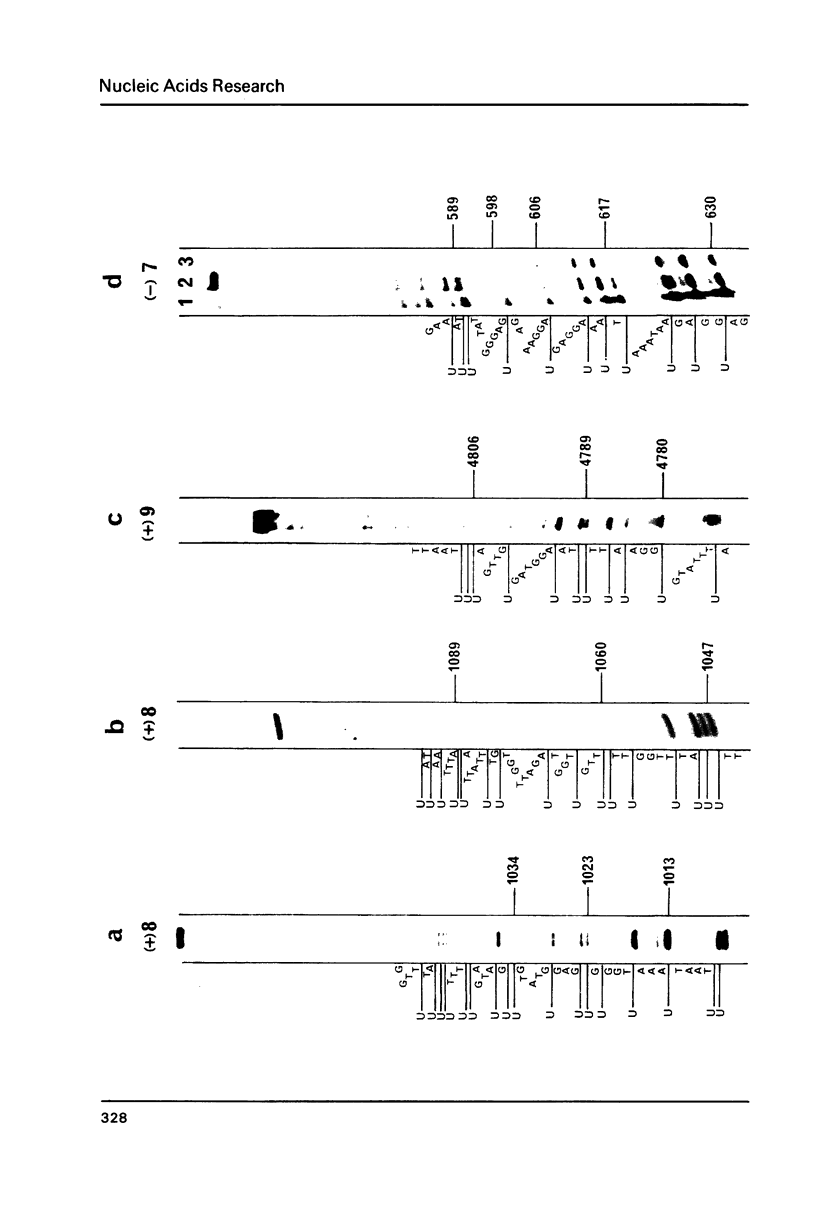
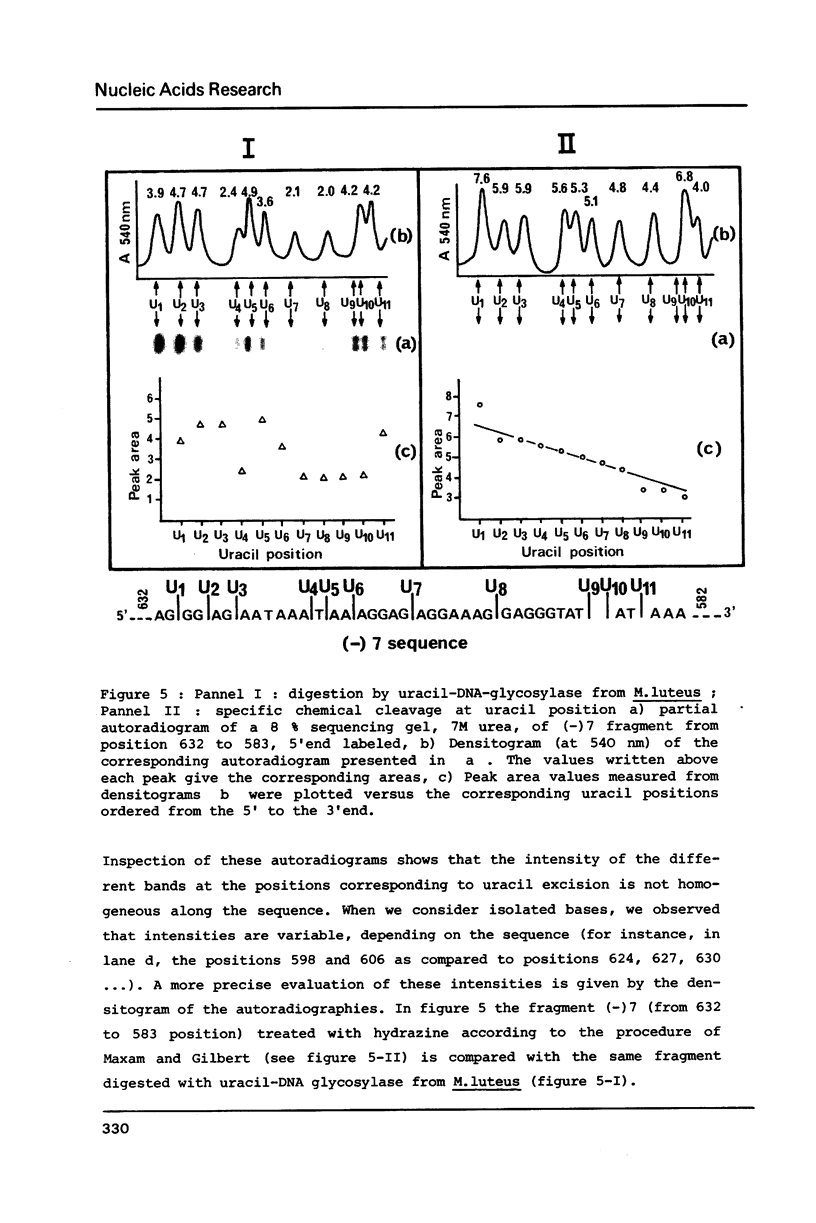
Abstract
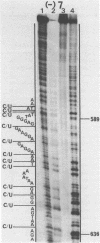
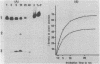
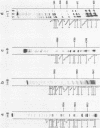
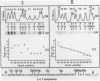
Various octadeoxynucleotides containing uracil at different positions were synthesized and submitted to the action of Escherichia coli and Micrococcus luteus uracil-DNA glycosylases. A uracil residue situated at the 5'-end was excised by the M.luteus enzyme but not by the E.coli one. Uracil residues located at the ultimate and penultimate positions at the 3'-end were not cleaved by either enzymes. At the other central positions, uracil was eliminated with different initial velocities. Single stranded phi X 174 DNA fragments were used to study the influence of the sequence. Cytosine bases were deaminated to give uracil by bisulfite treatment. It was shown that the initial excision velocity of two vicinal uracil residues was decreased. The same observation was made for two uracils separated by one base. A hypothetical scheme is suggested to explain the mechanism of action of uracil-DNA glycosylases.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arenaz P., Sirover M. A. Isolation and characterization of monoclonal antibodies directed against the DNA repair enzyme uracil DNA glycosylase from human placenta. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5822–5826. doi: 10.1073/pnas.80.19.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaszuk A. M., Deugau K. V., Sherwood J., Michalak M., Glick B. R. An efficient method for the sequence analysis of oligodeoxyribonucleotides. Anal Biochem. 1983 Feb 1;128(2):281–286. doi: 10.1016/0003-2697(83)90376-7. [DOI] [PubMed] [Google Scholar]
