Abstract
Proliferation and differentiation of hematopoietic stem/progenitor cells (HSPC) within bone marrow (BM) niches are regulated by adhesion molecules and cytokines produced by mesenchymal stem/progenitor cells (MPC) and osteoblasts (OB). HSPCs that egresses to peripheral blood are widely used for transplant and granulocyte-colony stimulating factor_(G-CSF) is used clinically to induce mobilization. The mechanisms, through which G-CSF regulates HSPC trafficking however, are not completely understood. Herein we show that G-CSF driven neutrophil expansion alters the BM niche that leads to HSPC mobilization. Alcam−Sca-1+MPC and Alcam+Sca-1− OB are reduced coincident with mobilization, which correlates inversely with BM neutrophil expansion. In mice made neutropenic by the neutrophil specific anti-Ly6G antibody, G-CSF mediated reduction in MPC and OB is attenuated and mobilization reduced without an effect on monocytes/macrophages. Neutrophils, expanded in response to G-CSF induce MPC and OB apoptosis leading to reduced production of BM HSPC retention factors including stromal cell derived factor-1 (SDF-1), stem cell factor (SCF) and vascular cell adhesion molecule-1(VCAM-1). Blockade of neutrophil reactive oxygen species (ROS) attenuates G-CSF mediated MPC and OB apoptosis. These data show that the expansion of BM neutrophils by G-CSF contributes to the transient degradation of retention mechanisms within the BM niche, facilitating enhanced HSPC egress/mobilization.
Keywords: G-CSF, HSPC mobilization, neutrophil expansion, osteolineage cells, hematopoietic niche disruption and ROS
INTRODUCTION
In adults, hematopoietic stem and progenitor cells (HSPC) are localized in specialized niches along the endosteal bone surface (1–3), or in perivascular sites adjacent to the endothelium (4–6), where they undergo self-renewal and differentiation, giving rise to all mature blood cells. The endosteal niche is composed primarily of mesenchymal stem/progenitor cells (MPC) and their progeny, which express numerous adhesion molecules and produce supportive cytokines, chemokines and other bioactive molecules essential for HSPC retention within the bone marrow (BM) (7, 8). At steady state, a small number of HSPC circulate in the peripheral blood (PB) (9–11), however agents with distinct cellular targets including hematopoietic growth factors, cytokines and chemokines can enhance HSPC trafficking to PB, a process termed mobilization (12–14). Mobilized peripheral blood stem cells (PBSC) are the primary source of HSPC for clinical hematopoietic transplantation and granulocyte-colony stimulating factor (G-CSF) is widely used clinically for PBSC mobilization (13). Evidence suggests that G-CSF induced HSPC mobilization involves a complex interaction of mechanisms, resulting in endosteal niche attenuation and HSPC egress to the periphery (5, 12, 15, 16). Soluble factors play a crucial role in HSPC retention in BM and include the interactions of stromal cell derived factor-1 (SDF-1) with its receptor CXCR4 expressed on HSPC and stromal vascular cell adhesion molecule-1(VCAM-1) with very late antigen 4 (VLA-4), which tether HSPC within the BM. Disruption of these axes induces HSPC mobilization (17–19). Earlier studies suggested that G-CSF-mediated HSPC mobilization was a consequence of proteolytic degradation of SDF-1 and VCAM-1 by serine and metallo-proteases primarily derived from polymorphonuclear neutrophils (20–23), however, the physiological role of these proteases remains unclear (24).
More recently, inhibition of MPC proliferation and osteoblast (OB) apoptosis by G-CSF has been implicated in HSPC mobilization (5, 16, 25), which is at least partly mediated through sympathetic nervous system signaling (26). Bone-marrow monocytes/macrophages provide positive support to osteolineage cells with the BM niche and reduction of this support in response to G-CSF results in HSPC mobilization (27–29). However, since G-CSF induced HSPC mobilization is only partially reduced in monocyte/macrophage depleted mice it is likely that G-CSF also acts on other contributing cell types.
The fact that G-CSF treatment induces a robust expansion of BM and PB neutrophils and that G-CSF receptor deficient mice, which are neutropenic, show impaired G-CSF-induced HSPC mobilization despite normal HSPC number and function, suggest that neutrophils are involved in G-CSF- induced HSPC mobilization (30). In this study we demonstrate that neutrophils contribute significantly to G-CSF induced mobilization of HSPC by reducing the number of BM MPC and OB and expression of factors involved in HSPC retention.
MATERIALS AND METHODS
Mice
C57BL/6 mice were purchased from Harlan Laboratories (Indianapolis, IN) and maintained in the Indiana University School of Medicine (IUSM) animal facility. All mouse experiments were approved by the Indiana University Institutional Animal Care and Use Committee.
G-CSF treatment and preparation of cell suspensions
Peripheral blood HSPC mobilization was induced by treating mice with 1 ug/mouse recombinant human G-CSF (NEUPOGEN; Amgen Inc. Thousand Oaks, CA) subcutaneously twice daily for three days (50 ug/kg, bid × 3 days). For some experiments mice were treated with the antioxidant N-acetyl-L-cystein (NAC; Sigma-Aldrich, St. Louis, MO) (subcutaneously at 50 mg/kg) and G-CSF together. Mice were sacrificed 16 hours after the last G-CSF injection and PB was obtained by cardiac puncture and placed in EDTA microtainers (BD Biosciences, San Diego, CA). Total blood counts were determined using a Hemavet hematology analyzer with veterinary software (Drew Scientific Inc., CT). Bone marrow cells were harvested by flushing femurs with α-modified Eagle medium (α-MEM; Lonza Inc, Allendale, NJ) containing 2% fetal bovine serum (FBS; Thermo Scientific HyClone, Logan, UT). The extracellular fluid in each femur was obtained by flushing with 1 mL ice-cold PBS followed by centrifugation at 400 × g for 3 minutes and collection of cell free supernatant.
Neutrophil depletion in vivo
To determine the role of neutrophils in G-CSF induced mobilization, mice were treated with anti-Gr-1 antibody (RB6-8C5 clone; eBiosciences, San Diego, CA) or anti-Ly6G antibody (1A8 clone; BioLegend, San Diego, CA) (both at 150 ug/mouse) on day 1 of a 3 day G-CSF treatment regimen. Control mice were treated with isotype antibody.
Competitive repopulation assay
Transplantable long-term PB hematopoietic stem cells (HSC) were quantitated by competitive repopulation transplantation analysis. Recipient congenic CD45.1/CD45.2 F1 hybrid mice were lethally irradiated with 11.0 Gy in two split doses approximately 5 hours apart. Twenty hours after irradiation mice were injected by tail vein with 50 uL RBC lysed blood samples from G-CSF mobilized normal mice or neutrophil-depleted CD45.2+ CD57BL/6 mice (5 mice/group) plus 200,000 competitive whole BM cells from untreated CD45.1+ BoyJ mice (in a total volume of 200 uL). Recipient mice were maintained on doxycycline feed for 1 month post-transplant. Chimerism in PB was determined at 6 months post-transplant and SLAM-LSK cell number in BM, defined as Lineageneg (CD3−Gr-1−CD11b−CD45R−Ter119−) Sca-1+ c-Kit+ CD150+ CD48−, was determined by flow-cytometry.
