Abstract
Objective:
To determine the proportion of pre-hypertension and hypertension in college students in Kuwait and their related risk factors.
Materials and Methods:
A total of 803, randomly selected students aged 17 to 23 years (346 male, 457 female) from different colleges in Kuwait, were included in the study between 2009 and 2010. Systolic and diastolic blood pressure measurements were taken by trained personnel. Pre-hypertension was defined as systolic pressure between 120 and 139 mm Hg or diastolic pressure between 80 and 89 mm Hg. Risk factor measurements that were determined, included smoking, body mass index (BMI), and family history of hypertension. Blood samples were collected and impaired glucose tolerance (IGT) and lipid profile levels were determined.
Results:
There were no hypotensive students. Normotensives constituted 53.5% (n = 430), pre-hypertensives formed 39.5% (n = 317), and hypertensive students comprised of 7% (n = 56). The overall proportions of hypertension and pre-hypertension were higher among male students (85.7 and 64.4%) than female students (14.3 and 35.6%), respectively. Hypertensive and pre-hypertensive students versus normotensive students had significantly higher levels of BMI-based obesity, smoking, glycated hemoglobin (HbA1c), and IGT. Also, hypertensive and pre-hypertensive, compared to normotensive students, had significantly higher proportions (21.4, 18.3, and 4.0%, respectively) of risky high-density lipoprotein (HDL) level (< 1 mg / dL), cholesterol (7.1, 3.8, and 1.4%, respectively), and triglycerides (TG) (17.9, 9.1, and 7.9%, respectively) where p was< 0.001, 0.016, and 0.051, respectively.
Conclusion:
Hypertensive and pre-hypertensive students showed elevated levels of lipids and BMI-based obesity more than normotensive students. TG, HDL, HbA1c, and cholesterol appeared to influence pre-hypertension.
Keywords: BMI-based obesity, hypertension, high-density lipoprotein, Kuwait, pre-hypertension, triglycerides
INTRODUCTION
It is well-documented that there is an increase in the prevalence of hypertension (HTN) in developed and underdeveloped nations.[1–4]
Several studies have shown that HTN and pre-hypertension (pre-HTN) can start in adolescence, perhaps in the early stages of life, and continue into adulthood.[5,6]
A recent study found that the prevalence of pre-HTN increased with age.[7] A regional study concluded that levels of blood pressure increased progressively with age in both boys and girls in Saudi Arabia.[8]
A number of studies have shown that baseline obesity is an independent risk factor for HTN.[9,10] A regional study, which reviewed the proportions of HTN and obesity in the Arabian Gulf States, showed that HTN levels in Kuwait, Oman, Bahrain, Qatar, and Saudi Arabia were very high and increased with age. It also demonstrated that the rate of increase in obesity was more pronounced in Saudi Arabia and Kuwait, especially among preschoolers (8 – 9%), with overweight and obesity in adolescents being among the highest in the world in Kuwait, with the worst estimates (40 – 46%).[11]
Moussa et al., concluded that a positive relationship existed between systolic and diastolic blood pressure and increased BMI, in both male and female students aged 7 – 18 years in Al Ain city, UAE.[12] Moreover, a recent study showed that the change in BMI between the ages of seven and fifteen years, had a significant effect on systolic blood pressure (SBP) at the age of 15.[13]
Several risk factors are well-recognized worldwide as contributors to the increase in blood pressure. They are a smoking habit, family history of hypertension, and increased glycemic and lipid levels.[14–16]
Most of the studies done in Kuwait, examined the prevalence of HTN in adults. Hence, little is known about the prevalence of HTN and pre-HTN in college students. In addition, it is well-known that HTN is an asymptomatic disease that is revealed only when there are complications. Hence, it is very important to diagnose elevated blood pressure (pre-HTN and HTN) at an early age. The purpose of this study was to examine the proportion of pre-HTN and HTN in college students, aged 17 – 23 years, and to determine the associated risk factors.
MATERIALS AND METHODS
The study was conducted during the academic year 2009 – 2010. The study population consisted of 803 students, aged 17 to 23 years, who were randomly selected from different colleges in Kuwait, and who agreed to participate by giving a written informed consent.
Sampling
To determine the sample size, the EPIINFO 6.0 program was used. Assuming that the prevalence of pre-HTN and HTN was 25%, within an accepted difference of 3% in the target population of 13,300 size, the estimated sample size at 5% level of significance and 80% power would be 755. Ten percent was added to the required sample for the sake of non-response. Out of a total of 825 students recruited for the study, who met the criteria for inclusion, 803 agreed to participate and were included in the analysis with a response rate of 97%.
