Abstract
Tuberculosis of the skull base and middle ear cavity is very rare. Infection with neurotropic fungi Fonsecaea pedrosoi is rare, which usually presents as brain abscess. We herein present an unusual case of concomitant tuberculosis and fungal (Fonsecaea pedrosoi) infections involving the middle ear cleft extending and destroying the craniovertebral junction.
Keywords: Fonsecaea pedrosoi, skull base tuberculosis, tuberculous otomastoiditis
Introduction
Tuberculous involvement of middle ear cavity accounts for less than 1% of central nervous system (CNS) tuberculosis with a low index of suspicion resulting in delay in diagnosis and treatment and hence complications such as hearing loss, facial palsy, destruction of skull base, and even instability at the craniovertebral junction.[1–4] Diagnosis is made from direct smear examination, culture, or histopathological examination. Fonsecaea pedrosoi are pigmented fungi with melanin in their cell walls and are very rare to cause fungal infection in immunocompetent individuals. Superadded or concurrent fungal infection in the present case added difficulties in the management. To the best of our knowledge, this is the first report of a concomitant tubercular and fungal infection in the central nervous system managed successfully.
Case Report
A 22-year-female patient presented to our hospital with complaints of progressive hearing loss, tinnitus, and purulent discharge from left ear and left facial paresis followed by headache and painful restriction of neck movements in over a week's duration. No history suggestive of any immune incompetency or high risk behavior. On examination, she was well built and nourished. She had enlarged cervical lymph nodes. Otoscopic examination showed grayish white, nonpulsatile mass, mixed with pus protruding from left ear with multiple perforations in the tympanic membrane. Examination of chest showed a small, nontender, nonmobile, nonfluctuant swelling at left sixth rib and intercostals space. There was no focal neurological deficit except for mild facial weakness (lower motor neuron type) which was gradually improving. Blood count cells showed a total white blood cells count of 17000/ mm3 and ESR was 30 mm/h. Liver function tests and renal function tests were within normal limits. Mantoux (10 TU) was reactive (24 mm). She was seronegative for HIV/HBsAg/ HCV. The CT scan of brain plain and contrast studies showed a well-defined isodense lesion, enhancing uniformly on contrast [Figure 1] in left posterior fossa extending from the mastoid to the petrous apex medially, inferiorly extending up to the C1 arch with destruction of the petrous bone and the C1 arch associated with sclerosis of mastoids. MRI brain and cervical spine showed iso on T1, hyper on the T2 well-defined lesion involving the left mastoids, petrous bone, extending up to the C1 with enhancement on the Gadolinium contrast study. Chest radiograph showed destruction of head of sixth rib. The CT scan chest showed destruction of the rib and extrapleural lesion. The bone scan was suggestive of inflammatory pathology. An excision biopsy from the easily accessible ear mass and rib lesion was performed for histopathology and culture (aerobic, tubercular, and fungal). Histopathology of the ear lesion was cholesteatoma [Figure 2]. Histopathology of the rib lesion was reported as a necrotising granulomatous lesion [Figure 3]. Microscopy of the tissue by Gram's stain for bacteria and yeasts, Zeihl Neelsen's stain for acid fast bacilli, and by 20% potassium hydroxide mounts for filamentous fungi were negative for any organism. The specimen was processed by culture on 5% sheep blood agar and chromogenic agar (incubated at 37°C for bacterial pathogens, Lowenstein-Jensen medium and BACTEC 12 medium for Mycobacteria and Sabaraud's dextrose agar (incubated at 25°C), Brain heart infusion agar (incubated at 37°C), Inhibitory mould agar (incubated at 25°C) and potato dextrose agar (incubated at 25°C) for fungal agents as per standard microbiology procedures. Bacterial cultures did not yield any organisms. A dry mycelial growth was observed on all the fungal media by the third day of incubation. However, the fungus failed to sporulate on prolonged incubation. The fungal isolate was sent to National Culture Collection of Pathogenic Fungi (Mycology Referral Centre, PGI, Chandigarh) with reference no. NCCPF 370012 and the same has been banked as a reference for Fonsecaea pedrosoi. Mycobacterium tuberculosis was grown on seventh week of rigorous culture in both solid and liquid media {Lowenstein-Jensen medium and BACTEC 12 B medium}. With histopathology features suggestive of a granulomatous lesion and culture form the ear specimen showing growth of a dematiaceous fungus, the patient was initiated with lyophilized Amphotericin (total dose 3.5 g, intravenously,) and oral voriconazole (400 mg q 12 h, 12 days). At the end of 7 weeks, culture showed growth of M. tuberculosis from both rib and ear lesion. The patient was then initiated on anti-tuberculosis treatment (ATT) with four drugs (INH, rifampicin, pyrazinamide, and ethambutol). She developed liver toxicity and her ATT was titrated by eliminating pyrazinamide. During follow up at 3 months the patient complained of increasing neck pain, subtle motor weakness in lower limbs with up going plantars. CT scans of the craniovertebral junction showed increase in the size of the lesion and bony destruction of the C1 arch. The lesion was surgically debrided by a far lateral approach, decompressing the cervical spinal cord and the brainstem. Anti-tubercular drugs were continued for 12 months. The neck pain subsided and on completion of the course of ATT, a repeat CT scan showed complete resolution of the lesion. After two years the patient is completely well both clinically ad radiologically.
