Abstract
Rationale
Addiction is a disease of learning and memory, as learning processes underlying acquisition, extinction, and reinstatement of drug-paired associations play central roles in addiction. Early developmental stress enhances risk for drug problems in adulthood. Environmental factors influencing learning and memory processes relevant to addiction remain incompletely characterized.
Objectives
To determine effects of prenatal immune activation and developmental stress on conditioned place preference to amphetamine, and reversal learning.
Methods
Pregnant Sprague Dawley rats were injected with polyinosinic:polycytidylic acid (poly I:C) or vehicle on gestational day 14. Half of the male offspring received two hours of restraint stress at postnatal day 35. Behavioral testing was performed in adulthood.
Results
Restraint stress inhibited acquisition of place preference to low dose amphetamine (0.5 mg/kg), while poly I:C treatment had no measurable effect on place preference acquisition. In contrast, drug-induced reinstatement of preference for drug-paired chamber was enhanced in offspring of poly I:C-treated dams [F(1,25) = 5.31, p = 0.03]. Performance on a Morris water maze reversal learning task was impaired in poly I:C offspring. Reversal learning performance was correlated with place preference reinstatement in non-stressed (r2 = 0.42, p = 0.0095), but not stressed rats (r2 =0.04, p = 0.49).
Conclusions
Prenatal immune activation enhances drug-induced reinstatement of conditioned place preference. These data demonstrate longstanding impact on behaviors with potential influence on risk for drug relapse as a consequence of prenatal immune activation. Further study is needed to determine clinical and epidemiological consequences of similar exposures in human populations.
Keywords: maternal, hippocampus, schizophrenia, animal model, prodrome, dopamine, stimulant, amphetamine
Introduction
Addiction has been described as a disease of learning and memory, as the learning processes underlying acquisition, extinction, and reinstatement of drug-paired associations play a central role in addictive disorders (Kelley2004;Hyman2005). The risk for addictions is determined by environmental and genetic factors acting in concert (Enoch2010;Goldman et al.2005), and epidemiological studies estimate early developmental stress accounts for more than half of the attributable risk for serious drug problems in adulthood (Dube et al.2003). Relapse to drug use is considered the major unmet need in the treatment of drug dependence (O'Brien2005). Despite this widespread recognition of their central importance to clinical outcome, identification of specific environmental factors influencing drug–associated learning and memory deficits of relevance to drug relapse remains incomplete.
Prenatal infection stimulates maternal cytokines, soluble polypeptides mediating the innate inflammatory response. Consequences to the offspring of maternal cytokine elevation have been studied in prenatal immune activation animal models using immunogens including the synthetic nucleic acid polyinosinic:polycytidylic acid (poly I:C), which activates Toll-like receptor TLR3, stimulating cytokine expression (Suh et al.2009). Maternal poly I:C injection alters dopamine function in offspring within nucleus accumbens core (Vuillermot et al.2010), the site of integration of drug-associated memories (Kalivas and O'Brien2008). Cognitive impairments resulting from prenatal immune activation include deficits in prefrontal cortically-mediated function (Bitanihirwe et al.2010), impaired reversal learning (Meyer et al.2006;Han et al.2011;Ito et al.2010;Lee et al.2007) and impaired extinction of a learned conditioned eye-blink response (Lee et al.2007). Repetitive drug use also impairs frontal cortical function, enhancing relapse to drug use (Everitt and Robbins2005;Kalivas2008;Kalivas and O'Brien2008). Prenatal immune activation and repetitive drug use may therefore exert similar adverse effects upon drug-associated learning and memory.
When combined with appropriate host genetic background (Clarke et al.2009), prenatal immune activation is a suspected environmental risk factor for schizophrenia (Patterson2009), an illness with 50% comorbidity for drug and alcohol dependence (Buckley2006). Based on estimates from studies of other disorders [(Brown and Derkits2010), Table 6], it is conceivable that as many as 1/3 of drug dependent patients have had relevant in utero exposures from maternal conditions inducing prenatal immune activation including pelvic inflammatory disease, influenza, or venereal disease. In order to determine whether prenatal immune activation alters drug-associated learning and memory, we tested the effects of poly I:C injection in conditioned place preference, a classical conditioning paradigm, which measures learned associations linking a drug experience and its environment. Because developmental stress is associated with elevated risk for drug dependence (Dube et al.2003;Andersen and Teicher2009), we also examined the combined effect of prenatal immune activation and a single peri-adolescent restraint stress exposure upon conditioned place preference. Finally, in order to determine if prenatal immune activation effects upon conditioned place preference and reversal learning (Meyer et al.2006;Han et al.2011;Ito et al.2010;Lee et al.2007) share common substrates, a Morris water maze reversal learning paradigm was used to identify relationships between the two measures. We hypothesized prenatal immune activation combined with peri-adolescent stress would enhance place preference conditioning, and would be correlated with reversal learning deficits.
Here we report a single peri-adolescent restraint stress exposure inhibited acquisition of place preference to a low dose of amphetamine (0.5 mg/kg), while poly I:C treatment had no measurable effect on place preference acquisition. In contrast, prenatal immune activation enhances drug-induced reinstatement of place preference. Reversal learning deficits were also observed following prenatal immune activation. These data demonstrate a longstanding impact on behaviors with potential impact on risk for drug dependence following in utero exposure to immune activation. Potential public policy implications of these findings for drug prevention and treatment programs are discussed.
