Abstract
Background
Completion of the human papillomavirus (HPV) vaccine in a large proportion of young females is an important goal to prevent anogenital cancers associated with HPV. This study examines whether the proportion of insured women who complete the vaccine series has changed across time, and how provider type and age at initiation affects completion.
Methods
This retrospective cohort study used administrative data from a private insurance company. This study included 271,976 female initiators of the HPV vaccine who had been continuously enrolled in their respective insurance plan 365 days after vaccine initiation. Multivariate logistic regression was used to determine the odds of completing the vaccine series within 365 days after initiation.
Results
Females 13–18 years, 19–26 years, and ≥27 years old were less likely than 9–12 year olds to complete their HPV vaccine series. Obstetricians/gynecologists were more likely to administer vaccines to completers than pediatricians, while clinics, nurses, family care practitioners, and specialists were less likely to administer initial vaccines to completers compared to pediatricians. This study also found that 9–12 year olds and 13–18 year olds had lower odds of completing the HPV vaccine series for each subsequent year than those 19–26 years and ≥27 years old.
Conclusions
Among insured females in the US, the proportion of females that complete the HPV vaccine is dropping over time, especially among younger females that are specifically targeted to receive the vaccine. Physicians need to stress the importance of completing all three vaccinations to their patients.
Keywords: HPV vaccines, human papillomavirus, papillomavirus vaccines, human papillomavirus vaccine L1, type 6, 11, 16, 18
BACKGROUND
Human papillomavirus (HPV) is a highly prevalent sexually transmitted disease that has been linked to genital warts as well as oropharyngeal and anogenital cancer.1, 2 A vaccine that is effective in preventing HPV 6-, 11-, 16-, or 18-related premalignant genital or anal lesions and genital warts in women3–6 was licensed by the Food and Drug Administration (FDA) for females 9–26 years of age in June, 2006. The quadrivalent vaccine is given as a series of three injections, with the recommendation that the second be given 1– 2 months after the first, and the third administered 6 months after the initial injection. It is important that the 3-dose series is completed within the recommended time frame because the efficacy and duration of protection are not well established for incomplete immunizations.7, 8
Recommendations that are based on age and varying policies about the requirement for HPV vaccination from state to state may affect completion rates. In 2006, the Advisory Committee on Immunization Practices (ACIP) recommended the vaccine for females between 11–12 years old, although it can be administered as young as nine years. The ACIP also recommends catch-up vaccination for females between 13 and 26 years for those who have not been vaccinated or have not received the full series. The HPV vaccination is not required to enroll in most public schools, with the exception of Washington D. C. and Virginia, which passed legislation in 2007 requiring vaccination for sixth grade girls with an option for parents to opt out. Since the HPV vaccine is not required in most states to be enrolled in schools, uptake and completion rates may be lower than other vaccines which are required.
Various characteristics may affect whether the HPV vaccine series is completed. For example, areas with a higher concentration of pediatricians have been shown to have a higher proportion of children who are up-to-date on all of their vaccinations.9 Pediatricians may be an important advocate for HPV vaccinations among the 11–12 year old age group who are presumably receiving the bulk of the vaccinations, and may be more likely to encourage completion of the series than other types of physicians. However, it is important to know whether there are differences in completion between pediatricians and other types of doctors who initiate the HPV vaccine series because this could affect older adolescents, who may be more likely to visit family physicians or gynecologists to receive their vaccinations. Furthermore, a study of claims data in Maryland found that publicly insured HPV vaccine initiators are more likely to complete the series than privately insured initiators.10 Therefore, it is important to determine what factors may be related to completing the vaccine series among privately insured females, and how completion among the privately insured has changed since the vaccine was licensed by the FDA.
The purpose of this study is to estimate the proportion of insured women that complete the HPV vaccine series over time. In addition, the effects of age and type of health provider on completion among enrollees of a private insurance program available in all states of the US are examined. This study also explores the off-label administration of the HPV quadrivalent vaccine among women older than age 26 when the initial dose was administered.
