Abstract
Objective:
To determine the seroprevalence rates of immunoglobulin G (IgG) and immunoglobulin M (IgM) to Chlamydia trachomatis in Saudi pregnant women.
Subjects and Methods:
Using enzyme-linked immunosorbent assay (ELISA), a total of 1600 serum samples were tested for antibodies to Chlamydia trachomatis known to cause a variety of clinical syndromes in women and newborn infants.
Results:
Chlamydia trachomatis IgG antibodies were detected in 8.7% and IgM antibodies were found in 1.5% of different age groups.
Conclusion:
Pregnant Saudi women have low prevalence rate of Chlamydia trachomatis IgG antibodies and lower prevalence for Chlamydia trachomatis IgM.
Keywords: Makkah, Pregnant women, ELISA, Saudi Arabia, Chlamydia trachomatis
INTRODUCTION
Chlamydia are small gram-negative eubacteria that grow intracellular. There are four species (Chlamydia trachomatis, C. psittaci, C. pneumonia and C. pecorum), the first three of which have been associated with various human diseases involving particular populations. Chlamydia trachomatis is a common bacterial cause of sexually transmitted disease worldwide and is responsible for high levels of morbidity.1,2 In the United States, Chlamydia trachomatis is the most common sexually transmitted pathogen, with an estimated 4.5 million new cases reported each year.3,4
It is also the major cause of genitourinary infection in developed countries.5 Chlamydia trachomatis can cause infections of the cervix, urethra and upper genital tract in women, infections of the urethra and epididymis in men, and the cause of conjunctivitis and pneumonia in newborns.6 It has been estimated that some 10-40% of inadequately treated women with chlamydial cervicitis develop pelvic inflammatory disease (PID) which is an important cause of infertility, chronic pelvic pain, ectopic pregnancy, and adverse outcome of pregnancy.7,9
In a majority of women, infections are asymptomatic and thus require laboratory testing for diagnosis. For this reason, laboratory screening of young women has been considered the cornerstone of chlamydial screening programmes. These programmes have been associated with subsequent reductions in the prevalence of chlamydia in a given population, as well as reduced the incidence of PID among the women screened.10
The most sensitive method for the diagnosis of a genital C. trachomatis infection was, until recently, based on tissue culture of the microorganism on McCoy cells, and was regarded as the “gold standard”. However, there are several disadvantages of cell culture.11–13 These have led to the search for alternative techniques for the detection of C. trachomatis. Besides the development of antigen detection techniques, such as direct fluorescent-antibody tests and enzyme immunoassays,14,15 nucleic acid amplification techniques have been developed.16,17
Nucleic acid amplification tests (NAAT's) have generally been more sensitive than traditional tests for the detection of C. trachomatis. They also have the advantage that urine can be substituted for the traditional swab specimen, thus reducing the dependence on invasive procedures and expanding the venues where specimens can be obtained. In the last few years, two such assays have been made available commercially: the urine LCx assay from Abbott Laboratories based on the ligase chain reaction (LCR), a test which has been withdrawn and therefore no longer available,18 and the AMPLICOR19 assay from Roche based on PCR. These assays are based on detection and amplification of C. trachomatis DNA in urine and have been proved to be reliable and reproducible alternatives.
The prevalence of chlamydia genital infection in women varies in different groups and communities. The incidence of C. trachomatis infection in asymptomatic unselected pregnant women varies from 4 to 21%.20 Very few reports on the prevalence of C. trachomatis in developing countries have been published.21 Only a few studies done in Saudi Arabia have been reported in the literature. In a study carried out by Forsey and Darougar, 10% of the females attending gynecology clinics were positive for chlamydia.22 In another study done by Jamjoom et al23 only 0.5% (4/820) of the women were positive for the culture of C. trachomatis and in another study which was conducted in Riyadh24 for direct antigen detection, no positive cases were found (0/57).
The aim of the present study was, therefore, to determine the seroprevalence of Chlamydia trachomatis in a group of randomly selected Saudi pregnant women, using the ELISA IgG and IgM assays.
SUBJECTS AND METHODS
The study was carried out in the period from January 2003 to January 2004. A total of 1600 randomly selected Saudi pregnant women in their first trimester attending the Maternity and Children's hospital and other main hospitals (al-Noor and Hera) in Makkah for ante-natal care were included in the study. The age range of the patients was 16-45 years with a mean age of 28.5 years. A 10ml clotted blood sample was obtained from each patient after obtaining informed consent. Serum was separated, aliquoted into two eppendorf tubes and stored at -20°C until tested.