Research Design and Methods
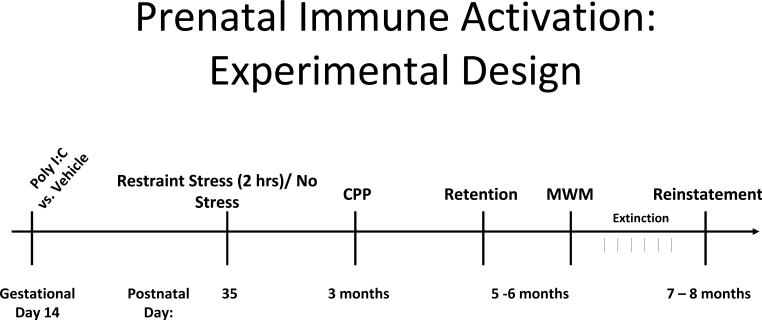
An experimental design summary is shown in Figure 1. Eight-week old male and nulliparous female Sprague-Dawley rats used as breeders were obtained from Harlan Laboratories (Indianapolis, IN). Following a minimum two week acclimatization, males and females were co-housed overnight, with the following morning defined as gestational day 0 (Taylor1986). Pregnant rats (identified by weight gain of ≥ 40 g) were injected with the synthetic nucleic acid analogue poly I:C (Sigma, St. Louis, MO, P1530; 8 mg/kg, i.p.) or vehicle (saline, 1 ml/kg) on gestational day 14 to stimulate a maternal inflammatory response. The timing of poly I:C injection was based upon the work of Zuckerman and colleagues describing outcomes following poly I:C injection on varying gestational dates in rats [(Zuckerman et al.2003). [Note gestational day 15 in Zuckerman's study is defined as gestational day 14 in some widely-referenced text books (Taylor1986); in our study we use the (Taylor1986) definitions]. The poly I:C dosage was based upon dosage ranges used by other investigators for rat intraperitoneal injection [reported dose range 0.75 to 20 mg/kg; mean dose 10 mg/kg (Fortier et al.2004b;Gilmore et al.2005)]. Based upon a study describing anorexia and weight loss associated with maternal immune activation (Fortier et al.2004b), weight change was determined in the pregnant dams over the 24 hour period following poly I:C injection. Offspring from 3 poly I:C-treated dams without weight loss were excluded from study. Poly I:C-injected dams included in the study lost 4.4 ± 0.67 grams, while vehicle-injected dams gained 5.2 ± 0.89 grams. There was no effect of prenatal treatment on miscarriage rate, litter size or offspring mortality. We have previously determined that offspring from poly I:C-injected dams without weight loss exhibit similar amphetamine-stimulated locomotion to offspring of saline-injected dams using this injection regimen (Bronson et al.2011).
Figure 1.
Experimental design summary. Animals received Restraint Stress or No Stress at postnatal day 35, and were tested for conditioned place preference to amphetamine at 3 months of age. Retention of place preference was tested at 5–6 months of age, followed by Morris water maze training. Extinction training preceded examination of drug-induced reinstatement at 7 – 8 months of age.
On postnatal day 1, litters were culled to 8 with no significant difference in number of male offspring retained (vehicle = 6.3 ± 0.8; poly I:C = 7.2 ± 0.5 SEM). Male pups were assigned to eight treatment groups (n=8 rats/group) with variables of Pre-treatment (poly I:C vs. vehicle); Post-treatment (stress vs. no stress); and drug dose (amphetamine 0.5 vs. 2.0 mg/kg). In order to guard against litter effect confounds, treatment groups were balanced across breeding cohorts. Each treatment group comprised offspring from 5–6 different dams, with no more than two offspring from a single dam assigned to the same treatment group. Rats were weaned on postnatal day 21 and housed 2/cage with same sex siblings.
Restraint stress
Half of the rats received 2 hours of restraint stress in Plexiglas restrainers as previously described (Ostrander et al.2003) at 10 am on postnatal day 35. The restraint stress regimen was based upon the prior observation that dopamine release in nucleus accumbens core is enhanced eight days following two hours of restraint stress (Pacchioni et al.2007). Rats were maintained on a 12-hour light:dark cycle (0500 on; 1700 off) and remained undisturbed in their home cages, except for routine cage changes, following restraint stress until adulthood.
All animal procedures were conducted in agreement with the Guide for the Care and Use of Laboratory Animals in accordance with NIH guidelines, and were approved by the University of Cincinnati and Cincinnati Veterans Affairs Medical Center Institutional Animal Care and Use Committees.
Drug treatment
D-amphetamine sulfate (Sigma, St. Louis, MO) was dissolved in 0.9% saline. Amphetamine concentration is described as free base. All injections were in a final volume of 1 ml/kg.
Conditioned Place Preference
Acquisition of conditioned place preference was begun at 3 months of age. Rats were singly housed in the testing room throughout the conditioning and testing phase of the study. The conditioned place preference chambers were separated from the housed rats by a plastic curtain to minimize disruptions. Testing occurred in the animals' light phase between 10am-12pm. Each conditioned place preference apparatus (Med Associates, St. Albans, VT) contains 2 large visually distinct compartments separated by a third smaller visually distinct connecting compartment. White and black compartments (each 28cm L) with either steel mesh or steel rod flooring were separated by a smaller gray compartment (12cm L) with Poly Vinyl Carbonate flooring. Dividing doors could be opened to allow exploration of other compartments. A video tracking system (CleverSys, Reston, VA) monitored the animals' position throughout each session. For initial habituation to the apparatus, rats were placed in the center compartment and allowed free exploration of all chambers for 20 minutes. The first 15 minutes of video were scored for time in each of the two large compartments for subsequent analysis, as previously described (Davis et al.2011). During the following 6 days, animals received alternating daily injections of amphetamine (0.5 or 2 mg/kg, subcutaneous) and saline and were immediately confined to the assigned compartment for 30 min. Each animal therefore received 3 amphetamine injections (0.5 or 2.0 mg/kg) in a visually distinct drug-paired compartment, and 3 saline injections in a visually distinct saline-paired compartment. An unbiased design was used to minimize potential for false-positive findings (Tzschentke1998), with animals balanced across drug-paired compartment, dam, and treatment. Each treatment group comprised 4 rats conditioned to the black compartment and 4 rats conditioned to the white compartment. When two offspring from a single dam were assigned to the same treatment group, one litter mate was conditioned to the black compartment and the other to the white compartment. Three animals randomized to the amphetamine 2.0 mg/kg group and two animals randomized to the amphetamine 0.5 mg/kg group had a strong preference (>70% total time) during the initial habituation session to the compartment to which they were subsequently drug-paired and were therefore excluded from data analysis. Twenty four hours following the last conditioning injection, place preference was determined by placing the rat in the center compartment and allowing free exploration of all chambers for 15 min. After test day, animals were returned to the animal colony paired with their original cage mate.
Animals conditioned to amphetamine 2.0 mg/kg were also analyzed for retention, extinction and reinstatement of conditioned place preference. Retention of the drug-associated preference was determined by place preference testing 2–3 months following conditioning (time interval equally balanced across treatments). Extinction training began 3 weeks after retention testing. Extinction of the conditioned preference for drug-paired chamber was achieved over 6 days in both groups by giving saline injections, followed by placement in the apparatus with full access to all compartments for 15 min. Drug-induced reinstatement was performed 24 hours following the last extinction training session by injecting a low amphetamine dose priming injection (0.2 mg/kg), followed by placement in the apparatus with full access to all compartments. Exploration was recorded for 15 min.