METHODS
Participants and Procedure
This retrospective cohort study utilized data from administrative health data from a large health insurance company. Enrollees in this private health insurance plan were located in the US, with a higher concentration in the southern states. During the study period, from 2006 to 2010, 20,492,526 individuals were included in the dataset. Records for 277,725 individual subjects were obtained that 1) contained a CPT code for HPV quadrivalent vaccine (90649), 2) had been enrolled continuously for 365 days from the initiation of the vaccine series, and 3) were at least 9 years of age. Males (n=987), cases that included claims for the bivalent HPV vaccine (n=53), subjects from an unknown region (n=1,033) of the US, and subjects that received their first vaccine from an unknown provider type (n=3,676) were excluded due to low frequencies and lack of information about focal variables. The final number of vaccine initiators included in this study was 271,976. Informed consent was not obtained because this study was an analysis of de-identified secondary data. This study was exempted from full review by the institutional review board of the University of Texas Medical Branch, Galveston, TX.
Measures
Vaccination
Subjects who had at least 3 claims for the quadrivalent HPV vaccine on 3 different dates (that were at least 10 days apart) within 365 days were considered completers. For data analysis, completed vaccination series were dichotomized as 1 versus 0 for those with completed versus non-completed vaccination series, respectively. Enrollees who received only one or two doses of the vaccine were also examined in this study. Initiating enrollees were counted as only one case across time, and were not included in the data set more than once regardless of the date of subsequent vaccine claims. For an enrollee to be classified as receiving two vaccines, they must have had claims for two HPV vaccine injections during the study period on two different dates.
Age group
Enrollees were categorized into four groups based on their age at first vaccination: 9–12 years, 13–18 years, 19–26 years, and >26 years. Nine to ten year olds were included with 11–12 year olds in the comparison group because of the small number of 9–10 year olds who received the vaccine. Thirteen to eighteen year olds are included in the catch-up recommendations issued by the ACIP, but are still likely to have substantial parental involvement in the decision to return for additional doses of vaccines. The 19–26 year age group is also included in the catch-up group, but may not have as much parental involvement in returning for additional doses as the younger groups. The 27+ group are the adults who received the vaccination on an off-label basis.
Provider type
There were 346 insurance codes for the type of providers that filed a claim for a HPV vaccination; these were grouped into 8 categories. “Allied health” included non-physician providers such as chiropractors and nutritionists. “Clinic, hospital, and other facilities” included hospitals, clinics, and other facilities, such as extended care or outpatient facilities. Although a nurse or doctor may have provided the vaccine in these facilities, that information was not available, hence their inclusion in a separate category. The “nurse” category included nurses, such as nurse midwives, nurse practitioners, or nurses with training in a medical specialty. “Obstetrician/gynecologist” included obstetricians or gynecologists. The “pediatrician” category included pediatricians and adolescent medicine specialists. The “family care practitioner, internist” category included family practitioners, general practitioners, or internal medicine physicians. The “specialist” category included physicians not already included who specialized in a specific area of medicine, such as allergists or cardiovascular disease specialists.
Geographic region
The US was divided into six regions: Northeast, Mid-Atlantic, South, Midwest, Southwest, and West. Northeast included Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont. Mid-Atlantic included Delaware, Maryland, New Jersey, New York, Pennsylvania, and Washington, D.C. South included Alabama, Arkansas, Florida, Georgia, Kentucky, Louisiana, Mississippi, North Carolina, South Carolina, Tennessee, Virginia, and West Virginia. Midwest included Illinois, Indiana, Iowa, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, and Wisconsin. Southwest included Arizona, New Mexico, Oklahoma, and Texas. West included Alaska, Colorado, California, Hawaii, Idaho, Montana, Nevada, Oregon, Utah, Washington, and Wyoming. South was the comparison category because it represented the largest proportion of enrollees.
