Abstract
Background:
Carbohydrate intolerance is the most common metabolic complication of pregnancy. Gestational Diabetes Mellitus (GDM) poses numerous problems for both mother and fetus. The objectives of this study are to find out the incidence of gestational diabetes mellitus in pregnant women and their pregnancy outcomes. It was also to discover the risk factors for the admission of neonates to the Neonatal Intensive Care Unit (NICU).
Design and Patients:
A hospital-based prospective study performed at King Khalid University hospital (KKUH), where 685 pregnant women who were diagnosed with gestational diabetes mellitus, out of 8000 pregnant women registered between January 2000 - December 2001, were followed and their outcomes studied.
Results:
The incidence of gestational diabetes mellitus was found to be 8.6% (95% C.I: 8.1, 9.3). There were 511 (74.6%) spontaneous vertex deliveries, and 148 (21.6%) were delivered by lower segment cesarean section. Maternal morbidity in these women was 1.2%. A total of 697 babies were delivered by these 685 women, out of whom 675 were singleton pregnancies, 9 sets of twins and one set of quadruplets. Six-hundred-eighty-seven babies were born alive, 7 babies died in utero and 3 died in the neonatal period. The incidence of neonatal intensive care admission was 4.9%. The mean length of stay in the NICU was 16 days. The commonest cause of neonatal NICU admission was hyperbilirubinemia (41.2%). The risk factors for NICU admission were delivery by non SVD procedure (RR: 4.6, 95% C.I:2.8, 7.7), preterm deliveries, (RR: 4.6, 95% C.I.:2.7, 7.7), and induction of labor (RR: 2.5, 95% C.I: 1.4, 4.5).
Conclusion:
The observation and quantification of maternal outcomes with gestational diabetes mellitus are necessary, so that proper measures could be taken to reduce complications during delivery and the neonatal period and thereby, minimize particularly NICU admission rate.
Keywords: Gestational diabetes mellitus, pregnant women, Neonate Intensive Care Unit, Saudi Arabia
INTRODUCTION
Diabetes mellitus is a global health problem which cuts across all age groups, of both sexes. Gestational diabetes mellitus (GDM) was first described as diabetes occurring “only during pregnancy, being absent at other times” by Duncan in 1982.1 The frequency of diabetes in pregnancy is highly variable but generally reflects the underlying pattern of type 2 diabetes in the particular population.1,2 Reported prevalence varies from 0.6% in China to 15% in Indian-born Australians. In the USA, the prevalence across all ethnic groups is 4 %.1 Even though, highly improved outcomes have been reported, reflected by a dramatic decline in maternal and perinatal morbidity and mortality over the past few years, debate persists on the care of pregnant women with gestational diabetes mellitus (GDM). The complications associated with GDM are macrosomia and stillbirth.2 But both of these complications are preventable as they are related to the degree of maternal glycaemic control. There is also a higher chance of pre-eclampsia (edema), pre-term delivery and polyhydramnios. Mothers with GDM are more likely to have large babies with higher birth weight. This increases the number of deliveries by cesarean section rather than vaginal delivery in order to reduce the risk of injury to the mother and baby. But again the chances of these complications are lower when women have good glycemic control. Another aspect of the outcome of GDM pregnancy relates to the babies born to these women. Although not all babies born to these women will have birth defects, there is a high probability of birth defects, if there has been no control of glucose level during pregnancy. Neonates born to women with gestational diabetes mellitus have a perinatal mortality rate similar to that of the control population,3 but with an increased rate of macrosomia and morbidity in terms of neonatal asphyxia, birth trauma, hypoglycemia, hypocalcaemia, hyperbilirubinemia and respiratory distress, among others.3,4
For pregnant women with poor diabetic control, the risk for a baby to be born with birth defects is about 6-10%; which is twice the rate when the mother's diabetes is well controlled. Some of the associated birth defects include spinal cord defects (spina bifida), heart defects, skeletal defects, and defects in the urinary, reproductive, and digestive systems. Also babies born to women with diabetes have an increased chance of having breathing difficulties, low blood sugar (hypoglycemia) and jaundice (yellowish skin) at birth.