Neutrophil isolation and co-culture with osteoblast precursors/osteoblasts
Bone marrow cells were stained with FITC conjugated anti-Ly6G antibody, APC-Cy7-conjugated anti-CD11b antibody, PE-conjugated anti-F4/80 antibody, APC-conjugated anti-CD115 and Ly6G+ CD115− F4/80neg (Ly6Gpos) neutrophils or Ly6Gneg non neutrophil cells were sorted by FACS. All antibodies were purchased from BD Biosciences (San Jose, CA) or eBiosciences (San Diego, CA). Dead cells were excluded with LIVE/DEAD Fixable Violet Dead Cell Staining dye (Invitrogen, Carlsbad, CA). The mouse preosteoblast MC3T3-E1 cell line (American Type Culture Collection. Manassas, VA) was maintained in a humidified 5% CO2 atmosphere at 37° C in α-MEM containing 10% FBS, 100 U/ml penicillin, 100 ug/ml streptomycin and 1 mM sodium pyruvate. In order to induce differentiation of MC3T3-E1 cells into OB, cells were plated at 2 × 105 cells/well in 6-well plates or 4 × 104 cells/well in 12-well plates in α-MEM with 10% FBS supplemented with 50 µg/ml ascorbic acid and 10 mM β-glycerol phosphate for 1–10 days (plating day was considered as day 0). For co-culture experiments, FACS sorted Ly6Gpos neutrophils or Ly6Gneg cells (2 × 106 cells) were added to preosteoblast MC3T3-E1 or differentiated MC3T3-E1 OB monolayers (2 × 105 cells) and cultured with G-CSF for 3 days. At the end of the incubation period cells were stained with UV-violet fluorescence reactive dye and lineage cocktail antibodies and followed by Annexin V staining (BD biosciences). Viability of preosteoblasts/OB was determined by flow-cytometry. Co-culture supernatants were collected for ELISA analysis.
Quantitative PCR
Total RNA was purified with Purelink™ RNA mini Kit and reverse transcribed with SuperScript™ III First-Strand Synthesis System for (Invitrogen, Grand Island, NY) RT-PCR. Primers for mouse actin, SDF1 and VCAM1 were designed to produce an amplicon size of 100 to 150 bp and synthesized by Integrated DNA technologies. Sequences of the primers were:
Actin F-AGGTGTGCACCTTTTATTGGTCTCAA;
Actin R-GTAGTAAGGTTTGGTCTCCCT.
SDF1-R-TTCTTCAGCCGTGCAACAATC; SDF1-F-TGCATCAGTGACGGTAAACCA.
VCAM1-F-GACCTGTTCCAGCGAGGGTCTA;
VCAM1-R- CTTCCATCCTCATAGCAATTAAGGTG.
QRT-PCR was performed by using SYBR advantage qPCR Premix kit (Clontech, Mountain View, CA) on MxPro-3000 (Agilent, LaJolla, CA). All experiments were performed in triplicates.
ELISA
SDF-1 and SCF levels in BM extracellular fluid (BMEF) and SDF-1 in culture supernatants were quantitated using R&D Systems Duo set ELISA kits according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Statistical Analysis
Data are presented as X ± SEM. Statistical significance was assessed using a 2-tailed Student’s t-test.
RESULTS
G-CSF-induced HSPC mobilization is impaired in neutrophil ablated mice
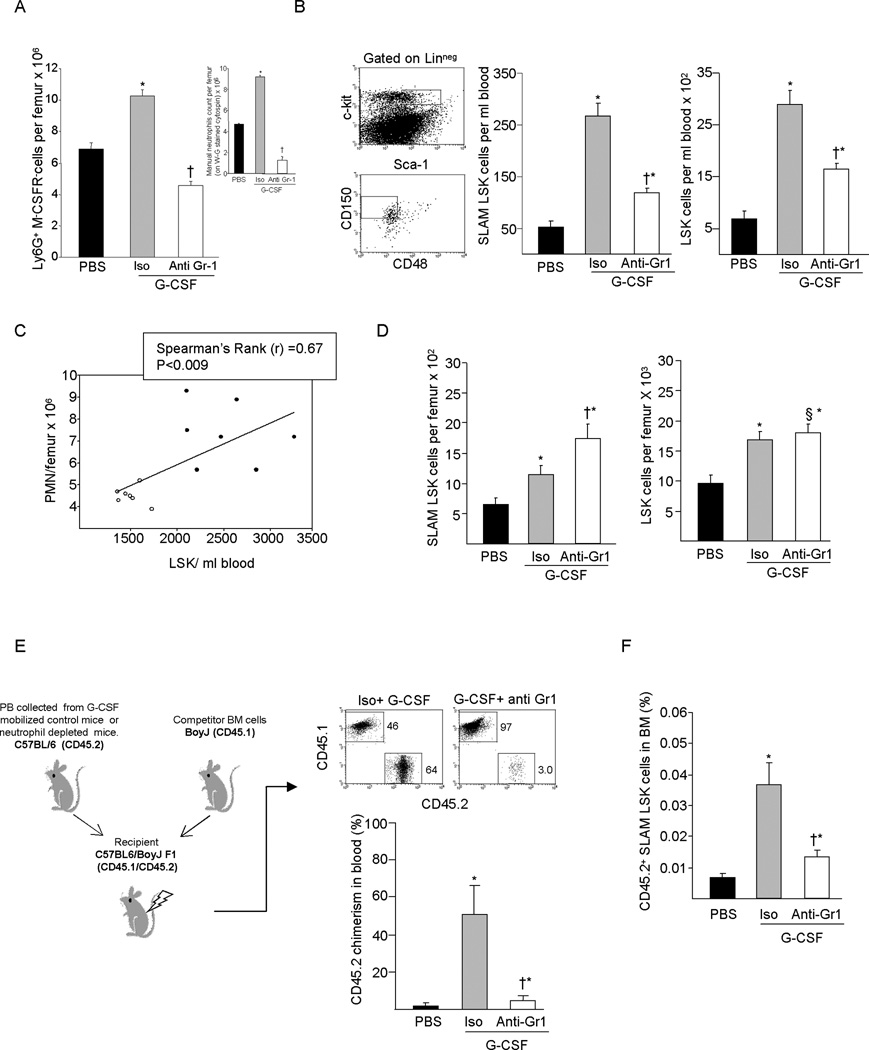
Mobilization by G-CSF is known to be a two compartment model mediated by trans acting signals affecting accessory cell populations. Because G-CSFR is expressed at high levels on mature and immature neutrophils and at an intermediate level on monocytes/macrophages, B-cells and NK cells, it is likely that one or more of these cell-types are involved in G-CSF induced HSPC mobilization. We have previously shown that neutrophil depletion reduces mobilization of myeloid colony forming cells and radioprotective cells (23). To further define a role for neutrophils in G-CSF induced HSPC mobilization, we evaluated HSPC number and function in neutrophil depleted mice. As we previously described (23), a 3 day regimen of G-CSF (100 ug/kg) significantly increases the proportion of Ly6G+ CD115− BM neutrophils, while administration of monoclonal