Year at first vaccination
The year at first vaccination included 2006 through 2009. The year 2010 was not included because of the requirement that subjects be enrolled continuously for 365 days in their respective insurance plan from the time of vaccination to be included in the study. Therefore, any subject who initiated vaccination after January 1, 2010 would not have been enrolled for 365 days from date of initiation because the data did not extend past December 31st, 2010. For analyses that examined trends across time for each age group, year at first vaccination was included as a continuous variable.
Statistical Analyses
Subjects were classified into four age groups. For each age group, the percent completion for year of initiation was graphed. Subjects were then stratified by the number of vaccinations that they received, and the proportions that received 1, 2, or 3 vaccinations were graphed by year of initiation. T-tests and chi-square tests were used for bivariate associations for continuous and categorical variables, respectively. Logistic regression was used to evaluate factors associated with vaccine completion. Since the trend of completion across time appeared to vary by age groups, we also examined the age and time interaction in the logistic regression model. The data analysis for this study was done using SAS R 9.2 (SAS Institute Inc., Cary, NC, USA.)
RESULTS
Descriptive characteristics were divided by vaccination completion (Table 1.) In this sample, 168,170 females received 1 or more vaccinations and did not complete within 365 days, while103,806 enrollees (38.2%) received three vaccinations within 365 days and were considered completers. It should be noted that 286 enrollees had claims for at least 3 doses, but these were not received within 365 days, and so were not considered complete. In addition, 1,153 females received >3 doses of the HPV vaccine. Of those women, 876 women were considered completers because two of those doses were received within 365 days of a previous dose. Most subjects (98.0%) were ≤26 years at vaccine initiation. Pediatricians administered a high proportion of initial vaccines (47.4%), and 29.2% of subjects lived in southern states. Females that received their first three vaccinations within 365 days received the first two vaccines on average 73.9 days (SD 26.4) apart and the second and third vaccinations 136.4 days (SD 35.5) apart. Subjects had been enrolled in their insurance plan for an average of 1,731 days (SD 950 days, range 365–3896), from the first day of enrollment until the time that the insurance was dropped, or the end of the study period (December 31st, 2010). Total vaccination initiation peaked in 2007, with a total of 115,193 subjects with at least one claim for a HPV vaccine that year.
Table 1.
Descriptive Characteristics of Females with 1 or More Gardasil Vaccinations (N=271,976)
|
Females
|
||
|---|---|---|
| Total1 | Completed2 | |
|
|
||
| n (% of total) | n (% of each group) | |
|
|
||
| Age Group | ||
| 9–12 years | 36,501 (13.4) | 13,797 (37.8) |
| 13–18 years | 147,818 (54.3) | 59,221 (40.1) |
| 19–26 years | 82,330 (30.3) | 29,442 (35.8) |
| > 26 years | 5,327 (2.0) | 1,346 (25.3) |
| Provider Type3 | ||
| Allied health provider | 648 (0.2) | 213 (32.9) |
| Clinic, hospital, and other facilities | 1,286 (0.5) | 260 (20.2) |
| Nurse | 2,130 (0.8) | 636 (29.9) |
| Obstetrician/Gynecologist | 57,636 (21.2) | 22,720 (39.4) |
| Pediatrician | 129,053 (47.4) | 50,676 (39.3) |
