Abstract
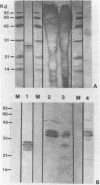
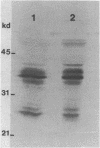
Antibodies were raised in rabbit against a pure subset of calf thymus single-stranded DNA binding proteins (ssDBPs) and purified by affinity chromatography on antigen-Sepharose. In Western blot experiments these antibodies were shown to react to the same extent with the whole family of bovine ssDBPs, as well as with ssDBPs from HeLa cells. When used to stain total cell extracts from both calf thymus and HeLa cells the antibodies reacted only with bands corresponding to the ssDBPs and with a set of bands of higher molecular weight, whose electrophoretic pattern matched that of the 40S hnRNP core proteins. In effect we observed that purified 40S hnRNP core proteins from HeLa cells were strongly reactive with the antibodies. Moreover after partial tryptic digestion HeLa cells ssDBPs and hnRNPs produced immunoreactive fragments of the same molecular weight and isoelectric point. Extensive structural homologies can thus be evidenced between these two classes of proteins, which share the property of selective binding to single-stranded nucleic acids.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer A. L., Christensen M. E., Walker B. W., LeStourgeon W. M. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell. 1977 May;11(1):127–138. doi: 10.1016/0092-8674(77)90323-3. [DOI] [PubMed] [Google Scholar]
- Douglass J., Civelli O., Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- Goldsmith M. R., Rattner E. C., Koehler M. M., Balikov S. R., Bock S. C. Two-dimensional electrophoresis of small-molecular-weight proteins. Anal Biochem. 1979 Oct 15;99(1):33–40. doi: 10.1016/0003-2697(79)90041-1. [DOI] [PubMed] [Google Scholar]
- Herrick G., Alberts B. Purification and physical characterization of nucleic acid helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2124–2132. [PubMed] [Google Scholar]
- Karn J., Vidali G., Boffa L. C., Allfrey V. G. Characterization of the non-histone nuclear proteins associated with rapidly labeled heterogeneous nuclear RNA. J Biol Chem. 1977 Oct 25;252(20):7307–7322. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leser G. P., Escara-Wilke J., Martin T. E. Monoclonal antibodies to heterogeneous nuclear RNA-protein complexes. The core proteins comprise a conserved group of related polypeptides. J Biol Chem. 1984 Feb 10;259(3):1827–1833. [PubMed] [Google Scholar]
- Martin T., Billings P., Pullman J., Stevens B., Kinniburgh A. Substructure of nuclear ribonucleoprotein complexes. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):899–909. doi: 10.1101/sqb.1978.042.01.091. [DOI] [PubMed] [Google Scholar]
- Planck S. R., Wilson S. H. Studies on the structure of mouse helix-destabilizing protein-1. DNA binding and controlled proteolysis with trypsin. J Biol Chem. 1980 Dec 10;255(23):11547–11556. [PubMed] [Google Scholar]
- Riva S., Clivio A., Valentini O., Cobianchi F. DNA binding proteins from calf thymus with an enhanced ability to stimulate DNA polymerase alpha in vitro. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1053–1062. doi: 10.1016/0006-291x(80)90059-5. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Taylor J. M., McKnight G. S., Palacios R., Gonzalez C., Kiely M. L., Schimke R. T. Isolation of hen oviduct ovalbumin and rat live albumin polysomes by indirect immunoprecipitation. J Biol Chem. 1974 Jun 25;249(12):3665–3671. [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini O., Biamonti G., Mastromei G., Riva S. Structural and functional heterogeneity of single-stranded DNA-binding proteins from calf thymus. Biochim Biophys Acta. 1984 Jun 16;782(2):147–155. doi: 10.1016/0167-4781(84)90018-6. [DOI] [PubMed] [Google Scholar]