All samples were screened using the indirect enzyme linked immunosorbent assay (ELISA) (Wampole Laboratories, New Jersey, USA) for anti-IgG antibodies using a group specific antigen to types D to K of C. trachomatis. All negative samples with the IgG ELISA test were then tested for anti-chlamydial specific IgM antibodies. The IgM ELISA test is specific for C. trachomatis antibodies and uses a purified antigen which has been coated onto the microtitre wells. The serum diluent provided in the kit contains rheumatoid factor sorbent to prevent interference by human IgG and thus prevent false positive results with the IgM test. Both ELISA tests were carried out according to standard procedures described previously.25
The results for both ELISA tests were interpreted by calculating the Antibody Index (AI) of each sample. This was determined by dividing the Optical Density (OD) value of each sample by the cut-off value. Specimens giving an AI value of less than 0.9 were regarded as negative. An antibody index of between 0.9-1.1 was considered a borderline positive or equivocal and the sample was repeat tested. Specimens with an AI of greater than 1.1 were considered positive for C. trachomatis.
ETHICAL CONSIDERATION
An informed consent was obtained from each individual before inclusion in the study. Every subject had been informed about the procedure before the blood samples were collected, making absolutely certain that she understood the procedure that was to be carried out. The subjects were aware that they had the right to refuse to be included in the study without any prejudicial effect.
RESULTS
One hundred and forty (8.7%) of the total number of 1600 sera tested were positive for IgG antibodies to C. trachomatis. The remaining 1460 samples that were negative were tested for specific anti-chlamydial IgM antibodies to detect recent or current infection with the organism (Table 1). Of the 1460 tested, 22(1.5%) were found to be positive for specific IgM using the ELISA test (Table 1).
Table 1.
Chlamydia trachomatis IgG and IgM positive among Saudi pregnant women

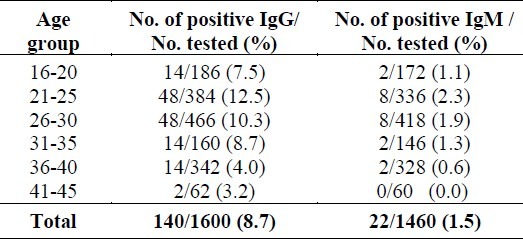
The prevalence of chlamydia IgG and IgM antibodies in the different age groups is summarized in (Table 2). The highest rate of positivity (12.5%) was in the age group 21-25 years. While 2.3% of the women in the same age group tested were also positive for anti-chlamydial IgM antibodies.
Table 2.
Chlamydia trachomatis IgG and IgM positive among different age groups
DISCUSSION
The prevalence of clinical and subclinical infections due to C. Trachomatis have been reported as high in both men and women in many countries. The World Health Organization estimated that 89 million cases of C. trachomatis infection occurred worldwide.26 In the United States, C. trachomatis infections are the most commonly reported bacterial disease, with an estimated 4-5 million cases occurring annually. The sequelae of C. trachomatis infections in women, namely pelvic inflammatory disease (PID), infertility and ectopic pregnancy, are the most costly outcome of any STD (except HIV/AIDS), resulting in an estimated 4 billion dollars in health care costs per annum.27 Neonates usually become infected with C. trachomatis during birth. Conjunctivitis, pneumonia, myocarditis, otitis media and other diseases may develop in neonates born to mothers infected with chlamydia.28,29
The prevalence of C. trachomatis infection in pregnant women ranges from 2 to 35%.30,32 In two studies, it was found that women with recent or invasive infection indicated by significant immunoglobulin M (IgM) antibody titers against C. trachomatis were at higher risk for preterm delivery and premature rupture of membranes.33
In our study, 8.7% of the women were positive for IgG antibodies to C. trachomatis with antibody indexes of between 1.4 and 2.0. Only 22/1460 (1.5%) of the women were positive for C. trachomatis specific IgM antibodies. These results are in keeping with other studies done in Saudi Arabia where prevalence rates of 0.5% to 10% have been reported previously.20,23 The low prevalence rate in our patient population may be due to the adherence of strict moral principles and code of ethics in Saudi Arabia. For these reasons, mucopurulent cervicitis is not commonly described among Saudi patients and very few patients suffer from this disease entity.23
In contrast, it has been shown in the USA and Europe that demographic factors which increase the risk of chlamydial infection include youth, non-white race, single marital status, multiple sexual partners and the use of oral contraceptives in women.20
Therefore, a closer attempt should be made to correlate risk factors and disease entity when screening for C. trachomatis and the choice of laboratory investigations. Thus, in the populations at high risk of the disease, it would be more effective to detect antigen especially in sexually active young women.