| Family care practitioner, internist | 79,300 (29.2) | 28,685 (36.2) |
| Specialist | 1,923 (0.7) | 616 (32.0) |
| Geographic Region | ||
| Northeast | 15,812 (5.8) | 5,678 (35.9) |
| Mid-Atlantic | 28,572 (10.5) | 11,819 (41.4) |
| Midwest | 78,078 (28.7) | 30,016 (38.4) |
| South | 79,410 (29.2) | 29,912 (37.7) |
| Southwest | 38,317 (14.1) | 14,077 (36.7) |
| West | 31,787 (11.7) | 12,304 (38.7) |
| Year at First Vaccination | ||
| 2006 | 12,782 (4.7) | 6,465 (50.6) |
| 2007 | 115,193 (42.3) | 54,121 (47.0) |
| 2008 | 85,615 (31.5) | 30,716 (35.9) |
| 2009 | 58,386 (21.5) | 12,504 (21.4) |
Total number of females in sample for each category
Completed HPV vaccine series in < 365 days
Type of provider that initiated HPV vaccine series
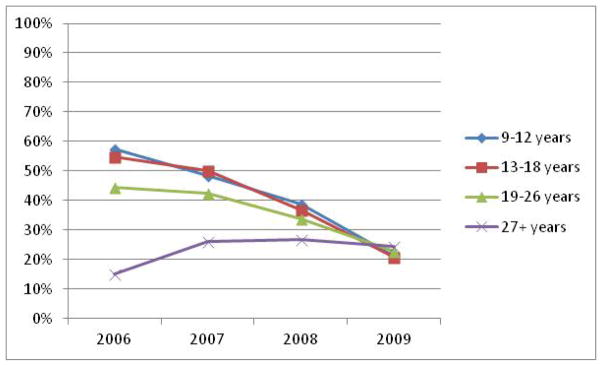
Across the time that was assessed for vaccine series completion, the proportion of females that completed dropped for 9–12 year olds, 13–18 year olds, and 19–26 year olds (Figure 1.) In the 9–12 year old age group, proportion of initiators who completed dropped from 57.45% in 2006 to 21.15% in 2009. In the 13–18 year age group, completion dropped from 54.91% in 2006 to 20.77% in 2009 and from 44.31% in 2006 to 22.61% in 2009 among the 19–26 year olds. In contrast, among the 27+ age group, there was an increase in completion, from 15% in 2006 to 26% in 2007 and 26.63% in 2008. However, in 2009, there was a slight decline to 24.47%.
Figure 1.
Proportion that received 3 vaccinations within 365 days of initiating of the quadrivalent HPV vaccine by age group
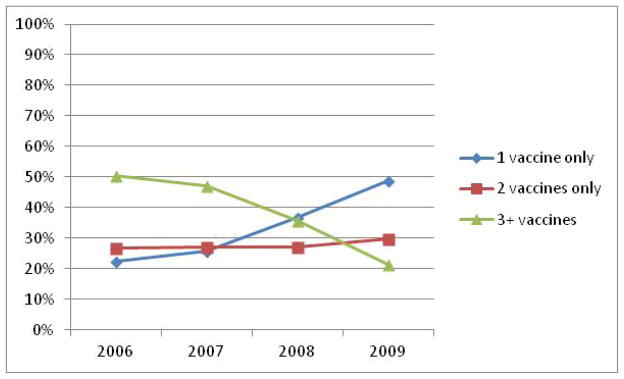
Some women received only 1 or 2 quadrivalent HPV vaccines during the 365 day period after initiation. The proportion of cases receiving only 1 vaccine in 365 days’ time increased between 2006 and 2009, from 22.4% of initiators in 2006 to 48.9% of initiators in 2009, crossing over the downward trajectory of patients who completed in 365 days between 2006 (50.6% of initiators) and 2009 (21.4%; Figure 2.) The proportion of initiators who received 2 vaccines only during the 365 days after vaccine initiation, however, remained relatively stable between 2006, with 26.8% of initiators receiving 2 HPV vaccinations in comparison with 29.7% of initiators in 2009 receiving 2 vaccinations.
Figure 2.
Proportion that received 1, 2, or 3 quadrivalent HPV vaccines.
After controlling for provider type, geographic region, year at first vaccination, and total length of continuous enrollment, enrollees who were 13–18 years at the time of initial vaccination had no significant difference in odds of completing the HPV vaccination series than enrollees 9–12 years old (Table 2.) Females 19–26 years of age had 0.76 (95% CI: 0.73–0.78) lower odds of completing, and the 27+ age group had 0.44 (95% CI: 0.41–0.47) lower odds of completing the HPV vaccination series compared to the 9–12 year olds. If the first vaccine was administered by a “clinic, hospital, or other facilities,” specialist, or by a nurse, subjects were less likely to complete the series than subjects who received the vaccination from a pediatrician. Subjects who went to a gynecologist or obstetrician for their first vaccine were more likely to complete than subjects who received their first vaccination from a pediatrician. In contrast, subjects who had a claim from a family care practitioner had slightly lower odds of completing than subjects who received their first vaccination from a pediatrician.
