Abstract
Intentional weight loss is an important component of treatment for overweight patients with type 2 diabetes, but the effects on bone density are not known. We used data from the Look AHEAD trial to determine the impact of an intensive lifestyle weight loss intervention (ILI) compared to diabetes support and education (DSE) on changes in bone mineral density (BMD) over 12 months. Overweight and obese adults with type 2 diabetes were randomly assigned to ILI or DSE. In a sub-study of BMD conducted at 5 of 16 clinical centers, hip, spine and whole body dual x-ray absorptiometry scans were obtained at baseline and one year later on 642 of 739 ILI and 632 of 740 DSE participants. At baseline, mean age was 58.4 years, and average body mass index was 35.2 kg/m2. Total hip BMD T-score was <−2.5 in 1% and <−1.0 in 8%. At one year, weight loss was greater in ILI than DSE (−8.6% versus −0.7%), and glycemic control and fitness were also improved. Bone loss over one year was greater in ILI at the total hip (−1.4% versus −0.4%; p<0.001) and femoral neck (−1.5% versus −0.8%; p=0.009), but change in BMD for the lumbar spine and whole body did not differ between groups. In ILI, bone loss at the total hip was independently associated with weight loss in men and women and with poorer glycemic control in men, but was not associated with changes in fitness. One year of an intensive lifestyle intervention in adults with type 2 diabetes, resulting in weight loss, was associated with a modest increase in hip bone loss despite improved fitness and glycemic control.
Keywords: bone mineral density, weight loss, type 2 diabetes, obesity, glycemic control, physical fitness
INTRODUCTION
Intentional weight loss in older adults without diabetes is associated with bone loss (1). However, there are no clinical trials assessing the effects of weight loss on bone loss in type 2 diabetes, and little has been published from observational data. Weight loss is a primary treatment strategy for type 2 diabetes, but this is also a population at higher risk of fracture (2). Although weight loss is associated with bone loss in other populations, weight loss in those with type 2 diabetes results in improved glycemic control, a change that may be beneficial for bone (3). Exercise may be effective as a means to prevent bone loss during weight loss, but only one study has examined this relationship in those with type 2 diabetes (4).
Look AHEAD is an ongoing trial assessing the long-term effects of intentional weight loss on cardiovascular disease in overweight and obese adults with type 2 diabetes. We examined the effect of the intensive lifestyle intervention in Look AHEAD, resulting in weight loss, on bone mineral density (BMD) during the first year of the trial. We also assessed the independent contributions of changes in weight, physical fitness and glycemic control to changes in BMD.
MATERIALS AND METHODS
Look AHEAD is a multi-center, randomized, clinical trial of the effects of an Intensive Lifestyle Intervention (ILI), aimed at loss of ≥7% of body weight, compared to a control condition of Diabetes Support and Education (DSE), in overweight and obese adults with type 2 diabetes (5). The ILI is designed to achieve weight loss in the first year and then maintain weight through decreased caloric intake and increased physical activity. The primary endpoint is the occurrence of major cardiovascular events.
A total of 5,145 participants were randomized into Look AHEAD at 16 U.S. clinical centers. As described previously (6,7), participation was open to persons with type 2 diabetes, 45 to 76 years old, with body mass index (BMI) ≥25 kg/m2 (or ≥27 kg/m2 if taking insulin). Applicants completed a maximal graded exercise test (GXT) (5,6), to ensure that they could safely adhere to the physical activity program in the ILI (8). At baseline and year 1 (Y1) clinic visits, weight and height were measured with a digital scale and a standard wall-mounted stadiometer. Participants brought all prescription medicines to the clinic for a medication inventory. Questionnaires were used to collect demographic characteristics, smoking history, and alcohol use. Calcium intake in the diet, but not supplement use, was assessed in a subset of participants using a 130-item food questionnaire. A submaximal GXT was administered at Y1. Change in fitness during the first year was the difference in estimated METS for the point during each test when >80% of age-predicted maximal heart rate was attained or, for those using a beta-blocker, when the participant achieved 16 on the rating of perceived exercise scale (9). HbA1c was measured at the Northwest Lipid Research Laboratories, University of Washington, using a dedicated ion-exchange high-performance liquid chromatography instrument (Biorad Variant II) (7).
Interventions
Eligible participants were randomized to the ILI or DSE. The DSE group received information on healthy eating and physical activity but did not receive the comprehensive components of the weight loss intervention or the specific strategies for weight loss provided in the ILI group. A full description of the ILI and DSE during the first year of Look AHEAD has been published (8). For all participants, general medical and diabetes care continued to be delivered by the participant’s personal physician.
