Sir,
Reversible posterior leukoencephalopathy syndrome (RPLS) is an acute encephalopathy syndrome characterized by headache, altered mental status, seizures, and visual loss associated with neuroimaging findings, suggestive of bilateral cortical and subcortical brain edema involving the posterior region of the cerebral hemispheres.[1,2]
A 28–year–old, unbooked, primigravida, presented with eclampsia at a period of gestation of 29.2 weeks and vaginally delivered a preterm stillborn child.She was managed with intravenous magnesium sulfate, antihypertensives (amlodipine and diuretics), and supportive care. On the day following the delivery she remained in altered sensorium and complained of blurring of vision. Her blood pressure had returned to normal. A central nervous system examination revealed cortical blindness and right side UMN hemiparesis. The rest of the general physical and systemic examination was normal. Her complete hemogram, coagulation profile, and biochemical parameters were normal, except her urine examination, which revealed 3+ albumin. Her non-contrast computed tomography (CT) head revealed hypodensities in the bilateral occipital lobes and left high parietal region. A magnetic resonance imaging (MRI) study revealed hypointensities on T1W images [Figure 1a], hyperintensities on T2W images, and Fluid Attenuated Inversion Recovery (FLAIR) sequences [Figure 1b and c] in the bilateral occipital lobes as well as left parietal lobe. There was restricted diffusion in these areas on diffusion weighted image (DWI). The calcarine and paramedian occipital lobe structures were spared [Figure 1d]. Her magnetic resonance (MR) venography was normal. The patient showed gradual improvement and she recovered fully on the fourth day. A follow-up MRI of the brain after six weeks showed significant resolution of the white matter lesions [Figure 2]. Hence, diagnosis of reversible posterior leukoencephalopathy syndrome was made.
Figure 1.
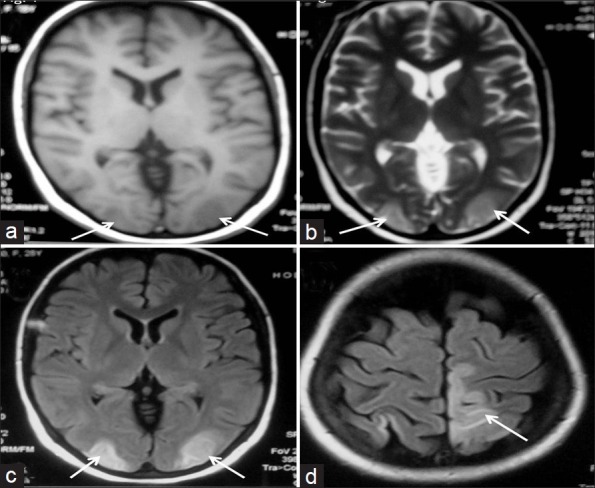
MR images showing hypointensities on TIW (a) and hyperintensities on T2W (b) in bilateral occipital lobes. FLAIR images (c, d) showing hyperintensities in the bilateral occipital lobes and left high parietal region with sparing of the calcarine and paramedian occipital lobe structures
Figure 2.
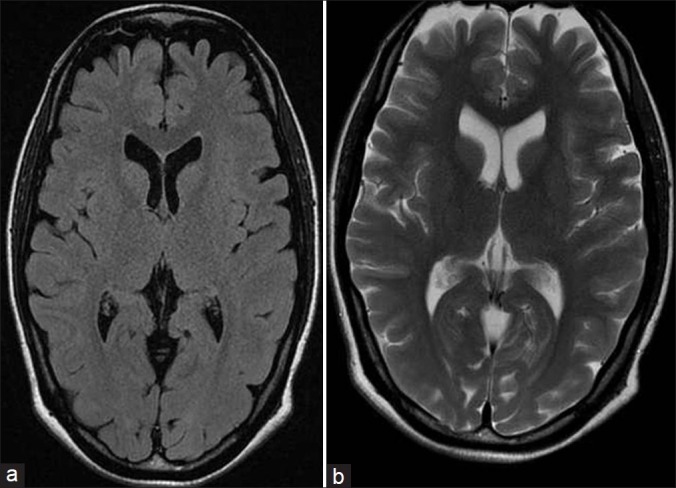
T1 (a) and T2 (b) weighted MR images showing resolution of lesions after six weeks of follow-up
The reversible posterior leukoencephalopathy syndrome (RPLS) is an increasingly recognized neurological disorder, with characteristic computed tomographic (CT) and magnetic resonance imaging (MRI) findings, associated with a multitude of diverse clinical entities.[2] The clinical entities associated with RPLS include acute glomerulonephritis, pre-eclampsia and eclampsia, systemic lupus erythematosus, thrombotic thrombocytopenic purpura, and the hemolytic-uremic syndrome, as well as drug toxicity from agents such as cyclosporine, tacrolimus, cisplatin, and erythropoietin. RPLS tends to strike the young more frequently than the elderly, and women more often than men. This is due to the natural history of the illnesses that predispose people to RPLS, many of which affect young women disproportionately, such as collagen vascular diseases, or exclusively eclampsia.[2]
The most common clinical symptoms and signs are headache, altered alertness, and behavior ranging from drowsiness to stupor, seizures, vomiting, mental abnormalities including confusion and diminished spontaneity and speech, and abnormalities of visual perception. The onset is usually subacute, but may be heralded by a seizure. Seizures are common at the onset of neurological symptoms, but can also develop later.[1] Seizures may begin focally, but usually become generalized. Lethargy and somnolence are often the first signs noted. Stupor and frank coma may develop, but usually patients remain responsive to stimuli. Abnormalities of visual perception are nearly always detectable. Patients often report blurred vision. Hemianopia, visual neglect, and frank cortical blindness may occur. Some patients with cortical blindness do not realize that they cannot see (Anton's syndrome). The tendon reflexes are often brisk, and some patients have weakness and incoordination of the limbs.