Table 2.
Binary Logistic Regression Estimating Odds of Completing HPV 3 Vaccine Series in 365 Days (N=271,976)
| OR (95% CI)1
|
|
|---|---|
| Age Group | |
| 9–12 years | Ref. |
| 13–18 years | 0.983 (0.959–1.007) |
| 19–26 years | 0.755 (0.733–0.778) |
| ≥ 27 years | 0.436 (0.407–0.468) |
| Provider Type | |
| Allied health provider | 0.873 (0.738–1.034) |
| Clinic, hospital, and other facilities | 0.467 (0.407–0.537) |
| Nurse | 0.800 (0.726–0.881) |
| Obstetrician/Gynecologist | 1.194 (1.164–1.225) |
| Pediatrician | Ref. |
| Family care practitioner, internist | 0.962 (0.942–0.982) |
| Specialist | 0.788 (0.714–0.870) |
| Geographic Region | |
| Northeast | 0.864 (0.833–0.896) |
| Mid–Atlantic | 1.180 (1.147–1.213) |
| Midwest | 1.010 (0.989–1.032) |
| South | Ref. |
| Southwest | 0.941 (0.917–0.965) |
| West | 1.060 (1.031–1.090) |
| Year at First Vaccination | |
| 2006 | 3.794 (3.645–3.950) |
| 2007 | 3.295 (3.220–3.372) |
| 2008 | 2.076 (2.027–2.128) |
| 2009 | Ref. |
| Odds of Completing for Each Age Group Across Time2 | |
| 9–12 years * year at first vaccination3 | 0.669 (0.664–0.675) |
| 13–18 years * year at first vaccination3 | 0.835 (0.832–0.838) |
| 19–26 years * year at first vaccination3 | 0.922 (0.920–0.923) |
| ≥ 27 years * year at first vaccination3 | 0.917 (0.915–0.918) |
OR = Odds Ratio, CI = Confidence Interval
Adjusted for provider type and region
“Year at first vaccination” included as continuous variable
Ref. = Reference Category
Visual observation of Figure 1 indicated that the 9–12 year and 13–18 year old groups appeared to have a more pronounced decline in the proportion of completers across time compared to the 19–26 year olds and 27+ year olds. This was tested, and it was found that the odds of completing for each subsequent year of initiation in the 9–12 age group (OR: 0.669 [95% CI: 0.664–0.675]) and the 13–18 age group (OR: 0.835 [95% CI: 0.832–0.838]) were smaller than for the 19–26 age group (OR: 0.922 [95% CI: 0.920–0.923]) and the 27+ age group (OR: 0.917 [95% CI: 0.915–0.918]) after controlling for provider type and geographic region. This indicates that the decrease in odds of completion by year is more pronounced among the younger vaccine initiators than among the older vaccine initiators.
CONCLUSION
Among females 9–26 years of age with private insurance, we observed a decrease in the proportion of initiators who completed the quadrivalent HPV vaccine series between 2006 and 2009. Two other studies from state–level data had similar results. One of these previous studies found comparable proportions of completion among initiators, while the other also showed a declining trend in HPV vaccine completion between 2006 and 2008, similar to our observations.11, 12 Furthermore, our study found that women in the 9–12 and 13–18 year old age groups have the steepest declines in completion compared with the other two age groups. This is concerning because the average age of sexual debut in the US is 17.4 years and may be lower than 14 years in 5% of females.13 Thus, most women need to complete the series before age 18 to obtain maximum efficacy from the vaccine.