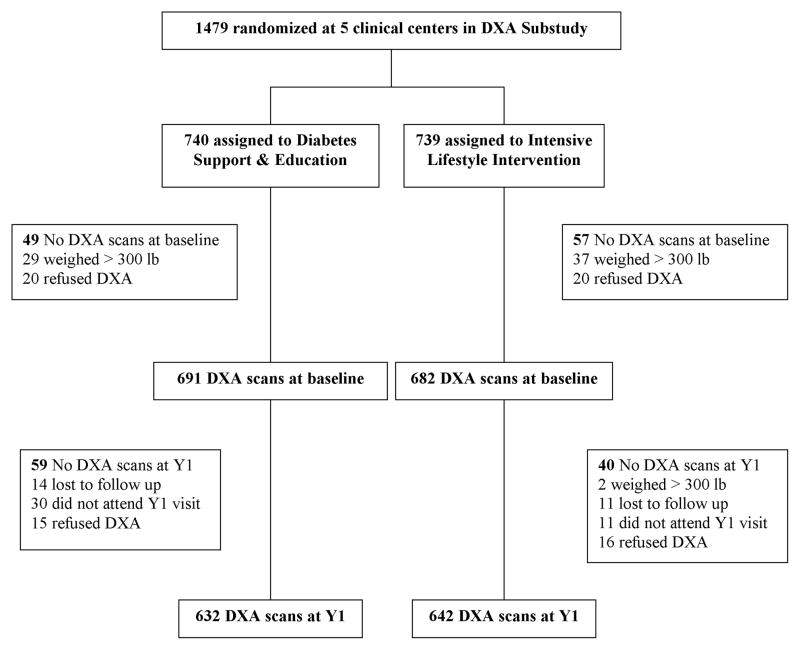
Dual energy x-ray absorptiometry (DXA) sub-study
In a sub-study, hip, spine and whole body DXA scans were obtained at baseline and Y1 at five clinical centers (Baton Rouge, Boston- Massachusetts General Hospital, Houston, Los Angeles, Seattle). Sites were selected based on availability of DXA scanners and interest of the local investigators. The recruitment goal was 1,260 participants, the minimum needed for 90% power to detect a difference of 0.5% in BMD change over one year between treatment groups. Of 1,479 enrolled participants, 68 were ineligible because their weight exceeded the DXA scanner limit (>300 lbs), 137 did not have scans at both visits, and 1,274 are included in these analyses (Figure). Mean baseline age, BMI, HbA1c, and insulin use of these included participants were not statistically different from the 137 participants without scans at both visits. All DXA sites used Hologic fan beam densitometers, and any software upgrades during the study were approved by a DXA quality assurance center (San Francisco Coordinating Center, University of California San Francisco). Longitudinal performance was monitored with regular scanning of a spine and a whole body phantom on each densitometer. The coefficient of variation for spine and whole body phantom BMD ranged from 0.36%–0.39% and 1.6%–2.4%, respectively, at the five sites. Longitudinal corrections were applied to spine and hip BMD results at the Los Angeles site and to whole body BMD at the Houston site. Soft tissue results were corrected to account for under-estimation of fat mass (10). The quality of participant scans was centrally monitored. T-scores were calculated using NHANES III as the reference for total hip and the manufacturer’s database for spine, with gender-specific references. If BMD loss was >10% for lumbar spine or total hip, the participant and primary care provider were notified.
Figure.
Flowchart for Look AHEAD DXA sub-study participants
Statistical analysis
For baseline characteristics, statistical tests for continuous variables were from linear models including gender, clinic, and randomization assignment. For categorical variables, the p-values were obtained from generalized linear models with a multinomial response probability distribution.
Changes in BMD, both absolute values and percent change, were compared across ILI and DSE groups. Analyses are also presented stratified by gender because of gender differences in BMD and bone loss. Differences by randomization assignment were tested with linear models adjusted for clinic, gender, baseline variables (Table 1) that differed across randomization assignment (p <0.10), and the baseline value of the outcome change variable. Adjusted means and standard errors are reported. The results were similar for absolute and percent change, and only the percent change is reported here.
Table 1.
Baseline Characteristicsa of Look AHEAD Trial Participants with Bone Density Scans at Baseline and One-Year Visits
| Variable | Diabetes Support and Education (DSE) | Intensive Lifestyle Intervention (ILI) | DSE vs. ILI P valueb | ||
|---|---|---|---|---|---|
| Men | Women | Men | Women | ||
| N | 246 | 386 | 237 | 405 | |
| Age (yr) | 60.0 ± 6.4 | 57.8 ± 6.5 | 60.4 ± 6.5 | 57.0 ± 6.6 | 0.39 |
| Ethnicity | 0.77 | ||||
| Black | 17 (7%) | 52 (13%) | 9 (4%) | 55 (14%) | |
| White | 178 (72%) | 192 (50%) | 167 (70%) | 208 (51%) | |
| Other | 51 (21%) | 142 (37%) | 61 (26%) | 142 (35%) | |
| Height (cm) | 175.5 ± 6.5 | 160.4 ± 6.7 | 174.3 ± 6.9 | 160.3 ± 6.9 | 0.14 |
| Weight (kg) | 104.9 ± 14.3 | 93.5 ± 16.0 | 102.9 ± 15.3 | 92.1 ± 16.7 | 0.07 |