Morris Water Maze
Rats in the amphetamine 2.0 mg/kg conditioned place preference groups began Morris water maze training and testing 3 days after conditioned place preference retention testing. Rats were moved to the Morris water maze room for acclimation 48 hours before testing and remained housed in that location until the end of all Morris water maze procedures. The water maze pool was enclosed by plastic curtains on two sides and white walls on the opposing sides. Extra-maze visual cues (42 cm × 76 cm posters printed with contrasting shapes and patterns) were placed at N, S, E, and W positions around the pool. The water maze was a circular, fiberglass pool (122 cm in diameter, 75 cm height) filled with room temperature tap water to 43 cm deep. A clear glass platform (10.5 × 10.5 cm square) was submerged 1.0 cm below the water surface in the SE pool quadrant. Food coloring was used to reduce visibility of the hidden platform. For each learning trial, the rat was placed into the water at one of four possible starting points with similar path lengths to the platform (e.g., SW, W, N, NE) (Vorhees et al.2009). The starting location for each trial was varied and all locations were used once before repeating. A trial was terminated and latency recorded when the rat found and climbed onto the platform for 5 sec. If the rat did not reach the platform within 90 sec., the trial was terminated and the rat placed upon the platform for 5 sec. Each rat received two trials per day, separated by at least one hour, for four consecutive days. On the fifth and final day of testing, a 90 sec. probe trial was performed with the rats started from a novel position (NW) and the escape platform removed from the pool.
The reversal learning phase of the Morris water maze was begun three days following the probe trial test. For this procedure the platform was moved to a novel location in the NW pool quadrant, with all procedures otherwise identical to the acquisition phase (2 trials/ day for 4 days). The starting points were shifted to SW, S, E, and NE. On the fifth day, 24 hours after the last learning trial, a reversal phase probe trial was performed with the rats started from a novel position (SE) with the platform again removed from the pool. Trials were digitally recorded using CleverSys TopScan software.
Statistical analysis
Conditioned place preference
Acquisition, retention, extinction (averaged over 6 training sessions) and reinstatement data were analyzed individually by two-way ANOVA using SAS ® System for Windows Version 9.2, (SAS Institute, Cary, NC) with experiment-wise error rate set at p < 0.05. The outcome measure for conditioned place preference data was analysis of between-group differences using the “difference score”, defined as the difference in preference for the drug-paired chamber on test day compared to the same chamber on habituation day (Navarro et al.2001). A positive difference score indicates a conditioned place preference for the drug-paired chamber. Pre-treatment (Poly I:C vs. Vehicle) and Post-treatment (Stress vs. No stress) were used as main factors and difference score as the dependent measure.
Morris water maze
Data for learning trials were analyzed by three-way repeated measures ANOVA, with Pre-treatment (Poly I:C vs. Vehicle) and Post-treatment (Stress vs. No stress) as main factors, individual day as the repeated measure and change in latency to platform from trial 1 to trial 2 on each of four individual days of training as the dependent measure. Subsequent multiple comparisons were conducted by post-hoc analysis using the Tukey-Kramer test. Treatment differences were considered statistically significant at p < 0.05. Probe trials were analyzed by Student's t-test with latency to former platform location; direct hits on platform location; percent time in a target area centered around the platform location; and number of entries into a target area centered around the platform location as outcome measures.
Conditioned Place Preference / Morris Water Maze Correlations
Data were analyzed by Pearson correlation to test for correlations between Morris water maze reversal learning performance and acquisition, retention, extinction, and reinstatement of conditioned place preference. Correlations were determined between decrease in latency from trial 1 to trial 2 averaged over the first three days of reversal learning, and the difference score on each day of testing for conditioned place preference.
Results
Conditioned place preference
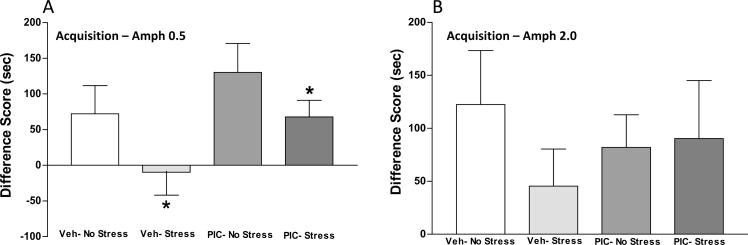
In order to determine the effect of prenatal immune activation on drug-associated learning and memory, conditioned place preference to amphetamine was tested at two amphetamine dosages (0.5 and 2.0 mg/kg) in offspring of vehicle- and poly I:C-injected dams. Because developmental stress may exert longstanding effects upon conditioned place preference to stimulant drugs (Mathews et al.2008;Campbell and Spear1999), half of the animals received two hours of restraint stress at postnatal day 35 in order to test for potential interactions between developmental stress and prenatal immune activation on outcome. During the initial habituation to the place preference apparatus prior to conditioning, ANOVA did not identify significant main effects or interactions between treatment groups (data not shown). The acquisition of conditioned place preference following amphetamine (0.5 mg/kg) exposure is shown in Figure 2A. Two-way ANOVA identified a significant effect of Post-treatment [F(1,26) = 4.38, p = 0.046], a trend for Pre-treatment which did not achieve statistical significance [F(1,26) = 3.87, p = 0.06], and no significant interaction [F(1,26) = 0.08, p = 0.78]. Post-treatment stress decreased place preference acquisition in both Vehicle/Stress and Poly I:C/Stress groups. Of interest, the negative difference score in the Vehicle/Stress group demonstrates lack of a conditioned response to the drug-paired compartment. Acquisition of conditioned place preference was also tested following exposure to a higher amphetamine dose (2.0 mg/kg). As shown in Figure 2B all groups demonstrated place preference conditioning, and there were no significant group differences identified by two-way ANOVA at the higher dose.
Figure 2.
Acquisition of chamber preference following six days of conditioning to amphetamine 0.5 (A) or 2.0 mg/kg (B). Difference score represents difference in preference for drug-paired chamber on test day compared to the same chamber on habituation day. Positive values indicate preference for drug-paired chamber. A): Restraint stress reduced acquisition of conditioned place preference. *(p<0.05 No Stress vs. Stress). The effect of Pre-treatment (Poly I:C) did not reach statistical significance [F(1,26) = 3.87, p = 0.06]. B): Two-way ANOVA did not identify significant group differences in acquisition of place preference to amphetamine 2.0 mg/kg. PIC: poly I:C.