Figure 1.
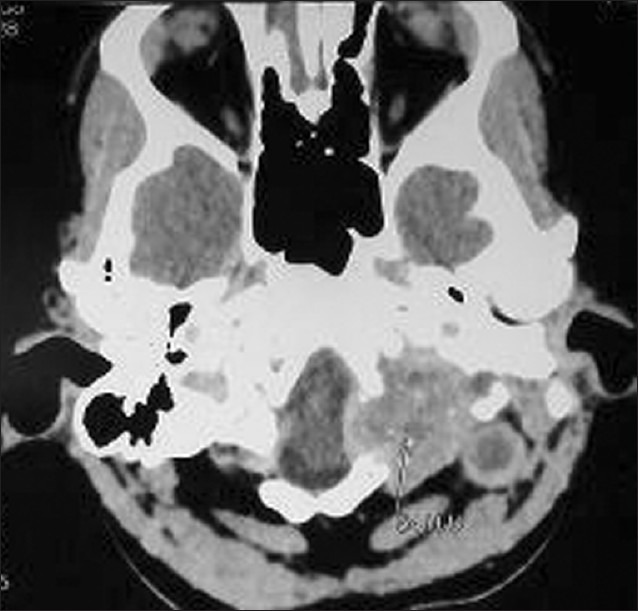
Computed tomography scan of brain plain study axial section is showing peripherally enhancing lesion in left posterior fossa extending from the mastoid to the petrous apex medially, inferiorly extending up to C1 arch with destruction of the petrous bone and the C1 arch associated with sclerosis of mastoids
Figure 2.
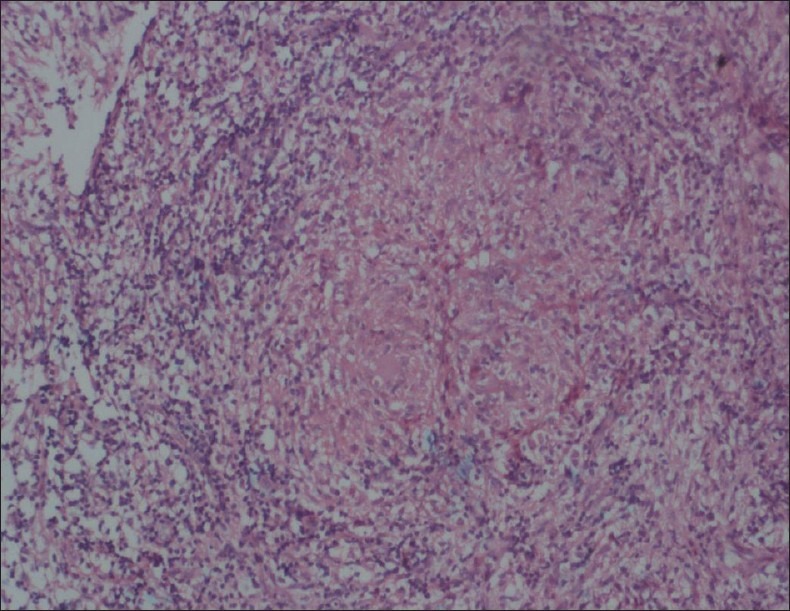
Histopathology of the ear lesion suggestive of cholesteatoma
Figure 3.