| BMI (kg/m2) | 34.0 ± 4.3 | 36.3 ± 5.5 | 33.9 ± 4.6 | 35.8 ± 5.7 | 0.20 |
| Obesec | 197 (80%) | 335 (87%) | 190 (80%) | 335 (83%) | 0.24 |
| Whole body lean soft-tissue mass (kg) | 64.9 ± 7.1 | 48.1 ± 6.2 | 63.6 ± 6.9 | 47.9 ± 6.7 | 0.07 |
| Whole body fat mass (kg) | 36.8 ± 8.8 | 43.0 ± 10.3 | 36.0 ± 9.7 | 42.0 ± 10.7 | 0.11 |
| Total hip BMD (g/cm2) | 1.099 ± 0.138 | 1.027 ± 0.142 | 1.077 ± 0.122 | 1.028 ± 0.140 | 0.31 |
| Total hip BMD T-score | |||||
| T-score < −2.5 | 4 (2%) | 4 (1%) | 1 (0%) | 3 (1%) | 0.23 |
| −2.5 < T-score < −1 | 17 (7%) | 27 (7%) | 16 (7%) | 30 (7%) | 0.88 |
| Femoral neck BMD (g/cm2) | 0.887 ± 0.123 | 0.865 ± 0.134 | 0.868 ± 0.120 | 0.865 ± 0.137 | 0.33 |
| Spine BMD (g/cm2) | 1.152 ± 0.175 | 1.079 ± 0.157 | 1.115 ± 0.143 | 1.068 ± 0.164 | 0.02 |
| Spine BMD T-score | |||||
| T-score < −2.5 | 2 (1%) | 10 (3%) | 2 (1%) | 10 (2%) | 0.94 |
| −2.5 < T-score < −1 | 40 (16%) | 64 (17%) | 39 (16%) | 73 (18%) | 0.68 |
| Whole body BMD (g/cm2) | 1.192 ± 0.118 | 1.111 ± 0.123 | 1.170 ± 0.103 | 1.110 ± 0.121 | 0.20 |
| Statin use | 120 (49%) | 134 (35%) | 120 (51%) | 150 (37%) | 0.39 |
| Insulin use | 34 (14%) | 60 (16%) | 46 (19%) | 59 (15%) | 0.47 |
| Metformin use | 152 (62%) | 216 (56%) | 155 (65%) | 261 (64%) | 0.01 |
| Sulfonylurea use | 129 (52%) | 171 (44%) | 126 (53%) | 178 (44%) | 0.99 |
| TZD use | 64 (26%) | 65 (17%) | 56 (24%) | 62 (15%) | 0.45 |
| Hormone therapy use | 0 (0%) | 239 (62%) | 0 (0%) | 257 (64%) | 0.62 |
| Osteoporosis medication use | 0 (0%) | 12 (3%) | 1 (0%) | 17 (4%) | 0.28 |
| Graded exercise test: Maximal value (MET) | 8.4 ± 2.1 | 6.9 ± 1.6 | 8.3 ± 2.1 | 7.1 ± 1.7 | 0.66 |
| Dietary calciumd (mg/day) | 850 ± 592 | 851 ± 536 | 825 ± 510 | 838 ± 436 | 0.67 |
| Smoking status | 0.71 | ||||
| Never | 97 (39%) | 250 (65%) | 93 (39%) | 259 (64%) | |
| Past | 138 (56%) | 120 (31%) | 129 (54%) | 131 (32%) | |
| Current | 11 (4%) | 16 (4%) | 15 (6%) | 15 (4%) | |
| Consumed alcohol in past year | 161 (65%) | 184 (48%) | 170 (72%) | 196 (48%) | 0.24 |
| Menopausal status | 0.06 | ||||
| Post- | 0 (0%) | 297 (77%) | 0 (0%) | 289 (71%) | |
| Pre- | 0 (0%) | 42 (11%) | 0 (0%) | 50 (12%) | |
| Unknown | 0 (0%) | 47 (12%) | 0 (0%) | 66 (16%) | |
| HbA1c (%) | 7.2 ± 1.2 | 7.4 ± 1.2 | 7.2 ± 1.2 | 7.2 ± 1.2 | 0.06 |
Mean ± standard deviation, or N(%)
P value for comparison between treatment groups (DSE and ILI) adjusted for gender and clinic
BMI ≥ 30 kg/m2
Calcium intake in diet, not including supplements. Available on a subset of participants (N= 697).
Within the ILI group, adjusted linear regression models were used to identify associations between change in hip BMD over one year and changes in other factors. Parameter estimates (Beta coefficients) were reported. The change in absolute value of BMD, not percent change, was used in these models. Analyses were carried out using SAS v 9.2 (Cary, NC).
RESULTS
Baseline characteristics
In this subgroup of Look AHEAD participants, average age was 58.4 years. Average BMI was 35.2 kg/m2, and 83% were obese, similar to the total Look AHEAD population (7). The proportion with osteoporosis, based on T-score, was low. Spine BMD, weight, lean mass, metformin use, menopausal status and HbA1c, but not other baseline characteristics, differed at baseline in the ILI and DSE groups (Table 1).
Changes over 12 months
During the first year, ILI participants lost more weight than the DSE group (p<0.001) (Table 2). The ILI group lost an average of 10.0 kg (men) and 8.3 kg (women), compared with 0.5 kg (men) and 0.8 kg (women) in the DSE group. The ILI group also experienced improved glycemic control (p<0.001) and fitness (p<0.001), compared with the DSE group.
Table 2.