Retention, extinction and reinstatement of conditioned place preference
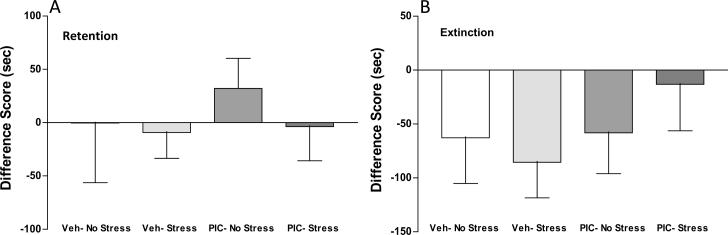
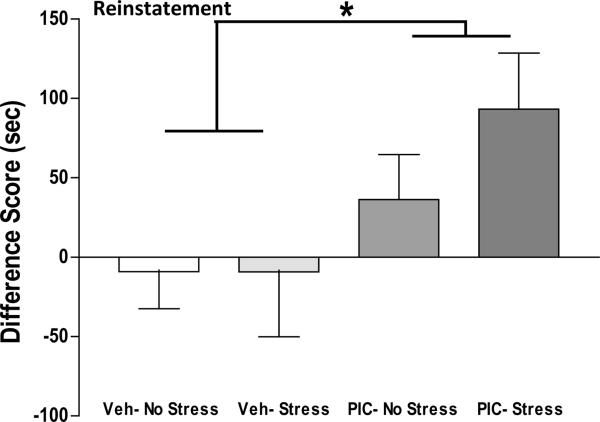
Animals conditioned to amphetamine 2.0 mg/kg were also analyzed for retention, extinction and reinstatement of conditioned place preference. Retention of the drug-associated preference was determined by place preference testing 2–3 months following conditioning (time interval equally balanced across the four Pre-treatment × post-treatment groups). As illustrated in Figure 3A, two-way ANOVA did not identify statistically significant group differences in difference scores at retention testing. Extinction of the conditioned preference for drug-paired compartment was achieved over 6 days in all groups by giving saline injections, followed by placement in the apparatus with full access to all compartments for 15 min. Two-way ANOVA did not identify statistically significant group differences in difference scores after extinction training. As seen in Figure 3B, negative difference scores in all groups evidences extinction of place preference following this regimen. Drug-induced reinstatement was performed by injecting a low dose of amphetamine priming injection (0.2 mg/kg), followed by placement in the apparatus with full access to all compartments. As shown in Figure 4, two-way ANOVA of reinstatement difference scores identified a significant main effect of Pre-treatment [F(1,25) = 5.31, p = 0.03], demonstrating a long-lasting effect of poly I:C treatment on reinstatement of preference for drug-paired compartment. ANOVA did not identify a significant Post-treatment effect [F(1,25) = 0.78, p = 0.39], or a Pre-treatment × Post-treatment interaction [F(1,25) = 0.79, p = 0.38]. Of interest, the Poly I:C/Stress group spent 2.5-fold more time in the drug-paired compartment compared to the Poly I:C/No Stress animals (PIC/No Stress = 36.4 ± 28.1 s, PIC/Stress = 93.2 ± 35.3 s SEM). Further study will be needed to determine if the trend towards enhanced reinstatement combining stress with prenatal immune activation achieves statistical significance with a larger sample size.
Figure 3.
(A): Retention of chamber preference. Testing for place preference was performed 2–3 months following conditioning to amphetamine 2.0 mg/kg. ANOVA did not identify group differences in Difference score (difference in preference for drug-paired chamber on test day compared to the same chamber on habituation day). (B). Chamber preference following extinction regimen delivered over 6 days by giving saline injections, followed by placement in the apparatus with full access to all compartments. Negative values indicate lack of preference for drug-paired chamber. ANOVA did not identify group differences. PIC: poly I:C
Figure 4. Poly I:C enhances reinstatement of drug-paired place preference.
All groups received a priming amphetamine injection (0.2 mg/kg) prior to placement in testing chambers. Positive values indicate a preference for drug-paired chamber. ANOVA demonstrates significantly greater difference score in poly I:C compared to vehicle groups *(p < 0.05).
Morris Water Maze
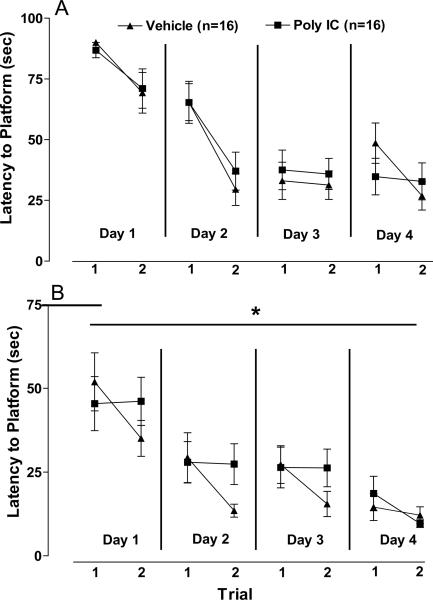
In order to determine if cognitive deficits in reversal learning were observed in offspring of poly I:C-injected dams, animals were tested for performance in a Morris water maze reversal learning paradigm. Rats were trained in a Morris water maze hidden platform task at 5–6 months of age as described in Methods above. Learning trials data were analyzed by three-way repeated measures ANOVA, with Pre-treatment (Poly I:C vs. Vehicle) and Post-treatment (Stress vs. No stress) as main factors, day as repeated measure and improvement in latency to platform between trial 1 and trial 2 each day as dependent measure. ANOVA identified a significant main effect of day [F(1,28) = 4.22, p =0.049]. Pre-treatment, Post-treatment, and Pre-treatment × Post-treatment interactions were not significant. Because Post-treatment and Pre-treatment × Post-treatment interactions were not significant, data were collapsed across Stress Post-treatment groups for further analyses of poly I:C effects. As seen in Figure 5A, offspring of vehicle- and poly I:C- injected dams learned the hidden platform location at similar rates and evidenced comparable improvements from trial 1 to trial 2 over the 4 training days. When the hidden platform was removed for probe trial testing, there were no significant group differences in latency to the former platform location [F(30) = 0.56, p = 0.58], hits on the platform location [F(30) = 0.00, p = 1.00], percent time in target area centered around hidden platform [F(30) = 0.33, p = 0.75] or entries into target area [F(30) = 0.36, p = 0.72].
Figure 5. Morris water maze hidden platform task learning (A) and reversal learning (B).
Latency to hidden platform location is shown for first and second learning trials. A): Vehicle and poly I:C offspring did not differ in change in latency from trial 1 to trial 2 during initial task learning. B): Poly I:C offspring demonstrate impaired improvement between daily trials 1 and 2 in learning the new platform location compared to vehicle offspring *(p<0.05).