Insulin-treated gestational diabetic patients seem to be affected more frequently than diet-only-treated patients.2 An abnormal glucose metabolism during pregnancy may lead to various types of adverse outcomes for both the mother and the fetus. As a result screening for diabetes mellitus during pregnancy is currently being offered to all pregnant women. The approach to gestational diabetes mellitus has altered markedly in the last decade. It is now guided by universal screening and an impetus to establish 24-hour normoglycaemia in these women through serial measurements of blood glucose by home monitoring and glycosylated hemoglobin. Improved outcome has been associated with improved levels of glycemia. Seventy-five percent of women with gestational diabetes respond to diet therapy alone. When glycemia is not achieved by diet alone, insulin therapy is recommended.5 With the background of 24% prevalence of diabetes6 in the Saudi general population, there is a need to quantify the magnitude of GDM and the pregnancy outcomes. Hence the primary objectives of this study are to quantify the incidence of gestational diabetes in pregnant women and the outcomes of NICU admission of their neonates.
MATERIAL AND METHODS
This is a hospital-based prospective study carried out between January 2000 and December 2001, where 8000 pregnant women who attended the department of Obstetrics and Gynecology at KKUH were studied. Screening with a 50-g oral glucose challenge was administered routinely to consecutive pregnant women at their first antenatal visit. Patients with abnormal screening results, defined as a serum glucose level > 7.8 mmol/l, were later given a 75-g oral glucose load, (glucose tolerance test) and the plasma glucose level was measured in the baseline fasting state, then one, two and three hour intervals after glucose ingestion. Gestational diabetes mellitus was considered if ≥ 2 values exceeded the following cutoff points: fasting, 5.8 mmol/l; one hour 10.8 mmol/l; two hours 8 mmol/l; and three hours 6 mmol/l. The tolerance test was repeated at 28 weeks in those patients at high risk of developing gestational diabetes, particularly those with a positive family history of diabetes, those who were obese, with a history of fetal macrosomia or unexplained neonatal death in previous pregnancies, with repeated urinary tract infections, repeated vaginal candidiasis or a history of neonatal hypocalcaemia, and hypoglycemia.
After the diagnosis of gestational diabetes mellitus was made, patients were prescribed a diabetic diet consisting of 1800 kcal/day, and after a week on the diet admitted to the day care unit (DCU) for a blood sugar series (BSS). The glycemic profile measuring the venous glucose level was performed in the fasting state, and also two hours after each main meal. If the fasting glucose concentration was < 5.8 mmol/l, and two hours after each meal ≤ 8 mmol/l dietary recommendation was considered enough. If these values were exceeded, provided there was good compliance by the patient to her diet, insulin treatment was initiated.
The women were offered clinic visits of two-week intervals, and ultrasound examinations were performed at the first antenatal visits, 28 weeks and 36-40 weeks. More ultrasound examinations were done as needed by each case. Labor was induced at 40 weeks of gestation using pessaries prostaglandin E2 pessaries or intra cervical gel if there is no spontaneous labor. Blood glucose was measured in the newborn of diabetic women 30 minutes after delivery. If there was hypoglycemia, measurements were repeated every second hour until stable values above 2.5 mmol/l were obtained. Macrosomia was defined as birth weight ≥ 4000 g; neonatal hypoglycemia was defined as a minimum blood glucose value < 2.0 mmol/l during the first 48 hours of life. Apgar scores < 7 after 5 minutes were considered low.
STATISTICAL ANALYSIS
The data was entered in MS Excel and analyzed by SPSSpc Version 10.0 statistical software. Univariate analysis was done by using Chi-square test, and relative risk (RR) was calculated for categorical variables, whereas students t-test for independent samples, was used for continuous variables. The 95% confidence intervals were provided for RR and for the difference in the mean values.