Change from Baseline to Year 1 by Randomized Groupa
| Adjusted Mean (SE) Changeb | Treatment P valuec | ||
|---|---|---|---|
| Diabetes Support and Education | Intensive Lifestyle Intervention | ||
| ALL | |||
| Total hip BMD (%) | −0.39 (0.12) | −1.45 (0.12) | <.001 |
| Femoral neck BMD (%) | −0.85 (0.19) | −1.50 (0.18) | 0.009 |
| Lumbar spine BMD (%) | −0.08 (0.14) | 0.01 (0.14) | 0.61 |
| Whole body BMD (%) | −0.16 (0.12) | 0.13 (0.12) | 0.06 |
| Weight (kg) | −0.87 (0.24) | −9.06 (0.24) | <.001 |
| HbA1c (%) | −0.11 (0.04) | −0.69 (0.04) | <.001 |
| Graded exercise test (MET) | 0.19 (0.06) | 1.17 (0.06) | <.001 |
| MEN | |||
| Total hip BMD (%) | 0.02 (0.15) | −1.48 (0.16) | <.001 |
| Femoral neck BMD (%) | −0.50 (0.23) | −1.18 (0.24) | 0.04 |
| Lumbar spine BMD (%) | 0.57 (0.22) | 0.80 (0.22) | 0.45 |
| Whole body BMD (%) | 0.27 (0.17) | 0.39 (0.18) | 0.63 |
| Weight (kg) | −0.49 (0.39) | −10.01 (0.40) | <.001 |
| HbA1c (%) | −0.12 (0.06) | −0.69 (0.06) | <.001 |
| Graded exercise test (MET) | 0.23 (0.10) | 1.27 (0.10) | <.001 |
| WOMEN | |||
| Total hip BMD (%) | −0.61 (0.17) | −1.44 (0.16) | <.001 |
| Femoral neck BMD (%) | −1.13 (0.26) | −1.74 (0.25) | 0.07 |
| Lumbar spine BMD (%) | −0.56 (0.18) | −0.52 (0.18) | 0.86 |
| Whole body BMD (%) | −0.52 (0.15) | −0.15 (0.15) | 0.07 |
| Weight (kg) | −0.84 (0.28) | −8.27 (0.27) | <.001 |
| HbA1c (%) | −0.08 (0.05) | −0.65 (0.05) | <.001 |
| Graded exercise test (MET) | 0.12 (0.07) | 1.09 (0.06) | <.001 |
Adjusted for clinical site, gender, baseline value of outcome, and baseline values of spine BMD, lean body mass, metformin use, HbA1c and, for women, menopausal status.
SE = standard error
P value for comparison between treatment groups
The average time between the baseline and Y1 DXA scans was 395 (SD 43) days, and did not differ between groups. Bone loss was greater in the ILI group compared with the DSE group for the total hip (−1.45% versus −0.39; p<0.001) and femoral neck (−1.50 versus −0.85%; p=0.009) (Table 2). Changes in spine BMD (p=0.61) and whole body BMD (p=0.06) did not differ by intervention group. At the total hip, the difference in bone loss between intervention groups was greater for men (−1.48% vs. 0.02%) than for women (−1.44% vs. −0.61%) (p for interaction = 0.04). There was no evidence of interaction at the other BMD sites.
Differences in bone loss between the ILI and DSE groups were similar in models excluding participants with thiazolidinedione (TZD) use at baseline (data not shown). During the first year of the trial, the proportion of participants using a TZD increased in the DSE group, 19.8% at baseline to 23.7% at Y1, and decreased in the ILI group, 18.4% to 15.9%. The proportion with osteoporosis increased in the ILI group and was stable in the DSE group, but remained small. At 12 months, 2.0% of the ILI group was osteoporotic based on total hip BMD, compared with 1% at baseline.
Factors affecting bone loss in the Intensive Lifestyle Intervention group
To assess the independent contributions of intentional weight loss, glycemic control and physical fitness, we analyzed the results for the ILI group separately. In unadjusted models, total hip bone loss increased with each increasing quartile of weight loss (data not shown). In linear regression analyses adjusted for age, gender, race and clinical site, weight loss correlated with total hip bone loss in the ILI group. Each additional kg of weight loss was associated with a decrease in total hip BMD of 0.0018 g/cm2. However, there were no statistically significant correlations between bone loss and weight loss for femoral neck BMD. In models additionally adjusted for HbA1c change, physical fitness change, baseline use of diabetes medications (separate adjustment for TZDs, insulin, metformin and sulfonlyureas), current or past smoking, and alcohol use, weight loss continued to be associated with bone loss at the total hip in men and women (Table 3). In men, but not women, a decrease in HbA1c was associated with an increase in total hip BMD, but was not associated with femoral neck BMD changes. Improved physical fitness, as determined by a graded exercise test, was not associated with bone changes for men or women. When weight change was separated into changes in fat mass and lean mass as measured by DXA, losses in both compartments were associated with total hip, but not femoral neck, bone loss for men and women in the ILI group. Results were similar when those reporting TZD use at baseline were excluded (data not shown).
Table 3.