There are many limitations of the ELISA test especially when testing for uncomplicated genital C. trachomatis infection and therefore, it should not be used for screening, because previous chlamydial infections frequently produces long-lasting antibodies that cannot be easily distinguished from the antibodies produced in a current infection. More specific than the ELISA test is the microimmunofluorescence (MIF) test, which uses type specific antigens and is nowadays regarded as the “gold serological standard” to which other serological tests should be compared.
Finally, it is important that future studies on the epidemiology of C. trachomatis be carried out in order to determine the true prevalence of this organism in Saudi Arabia.
The results endorse the fact that pregnant women should be screened routinely using the MIF test for the presence of Chlamydia to prevent adverse pregnancy outcome.
ACKNOWLEDGMENTS
This research was supported by a grant from Umm Al-Qura University, Makkah, Saudi Arabia. The authors would like to thank Ms. Mai Kaaki and Ms. Eman Sharaf for their excellent technical assistance.
REFERENCES
- 1.Kalman S, Mitchell W, Marathe R, et al. Comparative genomes of Chlamydia Pneumoniae and C. trachomatis. Nat Genet. 1999;21:385–9. doi: 10.1038/7716. [DOI] [PubMed] [Google Scholar]
- 2.Brunham RC. Human immunity to chlamydiae. In: Stephens ES, editor. Chlamydia intracellular biology, pathogenesis, and immunity. Washington, DC: ASM Press; 1999. pp. 211–38. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Chlamydiatrachomatis infection: policy guidelines for prevention and control. Morbid Mortal Weekly Rep. 1993;42:1–39. [Google Scholar]
- 4.Washington AE, Johnson RE, Sanders LL. Chlamydia trachomatis infections in the United States. What are they costing us? JAMA. 1987;257:2070–2. [PubMed] [Google Scholar]
- 5.Batteiger BE, Jones RB. Chlamydial infections. Infect Dis Clin North Am. 1987;1:55–81. [PubMed] [Google Scholar]
- 6.Stamm WE. Chlamydia Trachomatis Infections: progress and problems. J Infect Dis. 1999;179(suppl 2):380–3. doi: 10.1086/513844. [DOI] [PubMed] [Google Scholar]
- 7.Cates W, Wasserheit JN. Genital chlamydial infections: epidemiology and reproductive sequelae. Am J Obstst Gynecol. 1991;164:1771–81. doi: 10.1016/0002-9378(91)90559-a. [DOI] [PubMed] [Google Scholar]
- 8.McGregor JA, French JI. Chlamydia trachomatis infection in pregnancy. Am J Obstet Gynecol. 1991;164:1782–9. doi: 10.1016/0002-9378(91)90560-e. [DOI] [PubMed] [Google Scholar]
- 9.Ryan GM, Abdella SG, McNeely VS, et al. Chlamydia trachomatis infection in pregnancy and effect of treatment on outcome. Am J Obstet Gynecol. 1990;162:34–9. doi: 10.1016/0002-9378(90)90815-o. [DOI] [PubMed] [Google Scholar]
- 10.Scholes DA, Stergachis FE, Heidrich H. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N Engl J Med. 1996;344:1362–6. doi: 10.1056/NEJM199605233342103. [DOI] [PubMed] [Google Scholar]
- 11.Thejs H, Gnarpe J, Gnarpe H, et al. Expanded gold standard in the diagnosis of Chlamydia trachomatis in a low prevalence population: diagnostic efficacy of tissue culture, direct immunofluorescence, enzyme immunoassay, PCR and serology. Genitourin Med. 1994;70:300–3. doi: 10.1136/sti.70.5.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammerschlag MR. Implications of Inappropriate STD Testing Go Beyond Pure Diagnostics. ASM News. 2003;69(2):74–9. [Google Scholar]
- 13.Centers for Disease Control and Prevention. Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections. MMWR. 2002;51(RR-15):1–39. [PubMed] [Google Scholar]
- 14.Barnes RC. Laboratory diagnosis of human chlamydial infections. Clin Microbiol Rev. 1989;2:119–36. doi: 10.1128/cmr.2.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamm WE. Diagnosis of Chlamydia trachomatis genitourinary infections. Ann Intern Med. 1988;108:710–7. doi: 10.7326/0003-4819-108-5-710. [DOI] [PubMed] [Google Scholar]