RESULTS
Out of 8000 pregnant women admitted, 685 (8.6%, 95% confidence interval: 8.1, 9.3) were diagnosed with GDM. The characteristics of these women and the distribution of mode of delivery are given in Table 1. The commonest indication for LSCS whether elective or emergency was having had two or more previous LSCS. Polyhydramnious was found in 15 (2.2%) women, while pre-eclampsia (PET) was identified in 36 (5.3%) women. Maternal morbidity in these women was 1.2 % (8 out of 685), while maternal urinary tract infection (UTI) due to group-B streptococcus was detected in two (0.3%) women. Manual removal of placenta was performed in three (0.4%) women, one woman needed blood transfusion while one woman had pulmonary embolism for which Surgical Intensive Care Unit (SICU) admission was required.
Table 1.
Characteristics of gestational diabetic mothers and their mode of delivery (n=685)
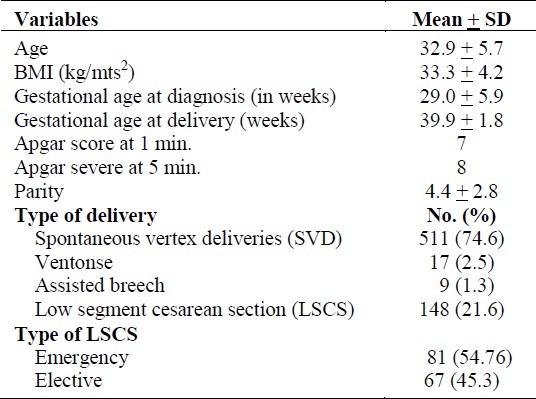
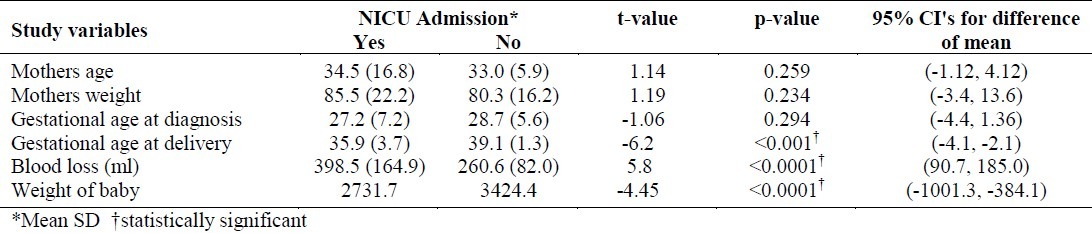
These 685 women delivered 697 babies, 51.6% of whom were males. The mean weight of these babies was 3389.9 grams (SD 600.7). Of these new born babies, 34 (4.9%, 95% C.I.:3.3, 6.5) were admitted to NICU. When these 34 cases were compared with a random selection of 68 babies who were not admitted, it was found that the maternal variables of onset of labor, mode of delivery, and pregnancy complications were highly statistically significantly associated with the NICU admission (Table 2). Thus, the risk of NICU admission was 2.5 times higher for the neonates of women who had non-spontaneouslabor, 4.6 times higher for the women who had non SVD procedures (Vento use/forceps, and CS) as the mode of delivery, 4.6 times higher for the women who had pregnancy complications (preterm labor) and 2.0 times higher for women who had other complications (polyhydramnios, oligohydramnios, IUGR and postdate pregnancy) when compared to mothers of those babies who had not been admitted to NICU. A comparison of mean values of variables of GDM mothers, in relation to the NICU admission by their babies has clearly demonstrated that, the mother's characteristics such as gestational age at delivery and blood loss at the time of delivery had a significant effect on the outcome of the baby (Table 3). The new born baby's weight also differed significantly in relation to the NICU admission.
Table 2.