Associations with One-Year Change in Hip BMD in the Intensive Lifestyle Intervention Group
| 1-year Change in Hip BMD (mg/cm2) | ||||
|---|---|---|---|---|
| Total Hip | Femoral Neck | |||
| Beta | P value | Beta | P value | |
| ALLa | ||||
| Weight change (per kg) | 1.77 | <.001 | 0.11 | 0.69 |
| HbA1c change (per %) | −1.58 | 0.21 | 1.78 | 0.29 |
| Graded exercise test change (per MET) | −1.24 | 0.20 | −0.12 | 0.93 |
| MENa | ||||
| Weight change (per kg) | 1.65 | <.001 | 0.02 | 0.95 |
| HbA1c change (per %) | −5.89 | <.001 | 0.88 | 0.65 |
| Graded exercise test change (per MET) | −1.77 | 0.17 | −1.26 | 0.37 |
| WOMENa | ||||
| Weight change (per kg) | 1.96 | <.001 | 0.04 | 0.93 |
| HbA1c change (per %) | 1.29 | 0.46 | 1.87 | 0.46 |
| Graded exercise test change (per MET) | −0.47 | 0.74 | 1.11 | 0.58 |
| ALLb | ||||
| Lean soft-tissue mass change (per kg) | 1.99 | <.001 | 0.34 | 0.68 |
| Fat mass change (per kg) | 1.81 | <.001 | 0.20 | 0.65 |
| HbA1c change (per %) | −1.44 | 0.26 | 1.74 | 0.31 |
| Graded exercise test change (per MET) | −1.04 | 0.28 | 0.00 | 0.10 |
| MENb | ||||
| Lean soft-tissue mass change (per kg) | 2.41 | 0.002 | 0.46 | 0.59 |
| Fat mass change (per kg) | 1.29 | 0.005 | −0.17 | 0.75 |
| HbA1c change (per %) | −5.00 | 0.005 | 1.20 | 0.54 |
| Graded exercise test change (per MET) | −1.94 | 0.12 | −1.25 | 0.38 |
| WOMENb | ||||
| Lean soft-tissue mass change (per kg) | 2.06 | 0.02 | 0.23 | 0.85 |
| Fat mass change (per kg) | 2.15 | <.001 | 0.26 | 0.69 |
| HbA1c change (per %) | 1.28 | 0.46 | 1.80 | 0.48 |
| Graded exercise test change (per MET) | −0.11 | 0.94 | 1.19 | 0.56 |
Model includes change in weight, HbA1c and graded exercise test, and baseline values for age, gender, race, clinic site, baseline use of insulin, metformin, sulfonylureas, TZDs, smoking, and alcohol use, and for women menopausal status and hormone therapy use.
Model includes change in lean soft tissue mass, fat mass, HbA1c and graded exercise test, and baseline values for age, gender, race, clinic site, baseline use of insulin, metformin, sulfonylureas, TZDs, smoking and alcohol use, and for women menopausal status and hormone therapy use.
DISCUSSION
We found that one year of intentional weight loss in overweight and obese adults with type 2 diabetes resulted in greater bone loss at the total hip and femoral neck, but not at the spine or whole body. To our knowledge, this is the first trial to assess the effects of an intentional weight loss intervention on bone in adults with type 2 diabetes.
Studies in broader populations have reported bone loss at the total hip, spine and whole body with weight loss (11,12). In obese patients undergoing bariatric surgery, bone loss appears to occur in proportion to the degree of weight loss (13). Bone loss with weight loss is likely due to less skeletal loading and reduced anabolic signals from muscle and fat mass (14–16). In observational studies, lower weight and intentional weight loss increase fracture risk (17,18). This relationship between lower weight and fracture is strong for BMI<25, but is weaker with higher BMI (17). In a small trial of a lifestyle intervention in obese older adults without diabetes, the intervention group had increased bone loss at the hip but not the spine or whole body, similar to our findings (19). Spine BMD changes are difficult to assess in older adults because of osteophytes and other degenerative changes. Whole body BMD has a higher proportion of cortical bone than hip BMD and may not be as sensitive to changes in weight.
A limitation of this study is the use of DXA to assess BMD changes in the setting of weight change, particularly in obese participants. With obesity, the reproducibility of BMD is reduced which is likely to attenuate any real associations between changes in weight and BMD (20). Changes in fat mass can affect the ability of DXA to identify bone edges and to accurately allow for the composition of soft tissue overlying bone, possibly introducing artificial changes in BMD (21). In a recent study using a Hologic densitometer, fat mass added to volunteers reduced the measured mean spine BMD but did not alter the results for mean hip BMD (22). In addition, with substantial weight loss, smaller hip circumference reduces the height of the bone above the densitometer table which may introduce fan beam magnification effects (23). The net effect of changes in fat mass on bone measurements vary with skeletal site and scanner model. However, in our analyses considering decreases in fat mass and in lean mass separately, we found that both were associated with total hip bone loss, suggesting that the observed association between weight loss and bone loss in this trial is not simply an artifact of changes in fat mass.
The ILI group experienced greater weight loss as well as improved physical fitness and glycemic control compared with the DSE group. Look AHEAD was not designed to identify whether these changes had separate effects on outcomes. However, it is plausible that weight loss results in bone loss while improved glycemic control and physical fitness have a positive effect on BMD. When we sought evidence for independent effects by considering the intervention group separately, we found that weight loss was associated with total hip bone loss in the intervention group even after adjustment for changes in HbA1c and physical fitness. TZD use, associated with bone loss and fracture in other studies (24), was greater in the DSE group during the first year and thus does not account for the differences in hip bone loss.