- Blaisdell P., Warner H. Partial purification and characterization of a uracil-DNA glycosylase from wheat germ. J Biol Chem. 1983 Feb 10;258(3):1603–1609. [PubMed] [Google Scholar]
- Chaconas G., van de Sande J. H. 5'-32P labeling of RNA and DNA restriction fragments. Methods Enzymol. 1980;65(1):75–85. doi: 10.1016/s0076-6879(80)65012-5. [DOI] [PubMed] [Google Scholar]
- Cone R., Duncan J., Hamilton L., Friedberg E. C. Partial purification and characterization of a uracil DNA N-glycosidase from Bacillus subtilis. Biochemistry. 1977 Jul 12;16(14):3194–3201. doi: 10.1021/bi00633a024. [DOI] [PubMed] [Google Scholar]
- Drake J. W., Baltz R. H. The biochemistry of mutagenesis. Annu Rev Biochem. 1976;45:11–37. doi: 10.1146/annurev.bi.45.070176.000303. [DOI] [PubMed] [Google Scholar]
- Efimov V. A., Reverdatto S. V., Chakhmakhcheva O. G. New effective method for the synthesis of oligonucleotides via phosphotriester intermediates. Nucleic Acids Res. 1982 Nov 11;10(21):6675–6694. doi: 10.1093/nar/10.21.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Binding of wheat germ ribosomes to bisulfite-modified reovirus messenger RNA: evidence for a scanning mechanism. J Mol Biol. 1980 Dec 15;144(3):291–304. doi: 10.1016/0022-2836(80)90092-3. [DOI] [PubMed] [Google Scholar]
- Krokan H., Wittwer C. U. Uracil DNa-glycosylase from HeLa cells: general properties, substrate specificity and effect of uracil analogs. Nucleic Acids Res. 1981 Jun 11;9(11):2599–2613. doi: 10.1093/nar/9.11.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc J. P., Laval J. Comparison at the molecular level of uracil-DNA glycosylases from different origins. Biochimie. 1982 Aug-Sep;64(8-9):735–738. doi: 10.1016/s0300-9084(82)80120-x. [DOI] [PubMed] [Google Scholar]