Risk factors for NICU admission among GDM pregnant women
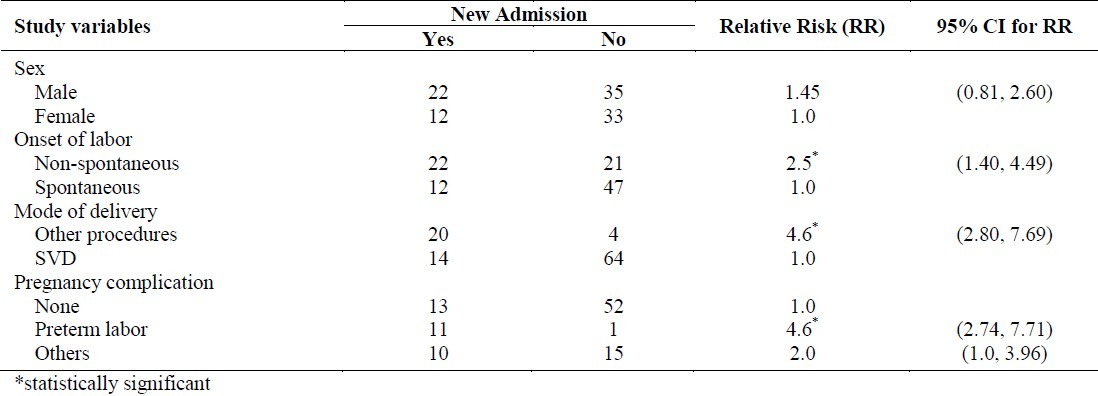
Table 3.
Comparison of mean values of variables of GDM mothers and mean weight of their babies in relation to the admission of their neonates in NICU
The mean length of stay in NICU by these 34 babies was 16 days. The complications which led to the admission of these babies into NICU were hyperbilirubinemia (14, 41.2%), respiratory complications (11, 32.3%), hydronephroses (2, 5.9%), congenital anomalies (2, 5.9%), and sepsis (2, 5.9%). Other complications were vomiting, anemia of the new born and hypoglycemia (3, 8.8%).
DISCUSSION
Gestational diabetes mellitus is known as glucose intolerance or diabetes mellitus which is diagnosed for the first time during pregnancy but disappears after the pregnancy. Pregnancy is a time of increasing insulin resistance because of great hormonal changes. Gestation may unmask a maternal defect, such as impaired insulin secretion and/or reduced glucose utilization, in insulin-sensitive tissues (e.g. skeletal muscle). Gestational diabetes ensues when the woman's insulin secretory capacity is inadequate to overcome the progressive insulin resistance. It is often associated with maternal risk factors, such as overweight, advanced age and a previous complicated obstetric history.3,4 It has been claimed that the higher rate of complications can be ascribed to these risk factors and not to the hyperglycemic condition itself.7 Our prospective study shows the magnitude of GDM as 8.6% in the pregnant mothers, reflecting the underlying pattern of diabetes in the Kingdom of Saudi Arabia (KSA). Factors such as maternal age, BMI and parity were of a higher order in our study sample. The presence of GDM has implications for both the baby and the mother. In our subjects, the mode of delivery by cesarean section was higher (21.6%) which is in agreement with the study which reported a higher rate of cesarean sections in GDM women in the USA.5 This study shows that the maternal variables: gestational age at the time of delivery, onset of labor, mode of delivery, pregnancy complications and blood loss during pregnancy were contributing factors to NICU admission of the newborn. This study also shows that the low birth weight of the new born was statistically significantly associated with NICU admission. Our results indicate a lower rate of NICU admission when compared with other studies, but the indications for such admissions are nearly the same.8–10 There is evidence that perinatal morbidity is higher in untreated GDM.14 In this study, the morbidity rate was low probably because all our patients were treated with insulin or diet. As in the Texas study, our study revealed that the commonest complication in babies of GDM mothers and the leading cause for NICU admission was hyperbilirubinemia, 41.2% compared to 6.4% for neonates of non-diabetic mothers.11 The rate of respiratory complications in babies of GDM mothers was 32.3% which could be considered high and in accord with previous studies.8,9 Our results agree with the findings of the study done by Hod M, et al which showed that, GDM is associated with increased perinatal morbidity, characterized by macrosomia, hyperbilirubinaemia, respiratory distress syndrome.15 Prematurity and growth retardation contribute mostly to the development of these respiratory complications which are also correlated to poor maternal metabolic control.9,10 In our study, there was a high incidence of hydronephrosis in babies of GDM mothers but we could not find any previous study that had looked at this complication for comparison. Cardiac malformations were the most common type of anomaly in these diabetic pregnancies, which is in agreement with previous results.12 Hypoglycemia was found to be a mild cause for NICU admissions as found in previous studies.13 Other complications like vomiting and anemia of the newborn were the other important indications for NICU admission.