Improved HbA1c was associated with preservation of total hip BMD in men but not women. The relationship between glycemic control and changes in BMD has not been clearly established. In an uncontrolled trial, Gregorio et al. reported increased femoral neck bone mineral content in 50 type 2 diabetic adults with poor glycemic control after a year of treatment that improved control (3). However, the investigators did not report on changes in weight. In Look AHEAD, most participants had good glycemic control at study entry (mean A1C ~ 7.2%) which may have limited any potential skeletal benefits of improved control.
Our analyses in the intervention group suggest that improved physical fitness during weight loss did not preserve bone. Physical activity was only measured on a subset of Look AHEAD participants using the Paffenbarger scale, but changes in physical activity over the first year of the trial correlated positively (R = 0.25, p<0.0001) with changes in physical fitness (9). Results from studies of the effects of exercise during weight loss on bone loss have been conflicting (25–27). One study in older type 2 diabetic adults reported that high-intensity resistance training reduced the effects of weight loss on total body BMD (4). The exercise intervention in Look AHEAD was not as intensive and did not emphasize resistance training which may account for the different findings.
Bone loss with intentional weight loss is primarily a concern because of the strong relationship between low BMD and fracture risk. However, the additional bone loss experienced in the ILI group averaged a modest 1%. Thus, the initial year of weight loss in Look AHEAD may not result in a clinically important increase in fracture risk, especially as subsequent years will focus on weight maintenance rather than additional weight loss. On the other hand, effects of weight loss on bone density might be greater in subsequent years as any skeletal remodeling response may lag behind the weight loss stimulus. This report is limited to one year of follow-up. For comparison, in a U.S. cohort of older women, an annual loss of 0.9% total hip BMD over 8 years, a cumulative loss of about 7%, was associated with a relative risk of subsequent hip fracture of 1.29 (28). Fracture events are not currently available for Look AHEAD, but are being collected and will be assessed at the end of the trial.
This study is the first to address the effects of a weight loss intervention on bone in a diabetic population. A strength of this study is the randomization of participants to the weight loss intervention. Thus, weight loss in the ILI group was generally intentional and not due to illness or frailty. A limitation of this study is the use of DXA in the setting of weight change. In addition, data were not collected on calcium or vitamin D supplement use. It is possible that the two groups used different amounts of these supplements. Another limitation is the lack of bone turnover markers to assess effects on bone resorption. These results may not apply to patients weighing more than 300 lbs, the weight limit for DXA measurements, or to patients with poor glycemic control.
In conclusion, one year of an intensive lifestyle intervention in overweight and obese men and women with type 2 diabetes that resulted in weight loss, improved glycemic control and enhanced physical fitness, also resulted in a modest increased bone loss at the hip. The extent to which this additional bone loss may increase fracture risk is not known. Further research, including fracture outcomes in Look AHEAD, is needed to elucidate the effects of intentional weight loss on fracture risk in older adults with type 2 diabetes.
Acknowledgments
This study is supported by the Department of Health and Human Services through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; Office of Research on Women’s Health; the Centers for Disease Control and Prevention; and the Department of Veterans Affairs. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center (M01RR01066); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (M01RR000056 44) and NIH grant (DK 046204); and the VA Puget Sound Health Care System/University of Washington, Medical Research Service, Department of Veterans Affairs; Frederic C. Bartter General Clinical Research Center (M01RR01346)
Look AHEAD Research Group for DXA Substudy at Year 1
Clinical Sites