- Leblanc J. P., Martin B., Cadet J., Laval J. Uracil-DNA glycosylase. Purification and properties of uracil-DNA glycosylase from Micrococcus luteus. J Biol Chem. 1982 Apr 10;257(7):3477–3483. [PubMed] [Google Scholar]
- Lindahl T. An N-glycosidase from Escherichia coli that releases free uracil from DNA containing deaminated cytosine residues. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3649–3653. doi: 10.1073/pnas.71.9.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Ljungquist S., Siegert W., Nyberg B., Sperens B. DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. J Biol Chem. 1977 May 25;252(10):3286–3294. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Smith H. O. Recovery of DNA from gels. Methods Enzymol. 1980;65(1):371–380. doi: 10.1016/s0076-6879(80)65048-4. [DOI] [PubMed] [Google Scholar]
- Talpaert-Borlé M., Campagnari F., Creissen D. M. Properties of purified uracil-DNA glycosylase from calf thymus. An in vitro study using synthetic DNA-like substrates. J Biol Chem. 1982 Feb 10;257(3):1208–1214. [PubMed] [Google Scholar]
- Téoule R., Derbyshire R., Guy A., Molko D., Roget A. A novel class of condensing reagents in phosphodiester oligodeoxyribonucleotides synthesis. Application of the constituents of free terminal carboxy oxytocine gene. Nucleic Acids Symp Ser. 1980;(7):23–37. [PubMed] [Google Scholar]
- Weiss R. B., Mineura K., Henderson E. E., Duker N. J., deRiel J. K. Enzymic detection of uracil in a cloned and sequenced deoxyribonucleic acid segment. Biochemistry. 1983 Sep 13;22(19):4501–4507. doi: 10.1021/bi00288a023. [DOI] [PubMed] [Google Scholar]