Although completion decreased over time, it is important to note that the proportion of females who received only one injection during the study period increased over the study interval. These trends may indicate that physicians are focusing more on initiating the vaccine series as time passes, but may not be following up with their patients for subsequent doses of the vaccine. It is also possible that adverse socioeconomic conditions discouraged patients from completion because the co-pay or deductible for the vaccination became burdensome for them as the recession progressed. To improve completion for the HPV vaccine series, doctors of all types should be encouraged to use various methods to remind parents and patients to return to the clinic to receive the remaining doses. Calling patients has been shown to be an effective method of reminding patients to return for immunizations of all types, although it was not found to be effective in one study of adolescents where there were problems with phone number discontinuity.14 Recently, texting has been shown to improve rates of return for a subsequent HPV vaccine dose.15 This may offer a less expensive alternative to phone calls to remind patients to return for vaccinations.
In this sample, there were differences in the odds of administering the first vaccine to completers by physician specialties. “Clinics, hospitals, and other facilities,” nurses, specialists, and family practitioners, were less likely to administer a first vaccination to completers than pediatricians. However, it was observed that obstetricians/gynecologists were more likely to administer the first dose of the vaccine to completers than pediatricians. Similar to the results in our study, vaccine initiators in North Carolina who received their injections from family providers or hospitals were less likely to complete than those who received them from pediatricians.12 This indicates that physicians who initiate the HPV vaccination, but may not be as familiar with the recommendations of the series or may not be the primary care provider of the patient, need to stress the importance of completing the series and follow up with their patients. This is particularly important in clinics, hospitals, and other facilities where patients may not seek continuous care. If the patient returns to these providers, the opportunity should be taken to administer remaining vaccinations, as it has been shown that when patients do return to the same clinic facilities, they often do not receive additional vaccinations, even if their visit coincides with the time the vaccinations are due.16 Providing the facilities with the means to flag the records of patients who have received the first vaccine may improve completion rates for patients who do return. The increasing use of electronic medical records may make it easier for facilities to do this.
This study also included data on those women greater than 26 years of age who received HPV vaccinations on an off-label basis. Older women who initiate this vaccine series may view themselves as having a higher risk for contracting HPV which may account for the demand we observed among this age group. Although the vaccine is currently not recommended for women over age 26, research indicates that it is safe and effective in the development of immunogenicity to HPV infections in women up to 45 years old.17 Future research should investigate the benefits of extending current vaccine recommendations to include older women so that physicians can adequately counsel those who express interest.
The insurance records we examined also indicated that some males initiated the HPV vaccine before it was licensed or recommended for use in this population. They were excluded from this study because the FDA did not license the quadrivalent vaccine for routine use in males until October of 2009. Its use in males was not recommended by the ACIP until October of 2011, at which time they changed their recommendations to include initial vaccinations for boys 11–12 years of age with catch-up vaccination for those 13–21 years old who had not already been vaccinated. They also stated that males as young as 9 years and up to 26 years of age could receive the vaccine. The males in this data base who received the vaccine must have been very motivated, considering the difficulties they likely would have encountered in finding a physician who would administer the vaccine outside of FDA licensure. Economic research has indicated that if an adequate proportion of women are not vaccinated, then vaccinating males is a cost-effective method of preventing genital warts and precancerous conditions related to HPV 6/11/16/18 among women.18, 19 The low prevalence of females who have initiated and completed the HPV vaccine series in the US20 thus far indicates that vaccination of males would not only decrease their risk of genital warts and several cancers, but also help reduce the burden of cervical cancer in the US.
This study was not able to capture enrollees who dropped out of the insurance plan they had when they received the first vaccine dose but completed the series later, which may have led to a loss of enrollees who completed the series of vaccinations. However, it is likely that the proportion that completed is higher among enrollees who were continuously enrolled in their insurance plan, as it indicates more stability in their financial situation. Thus, our results are likely conservative estimates of HPV vaccination completion among insured females. In addition, sociodemographic characteristics were not available, which may have introduced some bias into the study which cannot be addressed.