In conclusion, pregnancies complicated by gestational diabetes mellitus without treatment are associated with a higher frequency of adverse maternal and fetal outcomes. Besides checking on other maternal factors, diabetes during pregnancy should be controlled, in order to reduce both the maternal and neonatal complications, and accordingly reduce the number of NICU admissions.
REFERENCES
- 1.Duncan JM. On puerperal diabetes. Transactions of the Obstetrical Society of London. 1982;24:256–85. [Google Scholar]
- 2.Dandrow RV, O’Sullivan JB. Obstetric hazards of gestational diabetes mellitus. Am J Obstet and Gynecol. 1966;96:1144–7. doi: 10.1016/0002-9378(66)90525-4. [DOI] [PubMed] [Google Scholar]
- 3.King H. Epidemiology of glucose intolerance and gestational diabetes in women of child bearing age. Diabetes Care. 1998;21(Suppl 2):9–13. [PubMed] [Google Scholar]
- 4.Egelgau MM, Herman WH, Smith PJ, et al. The epidemiology of diabetes and pregnancy in the US. Diabetes Care. 1995;18:1029–33. doi: 10.2337/diacare.18.7.1029. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen JD, Cousins L. A population based study of maternal and perinatal outcome in patients with gestational diabetes. Am J Obstetric Gynecology. 1989;161:981–6. doi: 10.1016/0002-9378(89)90767-9. [DOI] [PubMed] [Google Scholar]
- 6.AL-Nozha MM, AL-Maatouq MA, Al-Mazrou YY, Al-Harthi SS, et al. Diabetes mellitus in Saudi Arabia. Saudi Med Journal. 2004;25(11):1603–10. [PubMed] [Google Scholar]
- 7.Jovanovic-Peterson L, Peterson MC. New strategies for the treatment of gestational diabetes. Isr J Med Sci. 1991;27:510–5. [PubMed] [Google Scholar]
- 8.Greene MF, Hare JW, Krache M, Phillippe M, Barss VA, Saltzman DH, et al. Prematurity among insulin-requiring diabetic gravid women. Am J Obstetric gynecology. 1989;161:106–11. doi: 10.1016/0002-9378(89)90244-5. [DOI] [PubMed] [Google Scholar]
- 9.Hanson U, Persson B. Outcome of pregnancies complicated by Type 1 insulin-dependent diabetes in Sweden: acute pregnancy complications, neonatal mortality and morbidity. Am J Perinatol. 1993;10:330–3. doi: 10.1055/s-2007-994754. [DOI] [PubMed] [Google Scholar]
- 10.Rosenn B, Miodovnik M, Combs CA, Khoury J, Siddiqi TA. Poor glycemic control and antepartum obstetric complications in women with insulin-dependent diabetes. Int J Gynaecol Obstet. 1993;43:21–8. doi: 10.1016/0020-7292(93)90269-3. [DOI] [PubMed] [Google Scholar]
- 11.Langer O, Rodriguez DA, Xenakis EM, McFarland MB, et al. Intensified versus conventional management of gestational diabetes. Am J Obstetric Gynecology. 1994;170:103–47. doi: 10.1016/s0002-9378(94)70097-4. [DOI] [PubMed] [Google Scholar]
- 12.Reece EA, Homko CJ. Infant of the diabetic mother. Semin Perinatol. 1994;18:459–60. [PubMed] [Google Scholar]
- 13.Moshe HOD, Meizner I. Diabetes in pregnancy. Ann. Isr Supper Sanita. 1997;33:317–22. [PubMed] [Google Scholar]
- 14.O’Sullivan JB, Charles D, Mahan CM, Dandrow RV. Gestational diabetes and perinatal mortality rate. Am J Obstetric Gynecology. 1973;136:901–4. doi: 10.1016/s0002-9378(16)33834-0. [DOI] [PubMed] [Google Scholar]
- 15.Hod M, Merlov P, Friedman S, Schoenfeld A. Gestational diabetes mellitus: a survey of perinatal complications in the 1980's. Diabetes. 1991;40(Suppl 2):74–8. doi: 10.2337/diab.40.2.s74. [DOI] [PubMed] [Google Scholar]