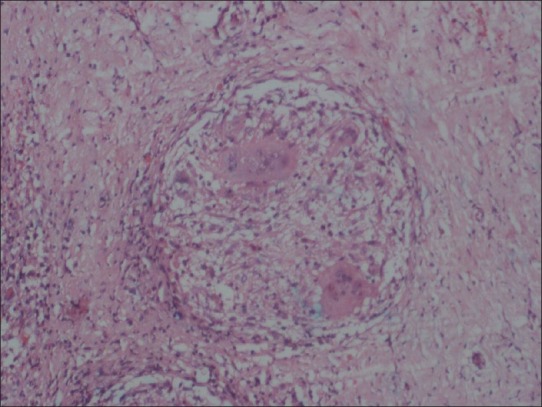
Histopathology of the rib lesion shows multinucleated giant cells and caseation suggestive of necrotizing granulomatous inflammation
Discussion
Tuberculosis of the middle ear is a rare entity and has been sparsely reported in the literature presenting as painless otorrhea, multiple tympanic perforations, abundant granulation tissue, bone necrosis, and severe hearing loss.[1] The disease is usually secondary to a primary infection in lungs, larynx, pharynx, and nose.[2,3] M. tuberculosis probably spreads contiguously or hematogenously, from these foci to the middle ear through the Eustachian tubes. Hematogenous spread often results in a direct involvement of the mastoid bone, producing necrosis which progresses to the middle ear.[3,5] Demonstration of acid fast bacilli in the ear discharge is quite tedious. The smear positivity varies from 5% to 35% and on repeated examination improves to 50%.[1] Tubercular petrositis is even rarer and occurs by involvement of venous channels.
Fungal infections of the CNS has seen a steep rise in numbers, largely, due to the advent of acquired immunodeficiency syndrome (AIDS), wide spread use of broad spectrum antibiotics, steroids, and immunosuppressive drugs for a variety of conditions. However, the dematiaceous fungal infections of the CNS continue to be uncommon. Fonsecaea pedrosoi usually presents with papules and verrucous like lesions in the subcutaneous plane following trauma and are commonly seen in the lower extremities. It is common in humid tropic areas. They have neurotropic potential but are known to produce brain abscess.[6] In the present case there was a combined infection with M. tuberculosis and F. pedrosoi involving the cranium and the rib cage. The vital lesson we share through this article is that odd combinations do occur and judicious treatment with appropriate drugs leads to cure. This is to our best knowledge, the first case of combined infection with these organisms in an immunocompetent individual managed successfully with surgical decompression, antifungals (amphotericin B and voriconazole) and anti-tubercular drugs. Recently, Criado et al. reported the use of oral voriconazole to treat three patients with extensive and long-standing cutaneous chromoblastomycosis with obtaining control of the disease. So, to the best of our knowledge, our case report is the second in the indexed literature (Pubmed/Medline) using oral voriconazole in the chromoblastomycosis treatment.[7]
Acknowledgment
We thank Dr. BP Sahu, Dr. AK Chakraborthy for their critical role in preparing this article.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Jeanes AL, Friedmann I. Tuberculosis of the middle ear. Tubercle. 1960;41:109. doi: 10.1016/s0041-3879(60)80003-7. [DOI] [PubMed] [Google Scholar]
- 2.Mahajan M, Agarwal DS, Singh NP, Gadre DJ. Tuberculosis of middle ear – A case report. Ind J Tub. 1995;42:55–6. [Google Scholar]
- 3.Miller FJ, Seal RM, Taylor MD. Tuberculosis in children: Evolution, control, treatment. London: J and A Churchill Ltd; 1963. [Google Scholar]
- 4.Windel-Taylar PC, Bailey CM. Tuberculous otitis media: A series of 22 patients. Laryngoscope. 1980;90:1039–44. doi: 10.1002/lary.1980.90.6.1039. [DOI] [PubMed] [Google Scholar]
- 5.Sinha A. Tuberculosis of Ear, Nose and Throat. In: Rao KN, editor. Text book of Tuberculosis. 2nd ed. New Delhi: Vikas Publishing House; 1981. p. 493. [Google Scholar]
- 6.Santosh V, Khanna N, Shankar SK, Pal L, Das S, Chandramukhi A, et al. Primary mycotic abscess of the brain caused by Fonsecea pedrosoi. J Neurosurg. 1995;82:128–30. doi: 10.3171/jns.1995.82.1.0128. [DOI] [PubMed] [Google Scholar]
- 7.Criado PR, Careta MF, Valente NY, Martins JE, Rivitti EA, Spina R, et al. Extensive long-standing chromomycosis due to Fonsecaea pedrosoi: Three cases with relevant improvement under voriconazole therapy. J Dermatolog Treat. 2011;22:167–74. doi: 10.3109/09546630903585074. [DOI] [PubMed] [Google Scholar]