This study only assessed the proportion of females that completed the quadrivalent HPV vaccine, and did not assess the proportion that completed the bivalent HPV vaccine. However, the bivalent vaccine is not commonly used in the US, and there were very few cases that initiated or included a code for the bivalent HPV vaccine in the claims dataset. Therefore, it is unlikely that including these cases would have changed the analyses. Furthermore, completion was defined as three vaccinations in 365 days, which means that 286 subjects did receive at least 3 doses, but not in one year’s time. Although these patients may gain adequate immunity from three vaccinations outside of one year’s time, the format that was chosen for this study follows more closely the guidelines of the ACIP.
Among insured females in this study, younger females had a noted decrease in the odds of completing each year compared to older females. This decrease could reduce the effectiveness of the vaccine in preventing HPV that is associated with anogenital malignancies. However, more females received one vaccine indicating a shift in emphasis from completion to simple initiation among providers of insured females. Thus, it is important that all providers who initiate the HPV vaccine series make an effort to follow up and stress the importance of completing the vaccine series to their patients. Calling or texting patients or the patients’ parents may be important methods of increasing HPV vaccine series completion among this population.
Acknowledgments
Federal support for this study was provided by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) as follows: Dr. Hirth, as an NRSA postdoctoral fellow under an institutional training grant (T32HD055163, PI: Berenson); Dr. Berenson, under a mid-career investigator award in patient-oriented research (K24HD043659, PI: Berenson).The content is solely the responsibility of the authors, and does not necessarily represent the official views of the NICHD or the National Institutes of Health.
Footnotes
There are no financial disclosures among the authors of this study.
Contributor Information
Jacqueline M. Hirth, Center for Interdisciplinary Research in Women's Health, University of Texas Medical Branch.
Alai Tan, Department of Preventive Medicine and Community Health, Senior Biostatistician, Sealy Center on Aging, University of Texas Medical Branch.
Gregg S. Wilkinson, Department of Preventive Medicine & Community Health, Senior Epidemiologist, Office of Biostatistics, Senior Fellow, Sealy Center on Aging, Senior Fellow, Center for Interdisciplinary Research in Women's Health, Associate Director of Research, Department of Family Medicine, University of Texas Medical Branch.
Abbey B. Berenson, Email: abberens@utmb.edu, Obstetrics & Gynecology, Director, Center for Interdisciplinary Research in Women’s Health University of Texas Medical Branch 301 University Blvd Rte 0587 Galveston, TX 77573, Phone: 409-772-2417, Fax: 409-747-5129.
References
- 1.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV Infection Among Females in the United States. JAMA. 2007;297(8):813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 2.Walboomers JMM, Jacobs MV, Manos MM, et al. Human Papillomavirus is a Necessary Cause of Invasive Cervical Cancer Worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Seoud M, Tjalma WA, Ronsse V. Cervical Adenocarcinoma: Moving Towards Better Prevention. Vaccine. 2011;29(49):9148–9158. doi: 10.1016/j.vaccine.2011.09.115. [DOI] [PubMed] [Google Scholar]
- 4.FUTURE I/II Study Group. Dillner J, Kjaer SK, et al. Four Year Efficacy of Prophylactic Human Papillomavirus Quadrivalent Vaccine Against Low Grade Cervical, Vulvar, and Vaginal Intraepithelial Neoplasia and Anogenital Warts: Randomised Controlled Trial. BMJ. 2010;341:c349334. doi: 10.1136/bmj.c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kjaer SK, Sigurdsson K, Iversen OE. A Pooled Analysis of Continued Prophylactic Efficacy of Quadrivalent Human Papillomavirus (Types 6/11/16/18) Vaccine against High-Grade Cervical and External Genital Lesions. Cancer Prevention Research. 2009;2(10):868–878. doi: 10.1158/1940-6207.CAPR-09-0031. [DOI] [PubMed] [Google Scholar]