Following completion of the initial platform training and probe trial, the hidden platform location was changed, and animals were trained and tested on learning a new platform location in a reversal learning paradigm. ANOVA of reversal learning data identified a significant main effect of Pre-treatment [F(1,28) = 7.21, p =0.012] and Pre-treatment × day interaction [F(1,28) = 5.33, p =0.029], but no effect of day, Post-treatment, or Pre-treatment × Post-treatment interactions. Because ANOVA did not identify significant effects of Post-treatment or Pre-treatment × Post-treatment interaction, data were collapsed across Stress Post-treatment groups for further analyses (Figure 5B). Post hoc analysis corrected for multiple comparisons identified a significant difference between poly I:C and vehicle offspring in improvement in latency to the hidden platform between trials 1 and 2 (p=0.041). The hidden platform was removed for probe trial testing after the fourth day of reversal learning. There were no significant group differences in latency to the new platform location [F(30) = 0.56, p = 0.54], hits on the platform location [F(30) = 1.24, p = 0.23], percent time in target area centered around hidden platform [F(30) = 0.06, p = 0.95], or entries into target area [F(30) = 0.10, p = 0.92]. Thus, while offspring of poly I:C-treated dams exhibit normal learning and retention of spatial object information as evidenced by normal Morris water maze acquisition and probe trial performance (5A), in the reversal learning paradigm poly I:C offspring demonstrate a delay in learning the new platform location (5B).
Conditioned Place Preference/ Morris Water Maze Correlations
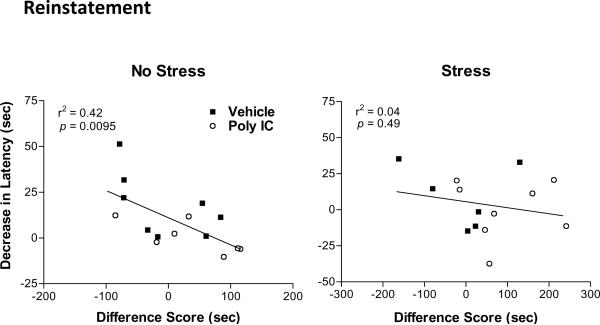
Data were analyzed to test for correlations between Morris water maze reversal learning performance and acquisition, retention, extinction, and reinstatement of conditioned place preference. No statistically significant correlations were identified analyzing the four different poly I:C vs. vehicle and stress vs. non-stress groups individually (Table I, Groups I – IV). Trends in correlation were observed in both non-stress groups between reversal learning performance and reinstatement of place preference [poly I:C/No-stress (p = 0.059); Vehicle/No-stress (p = 0.13)]. Collapsing the data across Stress/No-stress Post-treatment groups for analysis of poly I:C and vehicle effects failed to demonstrate any significant correlations between reversal learning and place preference performance (Table 1, Groups VII– VIII). In contrast, as seen in Table 1 (Group V) and Figure 6, collapsing the data across poly I:C/vehicle Pre-treatment groups identified a strong correlation in non-stressed animals between Morris water maze reversal learning performance and reinstatement of conditioned place preference (r2 = 0.42, p = 0.0095). Of interest, there was no correlation between reversal learning and reinstatement of conditioned place preference in restraint stressed animals (r2 =0.04, p = 0.49).
Table 1.
Correlations between Morris water maze reversal learning performance and acquisition, retention, extinction, and reinstatement of conditioned place preference to amphetamine. Acquisition, retention, extinction, and reinstatement of conditioned place preference are quantified as difference score for drug-paired compartment.
| Acquisition | Retention | Extinction | Reinstatement | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Group | r2 | p | r2 | p | r2 | p | r2 | p | |
| I. | Vehicle – No Stress | 0.049 | 0.60 | 0.026 | 0.73 | 0.025 | 0.71 | 0.33 | 0.13 |
| II. | Poly I:C – No Stress | 0.025 | 0.74 | 0.35 | 0.21 | 0.006 | 0.87 | 0.54 | 0.059 |
| III. | Vehicle - Stress | 0.38 | 0.20 | 0.61 | 0.12 | 0.18 | 0.40 | 0.051 | 0.67 |
| IV. | Poly I:C - Stress | 0.007 | 0.84 | 0.0001 | 0.99 | 0.008 | 0.83 | 0.0003 | 0.97 |
| V. | No Stress | 0.073 | 0.33 | 0.0015 | 0.90 | 0.008 | 0.75 | 0.42 | 0.0095* |
| VI. | Stress | 0.033 | 0.54 | 0.05 | 0.48 | 0.011 | 0.72 | 0.04 | 0.49 |
| VII. | Vehicle | 0.15 | 0.18 | 0.0004 | 0.95 | 0.007 | 0.78 | 0.13 | 0.20 |
| VIII. | Poly I:C | 0.009 | 0.73 | 0.011 | 0.73 | 0.007 | 0.77 | 0.026 | 0.56 |
Figure 6. Correlations between reinstatement of conditioned place preference and Morris water maze reversal learning performance.
Morris water maze reversal learning performance is calculated as decrease in latency to platform location between first and second learning trials averaged over training days 1 – 3. Reinstatement of conditioned place preference is measured as difference score for drug-paired compartment. Left: No stress animals. Right: Stress animals
Discussion
Because learning processes underlying the acquisition, extinction, and reinstatement of drug-paired associations play such a key role in human addictions, it has been suggested that drug dependence is best considered a disease of learning and memory (Kelley2004;Hyman2005). Drug dependence results from a combination of genetic and environmental influences, and it is therefore of interest to identify specific environmental factors impacting drug-related learning and memory. Here we report the effects of prenatal immune activation, a relatively common in utero occurrence (Brown and Derkits2010), on drug-associated learning and memory using the conditioned place preference classical conditioning paradigm. Because early stress exposure elevates adult vulnerability to drug dependence (Dube et al.2003;Andersen and Teicher2009), we also determined the influence of developmental stress on outcome measures. The data presented suggest four areas of potential relevance to human drug addiction. First, prenatal immune activation exerts a strong and lasting enhancement on drug-induced reinstatement of drug-associated memory (Figure 4), a finding which suggests this environmental exposure might potentially increase risk for relapse to drug dependence. Second, in agreement with some (Meyer et al.2006;Han et al.2011;Ito et al.2010;Lee et al.2007) [but not all (Zuckerman and Weiner2005;Meyer et al.2005)] studies, we identified deficits in reversal learning following prenatal immune activation (Figure 5), suggesting the potential for this domain of impaired cognition to contribute to enhanced reinstatement of conditioned place preference. Third, we identified an unexpected effect of stress on the relationship between reversal learning deficits and reinstatement of conditioned place preference (Figure 6). In non-stressed animals there is a direct and lasting relationship, suggesting a “trait” effect, between individual differences in reversal learning in the Morris water maze task and individual differences in reinstatement of conditioned place preference to amphetamine. In animals receiving postnatal day 35 restraint stress, this relationship is no longer apparent. Finally, in offspring of vehicle-treated dams a single episode of peri-adolescent restraint stress inhibited acquisition of place preference to a low dose of amphetamine (0.5 mg/kg, Figure 2A).