- 16.Ossewaarde JMM, Rieffe M, Rozenburg APM, et al. Development and clinical evaluation of a polymerase chain reaction test for detection of Chlamydia trachomatis. J Clin Microbiol. 1992;30:2122–8. doi: 10.1128/jcm.30.8.2122-2128.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battle TJ, Golden KL, Suchland JM, et al. Evaluation of laboratory testing methods for Chlamydia trachomatis infection in the era of nucleic acid amplification tests. J Clin Microbiol. 2001;39:2924–7. doi: 10.1128/JCM.39.8.2924-2927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chernesky MA, Lee J, Schachter J, et al. Diagnosis of Chlamydia trachomatis urethral infection in symptomatic and asymptomatic men by testing first-void urine in a ligase chain reaction assay. J Infect Dis. 1994;170:1308–11. doi: 10.1093/infdis/170.5.1308. [DOI] [PubMed] [Google Scholar]
- 19.Quinn TC, Welsh L, Lentz A, et al. Diagnosis by AMPLIC OR PCR of Chlamydia trachomatis in Urine Samples from Women and Men Attending Sexually Transmitted Disease Clinics. J Clin Microbiol. 1996;34:1401–6. doi: 10.1128/jcm.34.6.1401-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qadri SM, Akhter J, Ingacio K. Incidence of Chlamydia trachomatis in a large Metropolitan in Saudi Arabia. Saudi Med J. 1993;14:152–5. [Google Scholar]
- 21.Bashi SA. Chlamydial genital infection. Saudi Med J. 1986;7:484–9. [Google Scholar]
- 22.Forsey T, Darougan S. In: Clinical tropical medicine and communicable diseases- sexually transmitted diseases in the tropics. London: Bailliere Tindall; 1987. Chlamydia infections; pp. 59–75. [Google Scholar]
- 23.Jamjoom GA, Jesua CG, Sayed K. Low Rate of Isolation of Chlamydia trachomatis from Saudi Obstretrics/Gynaecology Patients. Saudi Med J. 1994;15:143–6. [Google Scholar]
- 24.Bashi SA, Siddique MN, Al-Shawaf TM, et al. Chlamydial infection in Riyadh, Saudi Arabia: A sero-epidemiological survey. Saudi Med J. 1987;8:387–90. [Google Scholar]
- 25.Bakir TMF, Hossain A, De-Silva S, et al. Enzyme Immunoassay in the diagnosis of Chlamydia trachomatis infections in diverse patient groups. J Hyg Epidem Microbiol Immun. 1989;33:189–97. [PubMed] [Google Scholar]
- 26.World Health Organization. Sexually transmitted diseases. Press release WHO/64,25. 1995 Aug [Google Scholar]
- 27.Washington, DC: National Academy Press; 1996. Institute of Medicine. The hidden epidemic: confronting sexually transmitted diseases. [Google Scholar]
- 28.Beem MO, Saxon EM. Respiratory tract colonization and a distinctive pneumonia syndrome in infants with Chlamydia trachomatis. N Engl J Med. 1977;163:306–10. doi: 10.1056/NEJM197702102960604. [DOI] [PubMed] [Google Scholar]
- 29.Grayston JT, Mordhorst CH, Wang SP. Childhood myocarditis associated with Chlamydia trachomatis infection. JAMA. 1981;246:2823–7. [PubMed] [Google Scholar]
- 30.Martin DHL, Koutsky DA, Eschenbach JR, et al. Prematurity and perinatal mortality in pregnancies complicated by maternal Chlamydia trachomatis infections. JAMA. 1982;247:1585–8. [PubMed] [Google Scholar]
- 31.Ryan GTN, Abdella SG, McNelly VS, et al. Chlamydia trachomatis infection in pregnancy and effect of treatment on pregnancy outcome. Am J Obstet Gynecol. 1990;162:34–9. doi: 10.1016/0002-9378(90)90815-o. [DOI] [PubMed] [Google Scholar]
- 32.Sweet RL, Sanders DV, Walker C, Schachter J. Chlamydia trachomatis infection and pregnancy outcome. Am J Obstet Gynecol. 1987;156:824–33. doi: 10.1016/0002-9378(87)90338-3. [DOI] [PubMed] [Google Scholar]
- 33.Black CM. Current Methods of Laboratory Diagnosis of Chlamydia trachomatis Infections. Clin Microbiol Rev. 1997;10:160–84. doi: 10.1128/cmr.10.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]


