Abstract
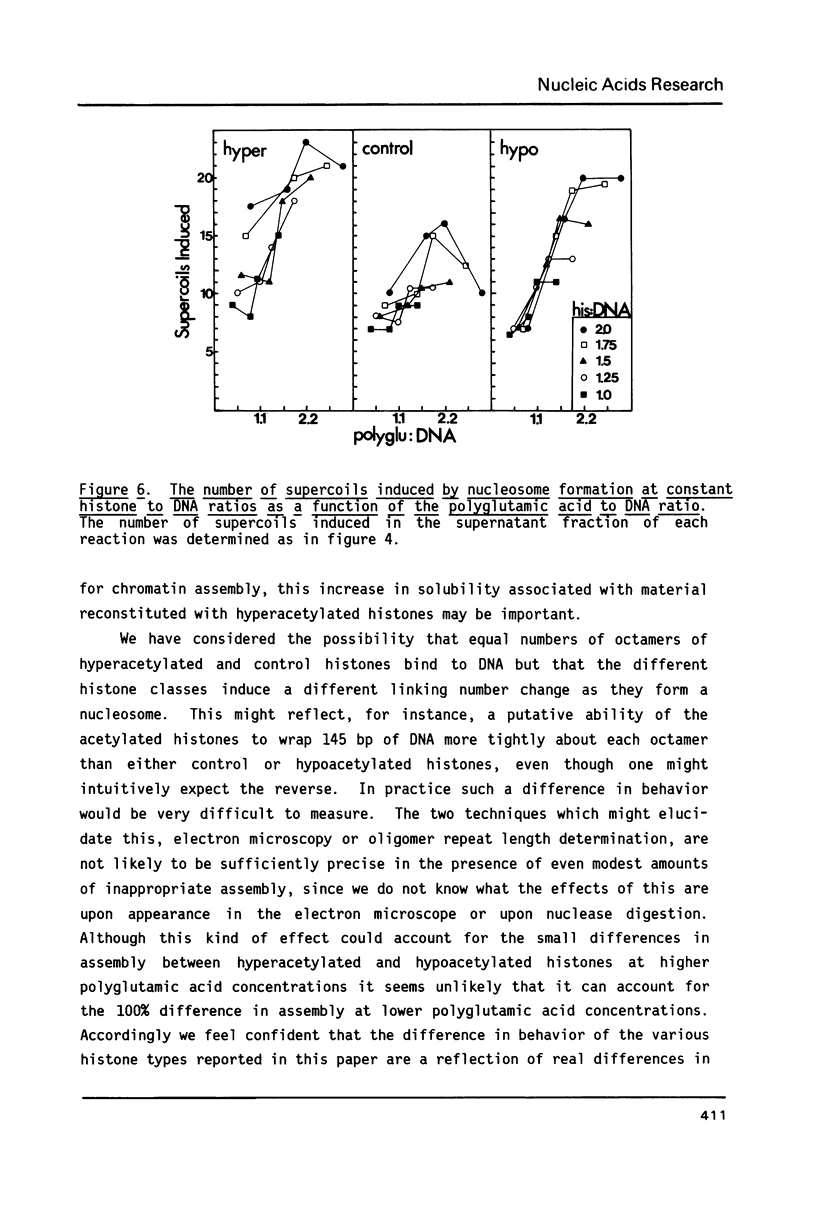
We have examined the effect of histone acetylation on the in vitro assembly of nucleosomes with DNA and purified histones at physiological ionic strength in the presence of polyglutamic acid. We have found that hyperacetylated histones assemble nucleosomes with greater efficiency, and to a greater extent, than either control or hypoacetylated histones. Assembly reactions were performed over a range of histone to DNA ratios (0.25 to 3.0, w/w) and polyglutamic acid to histone ratios (0 to 1.6, w/w). Although polyglutamic acid may act as a sink to prevent nonspecific histone-DNA interactions, our data suggest that the polyanion primarily facilitates the assembly of nucleosomes by organizing histones into a form that is amenable to deposition.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfageme C. R., Zweidler A., Mahowald A., Cohen L. H. Histones of Drosophila embryos. Electrophoretic isolation and structural studies. J Biol Chem. 1974 Jun 25;249(12):3729–3736. [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Fractionation and characterization of chromosomal proteins by the hydroxyapatite dissociation method. J Biol Chem. 1978 Jun 25;253(12):4446–4450. [PubMed] [Google Scholar]
- Covault J., Sealy L., Schnell R., Shires A., Chalkley R. Histone hypoacetylation following release of HTC cells from butyrate. J Biol Chem. 1982 May 25;257(10):5809–5815. [PubMed] [Google Scholar]
- Earnshaw W. C., Honda B. M., Laskey R. A., Thomas J. O. Assembly of nucleosomes: the reaction involving X. laevis nucleoplasmin. Cell. 1980 Sep;21(2):373–383. doi: 10.1016/0092-8674(80)90474-2. [DOI] [PubMed] [Google Scholar]
- Ellison M. J., Pulleyblank D. E. Internal structure of discrete nucleohistone complexes which form in vitro under conditions of physiological ionic strength. J Biol Chem. 1983 Nov 10;258(21):13314–13320. [PubMed] [Google Scholar]
- Ellison M. J., Pulleyblank D. E. Pathways of assembly of nucleohistone complexes formed in vitro under physiological conditions. Implications for the structure of the nucleosome. J Biol Chem. 1983 Nov 10;258(21):13321–13327. [PubMed] [Google Scholar]
- Ellison M. J., Pulleyblank D. E. The assembly of an H2A2,H2B2,H3,H4 hexamer onto DNA under conditions of physiological ionic strength. J Biol Chem. 1983 Nov 10;258(21):13307–13313. [PubMed] [Google Scholar]