Study limitations
The data do not identify an influence of prenatal immune activation on acquisition of place preference for amphetamine (Figure 2). It is possible that effects of prenatal immune activation on acquisition of conditioned place preference may be identified using a lower amphetamine dose, shorter acquisition training regimen, or larger sample size, since a trend towards enhanced acquisition following prenatal immune activation was observed in low dose amphetamine groups [Figure 2A, Pre-treatment effect (p = 0.06)]. We also did not observe a statistically significant effect of poly I:C injection or stress on extinction of the conditioned response to amphetamine. Because formal extinction training was performed several months following conditioning, the active learning process of extinction was likely occurring throughout the interval between acquisition and retention testing. Further study is needed to more adequately assess the extinction process following prenatal immune activation. Finally, training and testing were performed in the light phase of the circadian cycle, in order to assess animals in the same phase as prior studies demonstrating altered locomotor response to stimulant drugs following prenatal immune activation (Bronson et al.2011;Ozawa et al.2006;Richtand et al.2011). Since rats are a nocturnal species, it will be of great interest to determine drug-related learning and memory during the night phase of their light/dark cycle.
Potential interactions between stress and prenatal immune activation are also not fully addressed by the data presented above. While a single peri-adolescent stress exposure elevated the effect of prenatal immune activation by 2.5-fold (Figure 4.), this enhancement was not statistically significant with the sample size studied. The ANOVA did not identify statistically significant effects of stress (p = 0.39), or a poly I:C x stress interaction (p = 0.38). Further study with a larger sample size and stronger stress paradigms will be needed to adequately address potential effects of stress in this model.
Of interest, restraint stress inhibited acquisition of place preference for a low dose of amphetamine (Figure 2A). In offspring of vehicle-treated dams, the effect of stress resulted in a negative difference score, suggesting the restraint-stressed vehicle animals did not develop a conditioned place preference to the drug-paired chamber. While this outcome was unexpected, a prior study examining the effect of social stress on conditioned place preference to amphetamine observed a similar outcome: conditioned place preference to a low (0.5 mg /kg) amphetamine dose was decreased in rats exposed to a social stressor (1 hr of isolation, followed by housing with a new cage partner) throughout postnatal days 30–45 (Mathews et al.2008). While the two studies examined different rat strains (Long-Evans vs. Sprague-Dawley), stress paradigms, and stress timing, the findings in both studies are similar. Both restraint stress (Serrano et al.1989) and amphetamine 0.5 mg/kg (Kuczenski and Segal1989) elevate dopamine release in nucleus accumbens. It is unclear how restraint stress might decrease the sensitivity to subsequent acquisition of a conditioned association to injection of a low dose of amphetamine. Additional study will be needed to further define the inhibitory effects of stressors on acquisition of drug associated conditioning, identify underlying mechanism(s), and determine if this might have clinically meaningful correlates in human populations.
Conditioned place preference limitations
The conditioned place preference paradigm has inherent limitations which must be recognized in interpretation of this data. Conditioned place preference is a classical learning paradigm which measures the learned association linking a drug experience and its environment. The learned place preference induced by drugs of abuse can be extinguished, and then reinstated by drug or stress priming. Additionally, while conditioned place preference studies provide a measure of the reinforcing property of a learned association and have contributed to the current understanding of mechanisms underlying drug relapse (Epstein et al.2006;Liu et al.2008;Shaham et al.2003), as well as development of potential treatments (Tzschentke2007), conditioned place preference data does not provide a direct index of drug self-administration. The conditioned place preference data presented here therefore suggest it will be of great interest to determine the effects of prenatal immune activation on drug self-administration.
Future directions
The circuits and cellular mechanisms mediating drug-associated learning and memory have been well-described by research which has also identified the consequences of repetitive drug use upon these processes. These studies demonstrate important roles for glutamatergic excitatory neurotransmission, and dopaminergic modulation, in the acquisition and extinction as well as reinstatement of drug-associated memories, suggesting potential mechanisms mediating the behavioral effects identified above. Extinction of drug-associated memories is an active process, engaging circuits which include dopamine projections from ventral tegmentum to prefrontal cortex, and glutamate projections from prefrontal cortex to nucleus accumbens shell (McFarland et al.2004;McFarland and Kalivas2001;See2002). Reinstatement of drug use in addiction also results in part from impaired frontal cortical function as a consequence of repetitive drug exposure, resulting in a subsequent loss of inhibitory control. When coupled with enhancement of the incentive motivational properties of both drugs and stimuli associated with drug use, these adaptations act in concert to enhance relapse to drug addiction. Dopaminergic projections from ventral tegmentum to prefrontal cortex and nucleus accumbens, glutamatergic projections from prefrontal cortex to accumbens, and projections from basolateral amygdale to prefrontal cortex and accumbens have been identified as critically important components of the neural circuitry mediating drug relapse (Everitt and Robbins2005;Goldstein and Volkow2002;Jentsch and Taylor1999;Kalivas and O'Brien2008). Thus, potential neural mechanisms mediating the behavioral effects observed in our study have been previously described.
Of particular relevance to potential mechanisms of enhanced reinstatement, prior studies suggest prenatal immune activation alters function in circuits playing an important role in drug-associated learning and memory described above. Altered dopamine function within nucleus accumbens core, the site of integration of drug-associated memories (Kalivas and O'Brien2008), has been identified in offspring of poly I:C-injected mice (Vuillermot et al.2010), and abnormalities have been consistently observed in indices of dopamine system function following prenatal immune activation (Bakos et al.2004;Borrell et al.2002;Ling et al.2002;Ozawa et al.2006;Romero et al.2010;Zuckerman et al.2003). Additionally, abnormalities in glutamatergic systems required for extinction of drug-associated memories have been identified following prenatal immune activation including elevated basal extracellular glutamate in prefrontal cortex (Richtand et al.2010) and hippocampus (Ibi et al.2009), as well as decreased NMDA receptor-dependent synaptic current and plasticity (Lante et al.2007;Escobar et al.2011). Behavioral abnormalities of potential relevance to conditioned place preference to amphetamine have also been observed in offspring following maternal poly I:C injection, including reversal learning deficits (Meyer et al.2006;Lee et al.2007) and altered locomotor response to amphetamine and methamphetamine (Ozawa et al.2006;Fortier et al.2004a;Bakos et al.2004;Meyer et al.2005;Meyer et al.2008a;Meyer et al.2008b;Zuckerman et al.2003). It will be of particular interest to identify mechanistic linkages between these observations and long-standing alterations in glial cell function following prenatal immune activation (Bilbo and Schwarz2009;Bland et al.2010;Meyer et al.2011). Drugs of abuse stimulate cytokine production by glial cells within the nucleus accumbens, and the effect of early developmental environment upon this glial activation influences reinstatement of conditioned place preference to morphine (Schwarz et al.2011). These observations suggest changes in neuroimmune function following prenatal immune activation may play a critical role in altered function of the limbic pathways regulating reinstatement of conditioned place preference.