Pennington Biomedical Research Center George A. Bray, MD1; Kristi Rau2; Allison Strate, RN2; Brandi Armand, LPN2; Frank L. Greenway, MD3; Donna H. Ryan, MD3; Donald Williamson, PhD3; Amy Bachand; Michelle Begnaud; Betsy Berhard; Elizabeth Caderette; Barbara Cerniauskas; David Creel; Diane Crow; Helen Guay; Nancy Kora; Kelly LaFleur; Kim Landry; Missy Lingle; Jennifer Perault; Mandy Shipp, RD; Marisa Smith; Elizabeth Tucker
Harvard Center
Massachusetts General Hospital: David M. Nathan, MD1; Heather Turgeon, RN, BS, CDE2; Kristina Schumann, BA2; Enrico Cagliero, MD3; Linda Delahanty, MS, RD3; Kathryn Hayward, MD3; Ellen Anderson, MS, RD3; Laurie Bissett, MS, RD; Richard Ginsburg, PhD; Valerie Goldman, MS, RD; Virginia Harlan, MSW; Charles McKitrick, RN, BSN, CDE; Alan McNamara, BS; Theresa Michel, DPT, DSc CCS; Alexi Poulos, BA; Barbara Steiner, EdM; Joclyn Tosch, BA
Baylor College of Medicine John P. Foreyt, PhD1; Rebecca S. Reeves, DrPH, RD2; Henry Pownall, PhD3; Ashok Balasubramanyam, MBBS3; Peter Jones, MD3; Michele Burrington, RD; Chu-Huang Chen, MD, PhD; Allyson Clark, RD; Molly Gee, MEd, RD; Sharon Griggs; Michelle Hamilton; Veronica Holley; Jayne Joseph, RD; Patricia Pace, RD: Julieta Palencia, RN; Olga Satterwhite, RD; Jennifer Schmidt; Devin Volding, LMSW; Carolyn White
University of California at Los Angeles School of Medicine Mohammed F. Saad, MD1; Siran Ghazarian Sengardi, MD2; Ken C. Chiu, MD3; Medhat Botrous; Michelle Chan, BS; Kati Konersman, MA, RD, CDE; Magpuri Perpetua, RD
University of Washington/VA Puget Sound Health Care System Steven Kahn MB, ChB1; Brenda Montgomery, RN, MS, CDE2; Robert Knopp, MD3; Edward Lipkin, MD3; Matthew L. Maciejewski, PhD3; Dace Trence, MD3; Terry Barrett, BS; Joli Bartell, BA; Diane Greenberg, PhD; Anne Murillo, BS; Betty Ann Richmond, MEd; April Thomas, MPH, RD
University of Southern California Anne Peters, MD1; Valerie Ruelas, MSW, LCSW2; Siran Ghazarian Sengardi, MD2; Kathryn Graves, MPH, RD, CDE; Kati Konersman, MA, RD, CDE; Sara Serafin-Dokhan
Coordinating Center
Wake Forest University Mark A. Espeland, PhD1; Judy L. Bahnson, BA2; Lynne Wagenknecht, DrPH3; David Reboussin, PhD3; W. Jack Rejeski, PhD3; Alain Bertoni, MD, MPH3; Wei Lang, PhD3; Gary Miller, PhD3; David Lefkowitz, MD3; Patrick S. Reynolds, MD3; Paul Ribisl, PhD3; Mara Vitolins, DrPH3; Michael Booth, MBA2; Kathy M. Dotson, BA2; Amelia Hodges, BS2; Carrie C. Williams, BS2; Jerry M. Barnes, MA; Patricia A. Feeney, MS; Jason Griffin, BS; Lea Harvin, BS; William Herman, MD, MPH; Patricia Hogan, MS; Sarah Jaramillo, MS; Mark King, BS; Kathy Lane, BS; Rebecca Neiberg, MS; Andrea Ruggiero, MS; Christian Speas, BS; Michael P. Walkup, MS; Karen Wall, AAS; Michelle Ward; Delia S. West, PhD; Terri Windham
Central Resources Centers
DXA Reading Center, University of California at San Francisco Michael Nevitt, PhD1; Susan Ewing, MS; Cynthia Hayashi; Jason Maeda, MPH; Lisa Palermo, MS, MA; Michaela Rahorst; Ann Schwartz, PhD; John Shepherd, PhD
Central Laboratory, Northwest Lipid Research Laboratories Santica M. Marcovina, PhD, ScD1; Greg Strylewicz, MS
ECG Reading Center, EPICARE, Wake Forest University School of Medicine
RonaldJ. Prineas, MD, PhD1; Teresa Alexander; Lisa Billings; Charles Campbell, AAS, BS; Sharon Hall; Susan Hensley; Yabing Li, MD; Zhu-Ming Zhang, MD
Diet Assessment Center, University of South Carolina, Arnold School of Public Health, Center for Research in Nutrition and Health Disparities Elizabeth J Mayer-Davis, PhD1; Robert Moran, PhD
Hall-Foushee Communications, Inc.
Richard Foushee, PhD; Nancy J. Hall, MA
Federal Sponsors
National Institute of Diabetes and Digestive and Kidney Diseases: Barbara Harrison, MS; Van S. Hubbard, MD PhD; Susan Z. Yanovski, MD
National Heart, Lung, and Blood Institute: Lawton S. Cooper, MD, MPH; Jeffrey Cutler, MD, MPH; Eva Obarzanek, PhD, MPH, RD
Centers for Disease Control and Prevention: Edward W. Gregg, PhD; David F. Williamson, PhD; Ping Zhang, PhD
Footnotes
Principal Investigator
Program Coordinator
Co-Investigator
Clinical trial registration number NCT00017953, ClinicalTrials.gov.
A complete list of the Look AHEAD Research Group may be found in the online appendix.
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; Optifast ® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
All other Look AHEAD staffs are listed alphabetically by site.
Author Contributions
A.S. wrote manuscript, A.H., K.J., A.P., S.K. and G.B. researched data, contributed to discussion, and reviewed/edited manuscript, M.N., C.W. and J.S. contributed to discussion and reviewed/edited manuscript, M.W. researched data and contributed to discussion.