- Jackson V., Shires A., Tanphaichitr N., Chalkley R. Modifications to histones immediately after synthesis. J Mol Biol. 1976 Jun 25;104(2):471–483. doi: 10.1016/0022-2836(76)90282-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laskey R. A., Earnshaw W. C. Nucleosome assembly. Nature. 1980 Aug 21;286(5775):763–767. doi: 10.1038/286763a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Honda B. M., Mills A. D., Finch J. T. Nucleosomes are assembled by an acidic protein which binds histones and transfers them to DNA. Nature. 1978 Oct 5;275(5679):416–420. doi: 10.1038/275416a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D., Morris N. R. Assembly of SV40 chromatin in a cell-free system from Xenopus eggs. Cell. 1977 Feb;10(2):237–243. doi: 10.1016/0092-8674(77)90217-3. [DOI] [PubMed] [Google Scholar]
- Mills A. D., Laskey R. A., Black P., De Robertis E. M. An acidic protein which assembles nucleosomes in vitro is the most abundant protein in Xenopus oocyte nuclei. J Mol Biol. 1980 May 25;139(3):561–568. doi: 10.1016/0022-2836(80)90148-5. [DOI] [PubMed] [Google Scholar]
- Nelson T., Wiegand R., Brutlag D. Ribonucleic acid and other polyanions facilitate chromatin assembly in vitro. Biochemistry. 1981 Apr 28;20(9):2594–2601. doi: 10.1021/bi00512a035. [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Jorcano J. L., Eder G., Lurz R. In vitro core particle and nucleosome assembly at physiological ionic strength. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3284–3288. doi: 10.1073/pnas.76.7.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Allfrey V. G. Processing of newly synthesized histone molecules. Science. 1975 Oct 10;190(4210):117–128. doi: 10.1126/science.1166303. [DOI] [PubMed] [Google Scholar]
- Sealy L., Chalkley R. Modification of histones immediately following synthesis. Arch Biochem Biophys. 1979 Oct 1;197(1):78–82. doi: 10.1016/0003-9861(79)90221-2. [DOI] [PubMed] [Google Scholar]
- Shure M., Vinograd J. The number of superhelical turns in native virion SV40 DNA and minicol DNA determined by the band counting method. Cell. 1976 Jun;8(2):215–226. doi: 10.1016/0092-8674(76)90005-2. [DOI] [PubMed] [Google Scholar]
- Simon R. H., Felsenfeld G. A new procedure for purifying histone pairs H2A + H2B and H3 + H4 from chromatin using hydroxylapatite. Nucleic Acids Res. 1979 Feb;6(2):689–696. doi: 10.1093/nar/6.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A., Whitlock J. P., Jr, Bina M. Acidic polypeptides can assemble both histones and chromatin in vitro at physiological ionic strength. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5000–5004. doi: 10.1073/pnas.76.10.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]