- 6.Palefsky JM, Giuliano AR, Goldstone S, et al. HPV Vaccine against Anal HPV Infection and Anal Intraepithelial Neoplasia. N Engl J Med. 2011;365(17):1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 7.Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and Persistent Immunogenicity of a Quadrivalent Human Papillomavirus Types 6, 11, 16, 18 L1 Virus-Like Particle Vaccine in Preadolescents and Adolescents. Pediatr Infect Dis J. 2007;26(3):201–209. doi: 10.1097/01.inf.0000253970.29190.5a. [DOI] [PubMed] [Google Scholar]
- 8.Block SL, Nolan T, Sattler C, et al. Comparison of the Immunogenicity and Reactogenicity of a Prophylactic Quadrivalent Human Papillomavirus (Types 6, 11, 16, and 18) L1 Virus-Like Particle Vaccine in Male and Female Adolescents and Young Adult Women. Pediatrics. 2006;118:2135–2145. doi: 10.1542/peds.2006-0461. [DOI] [PubMed] [Google Scholar]
- 9.LeBaron CW, Massoudi M, Stevenson J, Lyons B. Vaccination Coverage and Physician Distribution in the United States, 1997. Pediatrics. 2001;107(3):E31. doi: 10.1542/peds.107.3.e31. [DOI] [PubMed] [Google Scholar]
- 10.Schluterman NH, Terplan M, Lydecker AD, Tracy JK. Human Papillomavirus (HPV) Vaccine Uptake and Completion at an Urban Hospital. Vaccine. 2011;29:3767–3772. doi: 10.1016/j.vaccine.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Chao C, Velicer C, Slezak JM, Jacobsen SJ. Correlates for Completion of 3-Dose Regimen of HPV Vaccine in Female Members of a Managed Care Organization. Mayo Clin Proc. 2009;84(10):864–870. doi: 10.4065/84.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan W, Viera AJ, Rowe-West B, Grimshaw A, Quinn B, Walter EB. The HPV Vaccine: Are Dosing Recommendations Being Followed? Vaccine. 2011;29:2548–2554. doi: 10.1016/j.vaccine.2011.01.066. [DOI] [PubMed] [Google Scholar]
- 13.Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, Family Planning, and Reproductive Health of U.S. Women: Data from the 2002 National Survey of Family Growth. National Center for Health Statistics. Vital Health Stat. 2005;23(25) [PubMed] [Google Scholar]
- 14.Vann JCJ, Szilagyi P. Patient Reminder and Recall Systems to Improve Immunization Rates (Review) Cochrane Database of Systematic Reviews. 2005;(3) doi: 10.1002/14651858.CD14003941.pub14651852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kharbanda EO, Stockwell MS, Fox HW, Andres R, Lara M, Rickert VI. Text Message Reminders to Promote Human Papillomavirus Vaccination. Vaccine. 2011;29:2537–2541. doi: 10.1016/j.vaccine.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 16.Neubrand TPL, Breitkopf CR, Rupp R, Breitkopf D, Rosenthal SL. Factors Associated with Completion of the Human Papillomavirus Vaccine Series. Clin Pediatr (Phila) 2009;48(9):966–969. doi: 10.1177/0009922809337534. [DOI] [PubMed] [Google Scholar]
- 17.Castellsaguè X, Muñoz N, Pitisuttithum P, et al. End-of-Study Safety, Immunogenicity, and Efficacy of Quadrivalent HPV (Types 6, 11, 16, 18) Recombinabt Vaccine in Adult Women 24–45 Years of Age. Br J Cancer. 2011;105(1):28–37. doi: 10.1038/bjc.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brisson M, van de Velde N, Franco EL, Drolet M, Boily M-C. Incremental Impact of Adding Boys to Current Human Papillomavirus Vaccination Programs: Role of Herd Immunity. The Journal of Infectious Diseases. 2011;204:372–376. doi: 10.1093/infdis/jir285. [DOI] [PubMed] [Google Scholar]
- 19.Elbasha EH, Dasbach EJ. Impact of Vaccinating Boys and Men against HPV in the United States. Vaccine. 2010;28:6858–6867. doi: 10.1016/j.vaccine.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. National and State Vaccination Coverage among Adolescents Aged 13 through 17 Years-United States, 2010. MMWR CDC Surveill Summ. 2011;60(33):1117–1123. [PubMed] [Google Scholar]