References
- 1.Shapses SA, Riedt CS. Bone, body weight, and weight reduction: what are the concerns? J Nutr. 2006;136(6):1453–6. doi: 10.1093/jn/136.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes-a meta-analysis. Osteoporos Int. 2007;18(4):427–44. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 3.Gregorio F, Cristallini S, Santeusanio F, Filipponi P, Fumelli P. Osteopenia associated with non-insulin-dependent diabetes mellitus: what are the causes? Diabetes Res Clin Pract. 1994;23:43–54. doi: 10.1016/0168-8227(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 4.Daly RM, Dunstan DW, Owen N, Jolley D, Shaw JE, Zimmet PZ. Does high-intensity resistance training maintain bone mass during moderate weight loss in older overweight adults with type 2 diabetes? Osteoporos Int. 2005;16(12):1703–12. doi: 10.1007/s00198-005-1906-4. [DOI] [PubMed] [Google Scholar]
- 5.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24(5):610–28. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 6.Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, Graves K, Haffner SM, Harrison B, Hill JO, Horton ES, Jakicic J, Jeffery RW, Johnson KC, Kahn S, Kelley DE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montgomery B, Nathan DM, Patricio J, Peters A, Redmon JB, Reeves RS, Ryan DH, Safford M, Van Dorsten B, Wadden TA, Wagenknecht L, Wesche-Thobaben J, Wing RR, Yanovski SZ. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray G, Gregg E, Haffner S, Pi-Sunyer XF, WagenKnecht LE, Walkup M, Wing R. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res. 2006;3(3):202–15. doi: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, Berkowitz RI, Kelley DE, Tomchee C, Hill JO, Kumanyika S. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14(5):737–52. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakicic JM, Jaramillo SA, Balasubramanyam A, Bancroft B, Curtis JM, Mathews A, Pereira M, Regensteiner JG, Ribisl PM. Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: results from the Look AHEAD Study. Int J Obes (Lond) 2009;33(3):305–16. doi: 10.1038/ijo.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoeller DA, Tylavsky FA, Baer DJ, Chumlea WC, Earthman CP, Fuerst T, Harris TB, Heymsfield SB, Horlick M, Lohman TG, Lukaski HC, Shepherd J, Siervogel RM, Borrud LG. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr. 2005;81(5):1018–25. doi: 10.1093/ajcn/81.5.1018. [DOI] [PubMed] [Google Scholar]
- 11.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc. 2003;51(12):1740–7. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- 12.Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res. 2005;20(3):455–63. doi: 10.1359/JBMR.041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleischer J, Stein EM, Bessler M, Della Badia M, Restuccia N, Olivero-Rivera L, McMahon DJ, Silverberg SJ. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. The Journal of clinical endocrinology and metabolism. 2008;93(10):3735–40. doi: 10.1210/jc.2008-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reid IR. Relationships between fat and bone. Osteoporos Int. 2008;19(5):595–606. doi: 10.1007/s00198-007-0492-z. [DOI] [PubMed] [Google Scholar]
- 15.Judex S, Carlson KJ. Is bone’s response to mechanical signals dominated by gravitational loading? Med Sci Sports Exerc. 2009;41(11):2037–43. doi: 10.1249/MSS.0b013e3181a8c6e5. [DOI] [PubMed] [Google Scholar]
- 16.Elkasrawy MN, Hamrick MW. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact. 2010;10(1):56–63. [PMC free article] [PubMed] [Google Scholar]
- 17.De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, McCloskey EV, Mellstrom D, Melton LJ, 3rd, Meunier PJ, Pols HA, Reeve J, Silman A, Tenenhouse A. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16(11):1330–8. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 18.Langlois JA, Mussolino ME, Visser M, Looker AC, Harris T, Madans J. Weight loss from maximum body weight among middle-aged and older white women and the risk of hip fracture: the NHANES I epidemiologic follow-up study. Osteoporos Int. 2001;12(9):763–8. doi: 10.1007/s001980170053. [DOI] [PubMed] [Google Scholar]
- 19.Villareal DT, Shah K, Banks MR, Sinacore DR, Klein S. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: a one-year randomized controlled trial. J Clin Endocrinol Metab. 2008;93(6):2181–7. doi: 10.1210/jc.2007-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel R, Blake GM, Rymer J, Fogelman I. Long-term precision of DXA scanning assessed over seven years in forty postmenopausal women. Osteoporos Int. 2000;11(1):68–75. doi: 10.1007/s001980050008. [DOI] [PubMed] [Google Scholar]
- 21.Tothill P. Dual-energy x-ray absorptiometry measurements of total-body bone mineral during weight change. J Clin Densitom. 2005;8(1):31–8. doi: 10.1385/jcd:8:1:031. [DOI] [PubMed] [Google Scholar]
- 22.Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2011 doi: 10.1002/jbmr.506. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blake GM, Fogelman I. Technical principles of dual energy x-ray absorptiometry. Semin Nucl Med. 1997;27(3):210–28. doi: 10.1016/s0001-2998(97)80025-6. [DOI] [PubMed] [Google Scholar]
- 24.Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. Cmaj. 2009;180(1):32–9. doi: 10.1503/cmaj.080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan AS, Treuth MS, Hunter GR, Elahi D. Resistive training maintains bone mineral density in postmenopausal women. Calcif Tissue Int. 1998;62:295–299. doi: 10.1007/s002239900434. [DOI] [PubMed] [Google Scholar]
- 26.Pritchard JE, Nowson CA, Wark JD. Bone loss accompanying diet-induced or exercise-induced weight loss: a randomised controlled study. Int J Obes Relat Metab Disord. 1996;20(6):513–20. [PubMed] [Google Scholar]
- 27.Svendsen OL, Hassager C, Christiansen C. Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am J Med. 1993;95(2):131–40. doi: 10.1016/0002-9343(93)90253-l. [DOI] [PubMed] [Google Scholar]
- 28.Hillier TA, Stone KL, Bauer DC, Rizzo JH, Pedula KL, Cauley JA, Ensrud KE, Hochberg MC, Cummings SR. Evaluating the value of repeat bone mineral density measurement and prediction of fractures in older women: the study of osteoporotic fractures. Arch Intern Med. 2007;167(2):155–60. doi: 10.1001/archinte.167.2.155. [DOI] [PubMed] [Google Scholar]