The pathogenesis of this syndrome is poorly understood. It is postulated that there is a temporary failure of the autoregulatory capabilities of the cerebral vessels leading to hyperperfusion breakdown of the blood brain barrier and consequent vasogenic edema.[3] In a healthy subject both the myogenic and neurogenic components of cerebral autoregulation maintain constant brain perfusion.The effectiveness of the neurological component of autoregulation is directly proportional to the degree of sympathetic innervation. In patients with RPLS the myogenic response is blunted by the effect of elevated blood pressure on the vessel wall or the direct toxic effect on the endothelium. Thus in the autoregulatory mechanism, which is more dependent on the neurogenic response, the poorly innervated posterior circulation areas are more vulnerable.[4] The most common abnormality on neuroimaging in RPLS is the edema involving the white matter in the posterior portion of the cerebral hemispheres, especially the bilateral parietal occipital regions.[1] The calcarine and paramedian occipital-lobe structures are usually spared; a fact that distinguishes RPLS from bilateral infarction of the posterior cerebral artery territory. Involvement of the additional areas of the brain, such as, the brain stem, cerebellum, basal ganglia, and frontal lobes has been reported in patients with RPLS. Abnormalities on imaging are defined as areas of low white matter attenuation on CT scans. An MRI will reveal hypointense areas on T1-weighted, hyperintense areas on T2-weighted, and isointense or hypointense areas on diffusion weighted imaging (DWI) that partially or completely resolve on follow-up scanning. These changes are characteristic of subcortical edema without infarction.[2]
The main differential diagnosis of RPLS is, ‘top of the basilar’ syndrome, due to simultaneous bilateral infarction of the posterior cerebral artery. The appropriate clinical scenario (e.g., acute hypertension, eclampsia, renal failure, transplant, use of immunosuppressive agent, etc.), seizures, and sparing of paramedian and calcarine regions distinguishes RPLS from ‘top of the basilar’ syndrome. On MRI the infarct is bright on DWI, whereas, in RPLS it is iso or hypointense. The hallmark of RPLS is rapid clinical and imaging resolution with appropriate treatment.
The treatment is to control the blood pressure, discontinue or decrease the dose of offending agents (immunosuppressive, cytotoxic), and treat seizures with anticonvulsants. Prognosis of RPLS is good. Most patients recover completely with prompt treatment, within hours to days. Imaging findings may persist for weeks. Resolutions of imaging findings have been reported within eight days to seventeen months after the first abnormal results.[1] It can lead to posterior circulation infarction or hemorrhage if not treated promptly.
The RPLS is often not suspected by clinicians. The common precipitant is acute elevation of blood pressure, such as in eclampsia, as was evident in our patient. As this diagnosis has important therapeutic and prognostic implications, clinicians as well as radiologists should be aware of the spectrum of imaging findings in RPLS. The clinical signs and findings on neuroimaging in patients with the reversible posterior leukoencephalopathy syndrome are consistent enough that this entity should be promptly recognized, as it is reversible and readily treated by controlling the blood pressure.
References
- 1.Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee P, McKinstry RC. Reversible posterior leukoencephalopathy syndrome: Evaluation with diffusion-tensor MR imaging. Radiology. 2001;219:756–65. doi: 10.1148/radiology.219.3.r01jn48756. [DOI] [PubMed] [Google Scholar]
- 3.Javed MA, Sial MS, Lingawi S, Alfi A, Lubbad E. Etiology of posterior reversible encephalopathy syndrome(PRES) Pak J Med Sci. 2005;21:149–54. [Google Scholar]
- 4.Covarrubias DJ, Luetmer PH, Campeau NG. Posterior Reversible Encephalopathy Syndrome: Prognostic Utility of Quantitative Diffusion Weighted MR images. Am J Neuroradiol. 2002;23:1038–48. [PMC free article] [PubMed] [Google Scholar]