anti-Gr-1 antibody at the time of initiation of the G-CSF mobilization regimen reduces Ly6G+ CD115− cells by ≥60% (Figure 1A) and circulating neutrophils by 98% (not shown). Manual differential counts performed to exclude interference of in vivo anti-Gr-1 treatment with FITC-labeled anti-Gr-1 used for flow cytometry analysis confirmed the expansion of BM neutrophils by G-CSF and the reduction in total BM polymorphonuclear neutrophil by anti-Gr-1 antibody treatment (Figure 1A insert). In control mice, G-CSF administration induced robust mobilization of HSC-enriched SLAM LSK and LSK cells enriched for multipotent progenitor cells; however, mobilization of circulating SLAM LSK and LSK cells was significantly attenuated in neutropenic mice (Figure 1B). The magnitude of HSPC mobilization directly correlated with the absolute number of BM neutrophils (Figure 1C). While G-CSF-induced mobilization was significantly reduced in neutropenic mice, neutrophil depletion did not affect the increase in SLAM LSK and LSK cells in the BM normally observed in response to G-CSF (Figure 1D). In fact, G-CSF treated neutropenic mice had significantly more SLAM LSK cells than control mice. In competitive transplant studies, PB chimerism and BM SLAM LSK content at 6 months post transplant was significantly lower in mice transplanted with PB from G-CSF mobilized neutropenic mice compared to G-CSF mobilized PB from control mice (Figure 1E & 1F). These results suggest that the neutrophil population is required for optimal G-CSF induced PBSC mobilization.
Figure 1. G-CSF mediated HSPC mobilization in neutrophil depleted mice.
Mice were treated with single dose of anti-Gr1 antibody or IgG isotype control antibody on day 1 of a 3 day G-CSF mobilization. (A) Ly6G+ CD115− neutrophils in BM and manual differential neutrophil counts (200 cells/slide) on Wright-Geimsa-stained cytospins (insert) (B) SLAM LSK and LSK cells in the PB of IgG isotype plus G-CSF or anti-Gr1 plus G-CSF treated mice. (C) Spearman’s rank correlation between the number of BM neutrophils and PB LSK cells. (D) SLAM LSK and LSK in the BM of G-CSF treated control or neutropenic mice. (E) Competitive transplantation of repopulating HSC using equal volumes of PB from G-CSF treated control or neutropenic mice (CD45.2 C57BL/6) in combination 2 × 105 BM competitor cells from CD45.1 BoyJ mice. Donor chimerism was measured by flow cytometry in peripheral blood at 6 months after transplantation. (F) Donor derived SLAM LSK cell number in BM of transplanted mice at 6 month (X ± SEM; N= ≥ 5 mice per group). *P< 0.05 compared to vehicle; † P< 0.05 compared to isotype plus G-CSF; § P > 0.05 compared to isotype plus G-CSF.
In vivo neutrophil depletion prevents G-CSF mediated disruption of the osteolineage cells
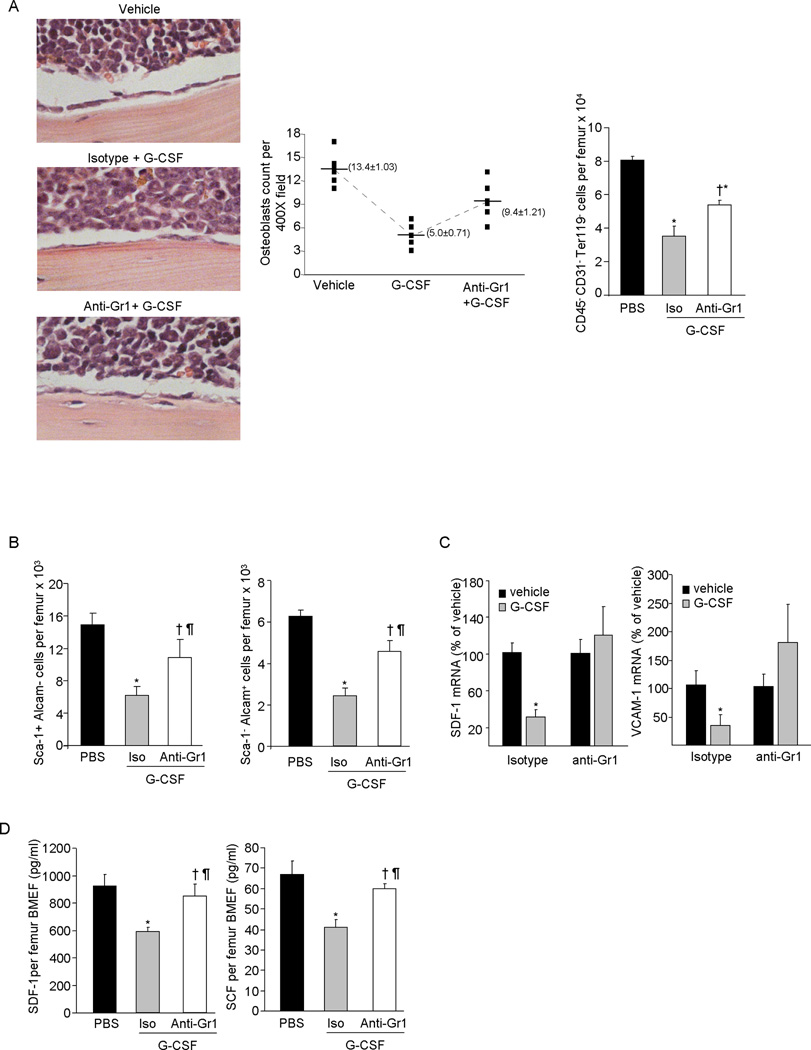
Long-term repopulating HSCs are localized in proximity to MPC and OB enriched endosteal regions within the BM (3). Previous studies have shown that G-CSF treatment decreases CD45− CD31− Ter119− osteolineage endosteal cells (16, 25) and inhibits OB differentiation (16), which may be responsible for increased HSC trafficking to PB. To examine the role of neutrophils in G-CSF-mediated disruption of the cellular components of the endosteal niche, we first quantitated osteolineage cells in the BM of G-CSF treated control and neutropenic mice. Similar to a previous report (16), bone adjacent OB and CD45− CD31−Ter119− osteolineage cells were substantially reduced in the BM of control mice after G-CSF treatment (Figure 2A). However, in neutropenic mice, the reduction in osteolineage cells in response to G-CSF was significantly attenuated.