Public health implications
Drug policy focuses in part upon intervening in younger populations prior to the onset of drug abuse, thereby lowering relapse risk by preventing consequences of repetitive drug use. Our data suggest an alternative possibility, that relapse risk might be elevated in individuals with a history of prenatal immune activation independent of repetitive drug exposure. That is consistent with reports from some drug-addicted patients of being “hooked” on their first drug exposure. Our findings may also represent an initial step in establishing maternal inflammatory pathways in learning effects associated with drug use in the offspring, which will inform gene x environment interaction studies needed to identify genetic contributions to complex diseases (Clarke et al.2009) such as drug dependence. The data presented may also suggest a potential link to underlying mechanisms for research implicating prenatal stress as a risk factor for drug dependence (Thomas et al.2009;Campbell et al.2009;Kippin et al.2008). And finally, mechanisms contributing to enhanced relapse may differ in individuals with a history of prenatal immune activation compared to other addicted patients. It will be of particular interest to determine the potential for new specific prevention and treatment interventions for individuals with this environmental exposure. These exposures impart long-lasting effects upon the offspring identifiable through altered immunological and inflammatory response (Surriga et al.2009), neuroimaging (Piontkewitz et al.2009;Piontkewitz et al.2010), and neuropsychological function (Bitanihirwe et al.2010). Thus, biological markers to identify individuals with a history of prenatal inflammatory exposures might potentially be utilized to improve prevention, treatment, and understanding of prognosis for drug addictions.
The results presented also suggest unaddressed questions. First, further study with a larger sample size is needed to determine if peri-adolescent stress enhances the effect of prenatal immune activation in elevating reinstatement of conditioned place preference. It will also be of interest to determine whether other types of stress, such as physiological stress, or stress during other developmental windows, interact with prenatal immune activation to influence drug associated learning and memory. Second, drug and stress-induced reinstatement of conditioned place preference may be mediated by different pathways (Aguilar et al.2009), with the prefrontal cortical – nucleus accumbens pathway mediating stimulant-induced reinstatement, while stress-induced reinstatement requires an intact prefrontal cortex – ventral tegmentum - nucleus accumbens pathway (Sanchez et al.2003). Because stress plays an important role in relapse to drug addiction (Sinha2001;Lu et al.2003), identifying the influence of prenatal immune activation on stress-induced reinstatement would further extend the relevance of this finding to human drug addiction. Finally, further study will be needed to extend the public health application of these findings by determining if similar outcomes occur with exposure during different pregnancy stages; with lower poly I:C dosages; in response to different cytokines; and can be blocked by blocking maternal inflammatory pathways.
Acknowledgements
This work was supported by the Department of Veterans Affairs Medical Research Service and National Institute of Mental Health (R21MH083192-01). The experiments described in this manuscript comply with the current laws of the United States of America. The authors disclose the following relationships which might potentially bias this work:
Neil M. Richtand: Consultant: Bristol-Meyers Squibb, Gerson Lehrman Group, Sunovion Pharmaceuticals Inc./ Sepracor. Speaker's Bureau: Bristol-Meyers Squibb, Otsuka America Pharmaceutical, Schering - Plough Corporation/ Merck, Novartis Pharmaceuticals, Sunovion Pharmaceuticals Inc./ Sepracor. Grant/Research Support: Ortho-McNeil Janssen Scientific Affairs, LLC; AstraZeneca Pharmaceuticals
Reference List
- Aguilar MA, Rodriguez-Arias M, Minarro J. Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res Rev. 2009;59:253–277. doi: 10.1016/j.brainresrev.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Desperately driven and no brakes: developmental stress exposure and subsequent risk for substance abuse. Neurosci Biobehav Rev. 2009;33:516–524. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakos J, Duncko R, Makatsori A, Pirnik Z, Kiss A, Jezova D. Prenatal immune challenge affects growth, behavior, and brain dopamine in offspring. Ann N Y Acad Sci. 2004;1018:281–287. doi: 10.1196/annals.1296.033. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. Epub@2009 Aug 24.:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Weber L, Feldon J, Meyer U. Cognitive impairment following prenatal immune challenge in mice correlates with prefrontal cortical AKT1 deficiency. Int J Neuropsychopharmacol. 2010:1–16. doi: 10.1017/S1461145710000192. [DOI] [PubMed] [Google Scholar]
- Bland ST, Beckley JT, Young S, Tsang V, Watkins LR, Maier SF, et al. Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain Behav Immun. 2010;24:329–338. doi: 10.1016/j.bbi.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell J, Vela JM, Arevalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;26:204–215. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- Bronson SL, Ahlbrand R, Horn PS, Kern JR, Richtand NM. Individual differences in maternal response to immune challenge predict offspring behavior: Contribution of environmental factors. Behav Brain Res. 2011;220:55–64. doi: 10.1016/j.bbr.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley PF. Prevalence and consequences of the dual diagnosis of substance abuse and severe mental illness. J Clin Psychiatry. 2006;67(Suppl 7):5–9. 5–9. [PubMed] [Google Scholar]
- Campbell J, Spear LP. Effects of early handling on amphetamine-induced locomotor activation and conditioned place preference in the adult rat. Psychopharmacology (Berl) 1999;143:183–189. doi: 10.1007/s002130050934. [DOI] [PubMed] [Google Scholar]
- Campbell JC, Szumlinski KK, Kippin TE. Contribution of early environmental stress to alcoholism vulnerability. Alcohol. 2009;43:547–554. doi: 10.1016/j.alcohol.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MC, Tanskanen A, Huttunen M, Whittaker JC, Cannon M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry. 2009;166:1025–1030. doi: 10.1176/appi.ajp.2009.08010031. [DOI] [PubMed] [Google Scholar]