Figure 2. Effect of neutrophils depletion on BM osteolineage cells in G-CSF treated mice.
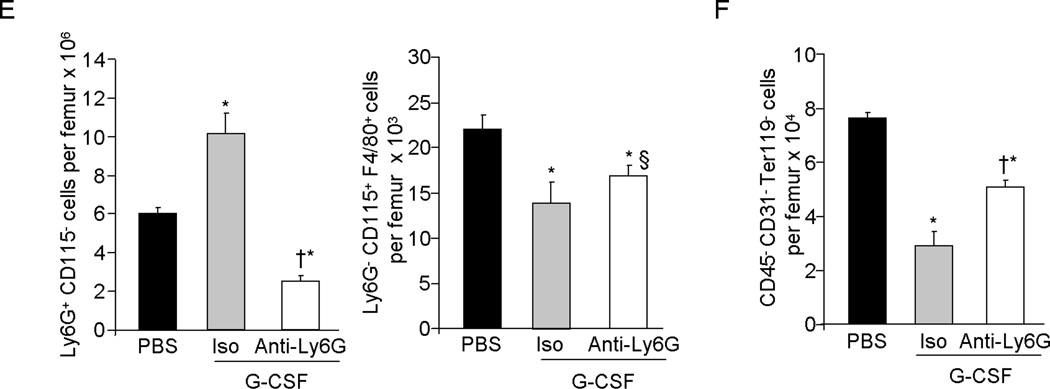
Mice were treated with G-CSF for 3 days and neutrophils were depleted by single injection of anti-Gr1 antibody on day 1 of the G-CSF regime. (A) Left: Representative photomicrographs show endosteal OB after H&E staining and graph show average OB counts per 400× field and Right: Bone associated cells were recovered from the femurs and tibiae of mice by flushing and treating with collagenase type 1 and CD45− CD31− Ter119− gated cell population quantitated by flow cytometry. (B) MPC enriched Sca-1+Alcam− cells and OB enriched Sca-1−Alcam+ cells in the collagenase treated BM fraction was determined by flow cytometry (X ± SEM; N=3 per group in each of 2 experiment). (C) SDF-1 and VCAM-1 m-RNA expression relative to β-actin in CD45− CD31− Ter119− enriched BM cells harvested from G-CSF treated control or neutropenic mice (X ± SEM; N=4 per group) (D) SDF-1, and SCF in the BM extracellular fluid of G-CSF treated control or neutrophil depleted mice as measured by ELISA (X ± SEM; N=3 per group in each of 2 experiment). (E) Effect of anti-Ly6G antibody plus G-CSF administration on BM Ly6G+ CD115− neutrophil and Ly6G− CD115+ F4/80+ macrophage and on (F) CD45− CD31− Ter119− osteolineage cells in the BM, analyzed by flow cytometry (X ± SEM; N=4 per group). *P< 0.05 compared to vehicle; † P< 0.05 compared to isotype plus G-CSF; § P > 0.05 compared to isotype plus G-CSF; ¶ P > 0.05 compared to vehicle.
The BM CD45− CD31−Ter119− osteolineage cell population is a heterogeneous population in terms of differentiation stage and function and can be divided into two subpopulations; the Sca-1+ Alcam− fraction enriched for MPC and the Sca-1− Alcam+ fraction enriched for OB (3). G-CSF treatment substantially reduced both MPC and OB in the BM of control mice (Figure 2B), but was significantly less effective in neutropenic mice.
Chemo-attracting cytokines, growth factors and adhesion molecules produced by the MPC and OB initiate signaling networks that regulate HSC retention in the BM (6, 31) and have been implicated in the mechanisms modulating HSPC mobilization particularly by G-CSF (5, 16, 18). To examine whether neutrophils alter expression of these retention factors, we measured SDF-1, SCF and VCAM-1 mRNA expression and/or protein in the BM of G-CSF treated control and neutropenic mice. SDF-1 and VCAM-1 mRNA expression were substantially decreased in CD45− CD31−Ter119− cells from G-CSF treated control mice, but were relatively unaffected in cells from neutrophil depleted mice (Figure 2C). While SDF-1 and SCF protein levels were significantly decreased in BM extracellular fluid of G-CSF treated control mice (15) (Figure 2D), in G-CSF treated neutropenic mice their production was unchanged.
Since the anti-Gr-1 monoclonal antibody (RB6-8C5) has been reported to also deplete some populations of monocytes (32), we injected mice with the anti-Ly6G antibody (clone 1A8), which has been shown to specifically deplete neutrophils without affecting monocytes (32). Co-treatment with anti-Ly6G during the G-CSF mobilization regimen, substantially depleted neutrophils in the BM of G-CSF treated mice, however the reduction of Ly6G− CD115+ F4/80int monocytes/macrophages was similar in G-CSF treated control and in neutropenic mice (Figure 2E). Similar to the affect seen with anti-Gr-1 dependent neutrophil depletion, the reduction in the CD45− CD31−Ter119− cell number was significantly less in G-CSF and anti-Ly6G treated neutropenic mice compared to G-CSF and control antibody treated mice (Figure 2F), further confirming a role of neutrophils in the disruption of the endosteal BM niche by G-CSF.
Neutrophils directly inhibit osteolineage cell SDF-1 production in vitro
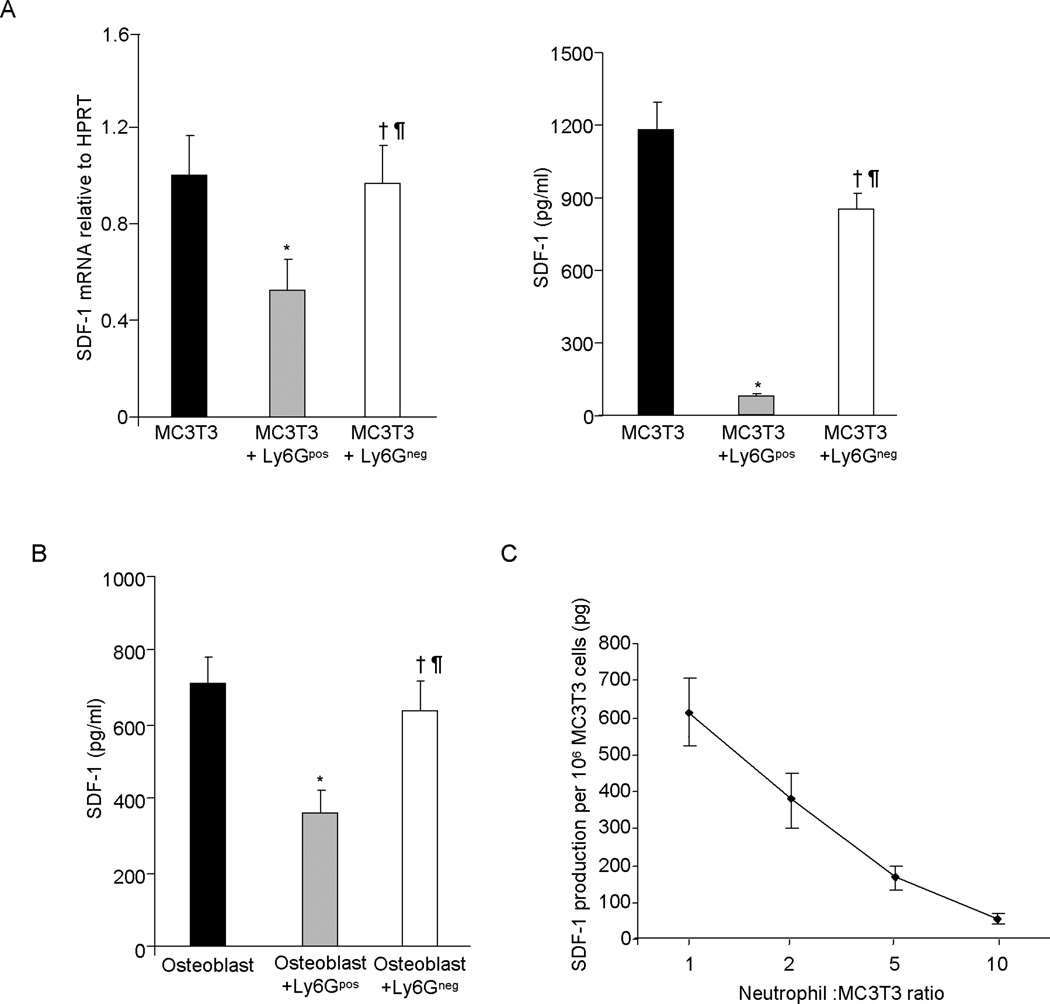
To determine whether neutrophils directly affect osteolineage progenitor cell and differentiated OB function, FACS sorted BM Ly6G+ CD115− neutrophils or Ly6Gneg non-neutrophil cells were co-cultured with MC3T3 pre-osteoblast cells or with differentiated OB, in the presence of G-CSF for 3 days and SDF-1 mRNA expression and SDF-1 protein levels in culture supernatant quantitated. Ly6G+ CD115− neutrophils substantially reduced MC3T3 cell SDF-1 mRNA and protein production; while Ly6Gneg non-neutrophil cells had no significant effect (Figure 3A). Similarly, Ly6G+ CD115− neutrophils also impaired SDF-1 production from differentiated OB (Figure 3B). Since G-CSF treatment induces extensive neutrophil expansion, we next examined whether SDF-1 production from the osteolineage cells is affected by neutrophil cell number. FACS sorted Ly6G+ CD115− BM neutrophils were co-cultured on MC3T3 pre-osteoblast cell monolayers at different ratios in the presence of G-CSF. We hypothesized that if neutrophil expansion is important for the impairment of SDF-1 production from pre-osteoblasts, then an increase in neutrophil number would dose dependently affect SDF-1 production. As shown in Figure 3C, increasing the number of neutrophils progressively reduced pre-osteoblast SDF-1 production. These observations suggest that the G-CSF mediated neutrophil expansion is likely responsible for impaired SDF-1 production in the BM.
Figure 3. SDF-1 production from osteolineage cells was inversely correlated with neutrophil cell number.
(A) Effect of Ly6GposCD115− neutrophil or Ly6Gneg non-neutrophil cells on SDF-1 production by MC3T3 cells, a pre-osteoblast and (B) by differentiated OB. FACS sorted Ly6Gpos neutrophil and Ly6Gneg non neutrophil BM cells were co-cultured with pre-osteoblast or with differentiated OB in presence of G-CSF for 3 days and SDF-1 expression in MCET3 cells was determined by Q-RT-PCR and SDF-1 levels in culture supernatant was measured in by ELISA (X ± SEM; N=3 experiments). (C) FACS sorted Ly6Gpos BM neutrophils were co-cultured at different ration with pre-osteoblast cells in presence of G-CSF for 3 days and SDF-1 was measured in culture supernatant by ELISA (X ± SEM; N=2 experiment). *P< 0.05 compared to vehicle; † P< 0.05 compared to isotype plus G-CSF; § P > 0.05 compared to isotype plus G-CSF; ¶ P > 0.05 compared to vehicle.
G-CSF mediated neutrophil expansion induces osteolineage cell apoptosis
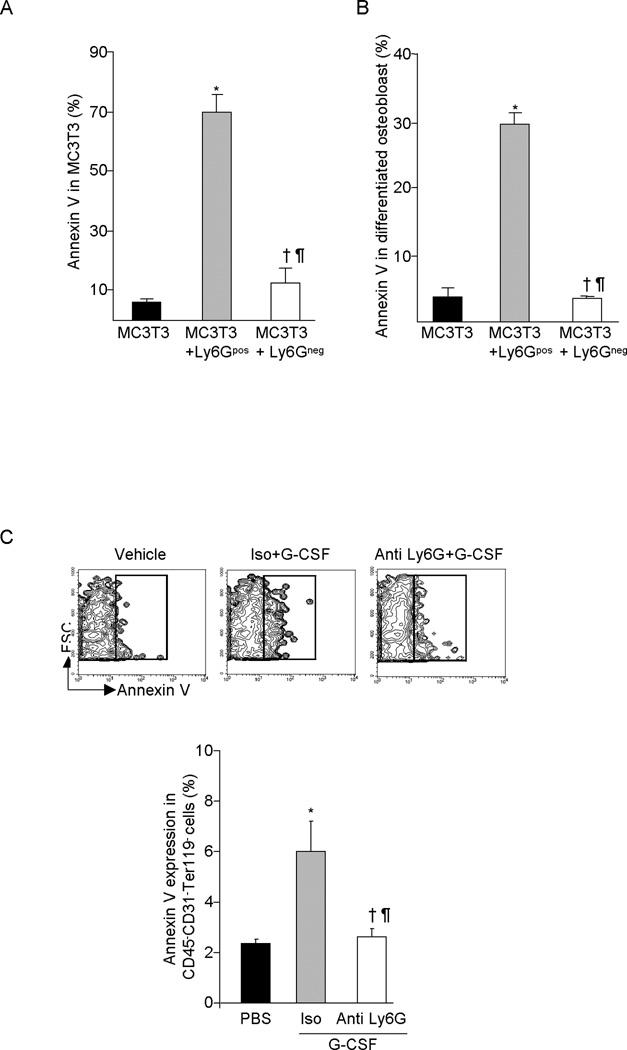
In order to evaluate mechanisms responsible for neutrophil-mediated reduction of MPC and OB in the BM of G-CSF treated mice, FACS sorted Ly6G+ CD115− or Ly6Gneg BM cells were co-cultured with MC3T3 pre-osteoblast cells or MC3T3-derived differentiated OB in the presence of G-CSF and the cell viability of gated MC3T3 cells analyzed by Annexin-V staining. Ly6G+ CD115− neutrophils significantly increased apoptosis of MC3T3 pre-osteoblast (Figure 4A) and mature OB (Figure 4B), while Ly6Gneg cells had no effect on survival. To further evaluate the role of neutrophils in regulating osteolineage cell apoptosis in response to G-CSF, we evaluated apoptosis in CD45− CD31−Ter119− gated BM cells from G-CSF treated control mice and mice made neutropenic by anti-Ly6G administration. G-CSF substantially increased CD45− CD31−Ter119− cell apoptosis in control mice as reported (25), however in neutropenic mice, CD45− CD31−Ter119− cell apoptosis was unaffected (Figure 4C), demonstrating that neutrophils mediate osteolineage cell apoptosis in response to G-CSF.