- Davis JF, Choi DL, Shurdak JD, Krause EG, Fitzgerald MF, Lipton JW, et al. Central melanocortins modulate mesocorticolimbic activity and food seeking behavior in the rat. Physiol Behav. 2011;102:491–495. doi: 10.1016/j.physbeh.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111:564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar M, Crouzin N, Cavalier M, Quentin J, Roussel J, Lante F, et al. Early, time-dependent disturbances of hippocampal synaptic transmission and plasticity after in utero immune challenge. Biol Psychiatry. 2011;70:992–999. doi: 10.1016/j.biopsych.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Joober R, Luheshi GN, Boksa P. Maternal exposure to bacterial endotoxin during pregnancy enhances amphetamine-induced locomotion and startle responses in adult rat offspring. J Psychiatr Res. 2004a;38:335–345. doi: 10.1016/j.jpsychires.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2004b;287:R759–R766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol. 2005;159:106–112. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Li N, Meng Q, Shao F, Wang W. Maternal Immune Activation Impairs Reversal Learning and Increases Serum Tumor Necrosis Factor-alpha in Offspring. Neuropsychobiology. 2011;64:9–14. doi: 10.1159/000322455. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Ibi D, Nagai T, Kitahara Y, Mizoguchi H, Koike H, Shiraki A, et al. Neonatal polyI:C treatment in mice results in schizophrenia-like behavioral and neurochemical abnormalities in adulthood. Neurosci Res. 2009;64:297–305. doi: 10.1016/j.neures.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Ito HT, Smith SE, Hsiao E, Patterson PH. Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain Behav Immun. 2010;24:930–941. doi: 10.1016/j.bbi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Szumlinski KK, Kapasova Z, Rezner B, See RE. Prenatal stress enhances responsiveness to cocaine. Neuropsychopharmacology. 2008;33:769–782. doi: 10.1038/sj.npp.1301447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal D. Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci. 1989;9:2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lante F, Meunier J, Guiramand J, Maurice T, Cavalier M, Jesus Ferreira MC, et al. Neurodevelopmental damage after prenatal infection: role of oxidative stress in the fetal brain. Free Radic Biol Med. 2007;42:1231–1245. doi: 10.1016/j.freeradbiomed.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Lee KH, Smith SE, Kim S, Patterson PH, Thompson RF. Maternal immune activation impairs extinction of the conditioned eye blink response in the adult offspring. 2007. [Google Scholar]
- Ling Z, Gayle DA, Ma SY, Lipton JW, Tong CW, Hong JS, et al. In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain. Mov Disord. 2002;17:116–124. doi: 10.1002/mds.10078. [DOI] [PubMed] [Google Scholar]
- Liu Y, Le Foll B, Liu Y, Wang X, Lu L. Conditioned place preference induced by licit drugs: establishment, extinction, and reinstatement. ScientificWorldJournal. 2008;8:1228–1245. doi: 10.1100/tsw.2008.154. 1228–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, Mills RG, McCormick CM. Chronic social stress in adolescence influenced both amphetamine conditioned place preference and locomotor sensitization. Dev Psychobiol. 2008;50:451–459. doi: 10.1002/dev.20299. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008a;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Schwarz MJ, Muller N. Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther. 2011;132:96–110. doi: 10.1016/j.pharmthera.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Meyer U, Spoerri E, Yee BK, Schwarz MJ, Feldon J. Evaluating Early Preventive Antipsychotic and Antidepressant Drug Treatment in an Infection-Based Neurodevelopmental Mouse Model of Schizophrenia. Schizophr Bull. 2008b doi: 10.1093/schbul/sbn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Carrera MR, Fratta W, Valverde O, Cossu G, Fattore L, et al. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21:5344–5350. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- Ostrander MM, Richtand NM, Herman JP. Stress and amphetamine induce Fos expression in medial prefrontal cortex neurons containing glucocorticoid receptors. Brain Res. 2003;990:209–214. doi: 10.1016/j.brainres.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Pacchioni AM, Cador M, Bregonzio C, Cancela LM. A glutamate-dopamine interaction in the persistent enhanced response to amphetamine in nucleus accumbens core but not shell following a single restraint stress. Neuropsychopharmacology. 2007;32:682–692. doi: 10.1038/sj.npp.1301080. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Risperidone Administered During Asymptomatic Period of Adolescence Prevents the Emergence of Brain Structural Pathology and Behavioral Abnormalities in an Animal Model of Schizophrenia. Schizophr Bull. 2010 doi: 10.1093/schbul/sbq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkewitz Y, Assaf Y, Weiner I. Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biol Psychiatry. 2009;66:1038–1046. doi: 10.1016/j.biopsych.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Richtand NM, Ahlbrand RL, Horn PS, Stanford KE, Bronson SL, McNamara RK. Effects of Risperidone and Paliperidone Pretreatment on Locomotor Response Following Prenatal Immune Activation. J Psychiatr Res. 2011;45:1194–1201. doi: 10.1016/j.jpsychires.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtand NM, Roenker N, Lindquist DM, Ahlbrand RL, Gudelsky GA. Early intervention In a Hypoglutamatergic model with relevance to Schizophrenia. 67 ed. 2010. [Google Scholar]
- Romero E, Guaza C, Castellano B, Borrell J. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia. Mol Psychiatry. 2010;15:372–383. doi: 10.1038/mp.2008.44. [DOI] [PubMed] [Google Scholar]
- Sanchez CJ, Bailie TM, Wu WR, Li N, Sorg BA. Manipulation of dopamine d1-like receptor activation in the rat medial prefrontal cortex alters stress- and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience. 2003;119:497–505. doi: 10.1016/s0306-4522(03)00078-2. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Hutchinson MR, Bilbo SD. Early-Life Experience Decreases Drug-Induced Reinstatement of Morphine CPP in Adulthood via Microglial-Specific Epigenetic Programming of Anti-Inflammatory IL-10 Expression. J Neurosci. 2011;31:17835–17847. doi: 10.1523/JNEUROSCI.3297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Serrano A, D'Angio M, Scatton B. NMDA antagonists block restraint-induced increase in extracellular DOPAC in rat nucleus accumbens. Eur J Pharmacol. 1989;162:157–166. doi: 10.1016/0014-2999(89)90616-x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Suh HS, Brosnan CF, Lee SC. Toll-like receptors in CNS viral infections. Curr Top Microbiol Immunol. 2009;336:63–81. doi: 10.1007/978-3-642-00549-7_4. 63–81. [DOI] [PubMed] [Google Scholar]
- Surriga O, Ortega A, Jadeja V, Bellafronte A, Lasala N, Zhou H. Altered hepatic inflammatory response in the offspring following prenatal LPS exposure. Immunol Lett. 2009;123:88–95. doi: 10.1016/j.imlet.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Taylor P. Practical Teratology. Academic Press; London: 1986. [Google Scholar]
- Thomas MB, Hu M, Lee TM, Bhatnagar S, Becker JB. Sex-specific susceptibility to cocaine in rats with a history of prenatal stress. Physiol Behav. 2009;97:270–277. doi: 10.1016/j.physbeh.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Johnson HL, Burns LN, Williams MT. Developmental treatment with the dopamine D2/3 agonist quinpirole selectively impairs spatial learning in the Morris water maze. Neurotoxicol Teratol. 2009;31:1–10. doi: 10.1016/j.ntt.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Vuillermot S, Weber L, Feldon J, Meyer U. A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. J Neurosci. 2010;30:1270–1287. doi: 10.1523/JNEUROSCI.5408-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]