Figure 4. Effect of neutrophil on osteolineage cells survival.
FACS sorted Ly6Gpos neutrophil and Ly6Gneg non neutrophil BM cells were co-cultured either with MC3T3 pre-osteoblast cells or differentiated OB in presence of G-CSF for 3 days. (A) MC3T3 cells apoptosis and (B) differentiated OB apoptosis was determined by flow cytometry after Annexin V staining (X ± SEM; N=3 experiment). (C) Annexin V expression on BM CD45− CD31− Ter119− osteolineage cell from the G-CSF treated control or neutropenic mice (X ± SEM; N=5 mice in each group). *P< 0.05 compared to vehicle; † P< 0.05 compared to isotype plus G-CSF; ¶ P > 0.05 compared to vehicle.
Reactive oxygen production by neutrophils is partially involved in osteolineage cell apoptosis
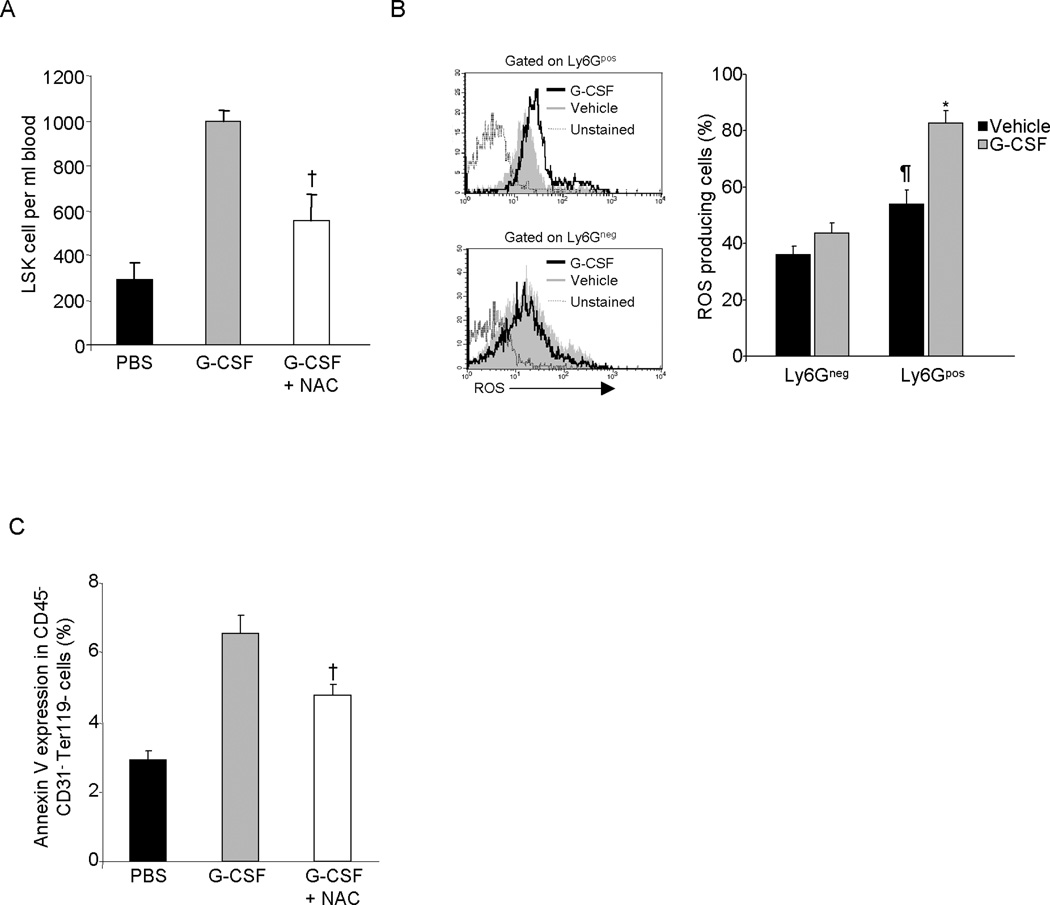
Several studies have shown a role for ROS in the apoptosis of osteolineage cells (33–35) and inhibition of ROS signaling has been shown to reduce G-CSF induced mobilization of HSPC (36). Since neutrophils induce osteolineage cell apoptosis, we evaluated whether ROS production by neutrophils was involved in this effect. Consistent with a previous report (36), treatment of mice with the ROS inhibitor NAC significantly reduced G-CSF induced HSPC mobilization (Figure 5A). To determine whether neutrophils play a role in ROS production in BM, mice were treated with G-CSF and ROS was measured in BM neutrophils and non-neutrophil cells. Bone marrow neutrophils generated significantly higher levels of ROS compared to non-neutrophil cells and G-CSF treatment substantially increased neutrophil ROS production, whereas non-neutrophil ROS production was not changed (Figure 5B). To examine the role of ROS in osteolineage cell apoptosis, we measured Annexin V expression in CD45−CD31−Ter119− BM cells from G-CSF and G-CSF plus NAC treated mice. G-CSF treatment substantially increased Annexin V expression in CD45−CD31−Ter119− gated BM cells, however treatment of mice with G-CSF and NAC together significantly reduced Annexin V expression (Figure 5C). These data link neutrophil-produced ROS in destruction of HSC niche components in the BM in response to G-CSF.
Figure 5. Reactive oxygen production by neutrophils is involved in osteoblastic cells apoptosis.
(A) Effect of ROS production blockade on G-CSF induced HSPC mobilization. Mice were treated with G-CSF or G-CSF plus NAC and LSK cell mobilization in PB was determined by flow-cytometry. (B) ROS production in G-CSF treated mice BM neutrophil and non-neutrophil cells. (X ± SEM; N= 4 mice). (C) Effect of ROS production blockade on G-CSF mediated oeteolineage cell apoptosis. Mice were treated with G-CSF alone or G-CSF plus NAC and Annexin V expression in CD45−CD31−Ter119− BM cells was determined by flow cytometry. † P< 0.05 compared to G-CSF; *P< 0.05 compared to Ly6Gpos vehicle (PBS); ¶ P< 0.05 compared to PBS treated Ly6Gneg cells.
DISCUSSION
Although it is well established that deregulation of BM niche components is a primary cause for G-CSF-induced HSPC mobilization, lack of G-CSFR expression on MPC and their progeny that make up the HSC endosteal niche (16, 26) suggests the involvement of accessory cells and/or factors in this process. While we have previously shown (23), and confirmed herein, that neutrophils are important for HSPC mobilization induced by G-CSF, we now provide evidence that the expansion of neutrophils in the BM that occurs in response to G-CSF, induces MPC and OB apoptosis through increased ROS production and reduces the expression of MPC and OB derived factors crucial for HSC retention in the BM, including SDF-1, SCF and VCAM-1. These data provide new mechanistic insight into the role of accessory neutrophils in the PBSC mobilization process.
Accumulating evidences suggest that the monocyte/macrophage cell lineage plays a role in HSPC retention in the BM and depletion of these cells induces HSPC trafficking (27–29). The marrow niche contains supportive endosteal macrophages and G-CSF treatment reduces this population coincident with mobilization and OB suppression. Depletion of F4/80+ Ly-6G+ CD11b+ macrophages with clodronate-loaded liposomes also results in mobilization, but to a much lower extent compared to G-CSF (27). Depletion of CD169+ resident macrophages also impairs HSPCs BM retention (28) and expression of G-CSFR under the control of CD68 promoter, which restricts G-CSF receptor expression to the monocyte/macrophage lineage, shows that G-CSF reduces BM macrophage, coincident with HSPC mobilization (29). While these studies show monocyte/macrophage depletion enhances HSPC mobilization, the magnitude of response seen after G-CSF administration is at least three times greater than that seen following monocyte depletion (28), indicating that G-CSF also acts on cells other than monocytoid cells. Since anti-Gr-1 antibody recognizes both Ly6G and Ly6C epitopes and Ly6C is expressed on a subset of monocyes (32), it is possible that anti-Gr-1 treatment during the G-CSF mobilization regimen also depletes some monocytes along with neutrophils. However, if monocytes/macrophages are primarily responsible for G-CSF induced HPC mobilization, then anti-Gr-1 antibody treatment during G-CSF mobilization should increase HSPC mobilization instead of decreasing it as we observed. More likely, mobilization occurs in concert coordinated by a balance between BM cell populations.
Evidence is accumulating that disruption of mesenchymal lineage cell function within the endosteal niche plays a key role in HSPC mobilization by G-CSF (5, 16, 25, 26). Reversal of G-CSF mediated reduction in osteolineage cells including progenitor cells and OB in anti-Gr-1 antibody treated neutropenic mice demonstrates that neutrophils are involved in G-CSF mediated endosteal niche disruption. Furthermore, Ly6G mediated neutrophil depletion, which does not ablate monocytes/ macrophages also afforded osteolineage cell protection similar to anti-Gr-1 mediated neutrophil depletion, demonstrating that neutrophils play a crucial role in endosteal niche degradation in response to G-CSF. Since previous studies have shown that G-CSF mediated monocyte/macrophage depletion in BM is involved in HSPC mobilization (27, 29), it is possible that simultaneous expansion of neutrophils and ablation of monocytes /macrophages in BM in response to G-CSF leads to maximum HSPC mobilization. Analysis of SDF-1 levels produced by OB in the presence of neutrophils clearly showed reduction in SDF-1 while others have shown that monocytes/macrophages increase SDF-1 production (27–29). Thus together, these findings also suggest that the balance between neutrophil and monocyte/macrophage populations is likely required for the appropriate maintenance of the niche and that altering the balance of these populations disrupts the niche, a process that can be used for therapeutic benefit.
Reactive oxygen species have been implicated in osteolineage cell apoptosis (33–35). G-CSF activates neutrophils to generate superoxide and other forms of ROS (37, 38). Significant inhibition of osteolineage cell apoptosis in the presence of NAC suggests that neutrophil-released ROS is involved, at least in part in the apoptosis of osteolineage cells. In support of our results, a recent finding shows that mice lacking ROS signaling have impaired HSPC mobilization in response to G-CSF (36). In addition, recent studies have shown that spingosine-1-phosphate (S1P) is involved in HSPC mobilization (39, 40) and S1P has been shown to induce ROS production in neutrophils (41). Therefore it is possible that G-CSF may regulate ROS production in neutrophils via S1P signaling. That fact that inhibition of ROS production in neutrophils only partially reduces osteolineage cell apoptosis suggests the involvement of additional mechanism(s). Evidence that neutrophil proteases decrease OB proliferation (42) and induce apoptosis in endothelial cells (43), argues that enhanced protease production by neutrophils in response to G-CSF may also be involved in osteolineage cell apoptosis. In addition, neutrophil proteases can activate the complement cascade that can lead to release of the bioactive lipids S1P and C1P (ceramide 1-phosphate) implicated in mobilization by G-CSF (44).
In conclusion, we provide evidence that neutrophil expansion, a physiological consequence of G-CSF administration, suppresses osteolineage cell populations in BM and impairs the production of HSC retention factors such as SDF-1, SCF and VCAM-1, thus disrupting the BM niche, leading to HSPC mobilization. These data clearly show that multiple cell populations are targeted by G-CSF and involved in altering the trafficking potential of HSPC. While single cell populations and mechanisms can lead to HSPC mobilization, optimal therapeutic mobilization is achieved in concert, and understanding the cells and signals involved will ultimately maximize therapeutic utility.
ACKNOWLEDGEMENTS
This work was supported by NIH grants HL069669 and HL096305 (to LMP) and partially supported by National Institute of Diabetes and Digestive and Kidney Diseases Center for Excellence in Molecular Hematology grant P30 DK090948. JH was supported sequentially by NIH Training Grants DK07519 and HL007910. Flow cytometry was performed in the Flow Cytometry Resource Facility of the Indiana University Simon Cancer Center (NCI P30 CA082709).
Footnotes
Conflict of Interest Disclosures
The authors declare no competing financial interests.
REFERENCES
- 1.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003 Oct 23;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003 Oct 23;425(6960):836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura Y, Arai F, Iwasaki H, Hosokawa K, Kobayashi I, Gomei Y, et al. Isolation and characterization of endosteal niche cell populations that regulate hematopoietic stem cells. Blood. 2010 Sep 2;116(9):1422–1432. doi: 10.1182/blood-2009-08-239194. [DOI] [PubMed] [Google Scholar]
- 4.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012 Jan 26;481(7382):457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010 Aug 12;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006 Dec;25(6):977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006 Feb;6(2):93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 8.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005 Sep 15;106(6):1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 9.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001 Nov 30;294(5548):1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 10.Abkowitz JL, Robinson AE, Kale S, Long MW, Chen J. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 2003 Aug 15;102(4):1249–1253. doi: 10.1182/blood-2003-01-0318. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharya D, Czechowicz A, Ooi AG, Rossi DJ, Bryder D, Weissman IL. Niche recycling through division-independent egress of hematopoietic stem cells. J Exp Med. 2009 Nov 23;206(12):2837–2850. doi: 10.1084/jem.20090778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoggatt J, Pelus LM. Many mechanisms mediating mobilization: an alliterative review. Curr Opin Hematol. 2011 Apr 30; doi: 10.1097/MOH.0b013e3283477962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelus LM, Horowitz D, Cooper SC, King AG. Peripheral blood stem cell mobilization. A role for CXC chemokines. Crit Rev Oncol Hematol. 2002 Sep;43(3):257–275. doi: 10.1016/s1040-8428(01)00202-5. [DOI] [PubMed] [Google Scholar]
- 14.Thomas J, Liu F, Link DC. Mechanisms of mobilization of hematopoietic progenitors with granulocyte colony-stimulating factor. Curr Opin Hematol. 2002 May;9(3):183–189. doi: 10.1097/00062752-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006 Jun;12(6):657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 16.Semerad CL, Christopher MJ, Liu F, Short B, Simmons PJ, Winkler I, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005 Nov 1;106(9):3020–3027. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003 Jan;111(2):187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002 Jul;3(7):687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch E, Iglesias A, Potocnik AJ, Hartmann U, Fassler R. Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature. 1996 Mar 14;380(6570):171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- 20.Levesque JP, Hendy J, Takamatsu Y, Williams B, Winkler IG, Simmons PJ. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002 May;30(5):440–449. doi: 10.1016/s0301-472x(02)00788-9. [DOI] [PubMed] [Google Scholar]
- 21.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001 Sep 1;98(5):1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 22.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002 May 31;109(5):625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelus LM, Bian H, King AG, Fukuda S. Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT/CXCL2delta4. Blood. 2004 Jan 1;103(1):110–119. doi: 10.1182/blood-2003-04-1115. [DOI] [PubMed] [Google Scholar]
- 24.Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C, et al. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood. 2004 Jul 1;104(1):65–72. doi: 10.1182/blood-2003-05-1589. [DOI] [PubMed] [Google Scholar]
- 25.Christopher MJ, Link DC. Granulocyte colony-stimulating factor induces osteoblast apoptosis and inhibits osteoblast differentiation. J Bone Miner Res. 2008 Nov;23(11):1765–1774. doi: 10.1359/JBMR.080612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006 Jan 27;124(2):407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 27.Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010 Dec 2;116(23):4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 28.Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011 Feb 14;208(2):261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011 Feb 14;208(2):251–260. doi: 10.1084/jem.20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu F, Poursine-Laurent J, Link DC. Expression of the G-CSF receptor on hematopoietic progenitor cells is not required for their mobilization by G-CSF. Blood. 2000 May 15;95(10):3025–3031. [PubMed] [Google Scholar]
- 31.Thoren LA, Liuba K, Bryder D, Nygren JM, Jensen CT, Qian H, et al. Kit regulates maintenance of quiescent hematopoietic stem cells. J Immunol. 2008 Feb 15;180(4):2045–2053. doi: 10.4049/jimmunol.180.4.2045. [DOI] [PubMed] [Google Scholar]
- 32.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008 Jan;83(1):64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 33.Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007 Sep 14;282(37):27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, et al. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest. 2003 Sep;112(6):915–923. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almeida M, Han L, Ambrogini E, Bartell SM, Manolagas SC. Oxidative stress stimulates apoptosis and activates NF-kappaB in osteoblastic cells via a PKCbeta/p66shc signaling cascade: counter regulation by estrogens or androgens. Mol Endocrinol. 2010 Oct;24(10):2030–2037. doi: 10.1210/me.2010-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tesio M, Golan K, Corso S, Giordano S, Schajnovitz A, Vagima Y, et al. Enhanced c-Met activity promotes G-CSF-induced mobilization of hematopoietic progenitor cells via ROS signaling. Blood. 2011 Jan 13;117(2):419–428. doi: 10.1182/blood-2009-06-230359. [DOI] [PubMed] [Google Scholar]
- 37.Sattler M, Verma S, Shrikhande G, Byrne CH, Pride YB, Winkler T, et al. The BCR/ABL tyrosine kinase induces production of reactive oxygen species in hematopoietic cells. J Biol Chem. 2000 Aug 11;275(32):24273–24278. doi: 10.1074/jbc.M002094200. [DOI] [PubMed] [Google Scholar]
- 38.Sattler M, Winkler T, Verma S, Byrne CH, Shrikhande G, Salgia R, et al. Hematopoietic growth factors signal through the formation of reactive oxygen species. Blood. 1999 May 1;93(9):2928–2935. [PubMed] [Google Scholar]
- 39.Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, et al. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010 May;24(5):976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007 Nov 30;131(5):994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Florey O, Haskard DO. Sphingosine 1-phosphate enhances Fc gamma receptor-mediated neutrophil activation and recruitment under flow conditions. J Immunol. 2009 Aug 15;183(4):2330–2336. doi: 10.4049/jimmunol.0901019. [DOI] [PubMed] [Google Scholar]
- 42.Fornoni A, Cornacchia F, Howard GA, Roos BA, Striker GE, Striker LJ. Cyclosporin A affects extracellular matrix synthesis and degradation by mouse MC3T3-E1 osteoblasts in vitro. Nephrol Dial Transplant. 2001 Mar;16(3):500–505. doi: 10.1093/ndt/16.3.500. [DOI] [PubMed] [Google Scholar]
- 43.Yang JJ, Kettritz R, Falk RJ, Jennette JC, Gaido ML. Apoptosis of endothelial cells induced by the neutrophil serine proteases proteinase 3 and elastase. Am J Pathol. 1996 Nov;149(5):1617–1626. [PMC free article] [PubMed] [Google Scholar]
- 44.Ratajczak MZ, Kim CH, Abdel-Latif A, Schneider G, Kucia M, Morris AJ, et al. A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia. 2012 Jan;26(1):63–72. doi: 10.1038/leu.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]