Data collection
The study began with an early interview by trained personnel using a pre-designed questionnaire. Information such as: age, sex, history of chronic disease [epilepsy, asthma, respiratory allergy, migraine, thalassemia, chronic irritated colon, persistent colitis, and renal disease], family history of hypertension and / or diabetes mellitus, and history of smoking were collected. Height (cm) and weight (kg) were assessed using a scale (Detectoscale, MO USA). BMI was calculated using the formula [weight (kg) / [height (m)].[2] According to the definition by the World Health Organization (WHO), a BMI ≥25 kg / m2 was overweight and a BMI ≥ 30 kg / m2 was obese.[17]
A pilot study was carried out on 15 college students. Its aim was to test the suitability of the questionnaire to be used regarding its phrasing, the culture of interviewees, estimated time of completing the questionnaire, testing the analytic procedure, and the overall response of the students. This study revealed that the questionnaire was on the whole suitable. A urine analysis was omitted owing to a low response rate, especially among females.
The average of two readings of systolic and diastolic blood pressures was calculated using an electric sphygmomanometer (OMRON). According to the Joint National Committee (JNC) 7 report,[18] blood pressure (BP) categories were defined as normal blood pressure if the observed systolic blood pressure (SBP) was between 91 and 120 mm Hg or diastolic blood pressure (DBP) was between 61 and 80 mm Hg; pre-HTN if the observed SBP was between 121 and 139 mmHg or DBP was between 81 and 89 mmHg; and considered as HTN if the observed SBP was equal to or above 140 mmHg and DBP was equal to or above 90 mmHg; and finally hypotension was defined as SBP being equal to or less than 90 mm Hg or DBP being equal to or less than 60 mm Hg.[19]
Clinical measurements of blood glucose and lipid levels were completed. The students were asked to fast overnight for 12 – 14 hours before the day of the examination and an oral glucose tolerance test (OGTT) was completed using 75 g of glucose (Trutol™75, Glucose Tolerance beverage, Thermo Fisher Scientific Inc.). A first finger prick, 5 μl blood sample, was used for fasting blood glucose, fasting Hb1Ac and lipid measurements. Blood glucose levels were tested using the HemoCue Blood Glucose Analyzer (HemoCue Inc). According to the American Diabetes Association (ADA) criteria,[20] the students were considered normoglycemic if they had a fasting glucose < 5.6 mmol / l; pre-diabetic if it was between 5.6 and 6.9 mmol / l; and diabetic if it was ≥ 7 mmol / l. For the diagnosis of pre-diabetes, the measurement of glycated hemoglobin (HbA1c) was used. The desirable range of HbA1c between 5.7 and 6.4% was recently approved by the ADA. The HbA1c was calculated using the Afinion™ AS100 Analyzer (Norway). For the most part, lipid profiles of total cholesterol, triglycerides, high density lipoprotein (HDL), and low density lipoprotein (LDL) were measured using Reflotron® Plus, (Roche Diagnostics GmbH, Germany). A second blood sample (5 μl) was drawn after two hours for postprandial blood glucose measurement. According to the ADA criteria, the study participants were considered to have impaired glucose tolerance (IGT) in the presence of one or more of the following; fasting plasma glucose of > 5.6 and < 7 mmol / L, two-hour postprandial glucose between 7.8 to 11.0 mmol / L, and HbA1c > 5.6% and < 6.4%. Type 2 diabetes was diagnosed if any one of the following was found; fasting blood glucose ≥ 7 mmol / L, two-hour post prandial glucose ≥ 11.1 mmol / L and HbA1c ≥ 6.5%.
The study was approved by the Ethical Committee of the Ministry of Health of Kuwait, and informed consent was obtained from all the participants. The inclusion criteria were: (1) Kuwaiti students in college and (2) aged between 17 and 23 years, while the exclusion criteria were: (1) being pregnant and (2) being diabetic or having renal disease.
Statistical analysis
Data were collected, coded, and entered into an IBM compatible computer, using the SPSS version 17 for Windows. The entered data were checked for accuracy and then for normality, using the Kolmogorov-Smirnov test. Qualitative variables were expressed as numbers and percentages, while quantitative variables were expressed as median, mean (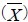 ), and standard deviation (S). The arithmetic mean and median were used as measures of central tendency, while the standard deviation and interquartile range were used as measures of dispersion.
), and standard deviation (S). The arithmetic mean and median were used as measures of central tendency, while the standard deviation and interquartile range were used as measures of dispersion.
The following statistical tests were used:
The χ2 -test (or likelihood ratio = LLR) was used as a non-parametric test of significance, for comparison between the distribution of two qualitative variables.
The one-way ANOVA (F-test) was used as a parametric test of significance for comparison of more than two sample means, using either Scheffe's or Tamhane's post hoc tests for paired comparison according to the results of homogeneity testing.
A 5% level was chosen as a level of significance in all statistical significance tests used.
RESULTS
Students were classified into three groups according to the status of their blood pressure: normotensive, pre-hypertensive, and hypertensive. No hypotensive cases were found. Normotensive students constituted 53.5% (n = 430), prehypertensive 39.5% (n = 317), and hypertensive students represented 7% (n = 56). Some descriptive data of the SBP and DBP of normotensive, pre-hypertensive, and hypertensive students, such as, the arithmetic mean, 5% trimmed mean, standard deviation, median, interquartile range, minimum, maximum, and range, are shown in Table 1.
Table 1.
Description of SBP and DBP of normotensive, pre-hypertensive, and hypertensive students
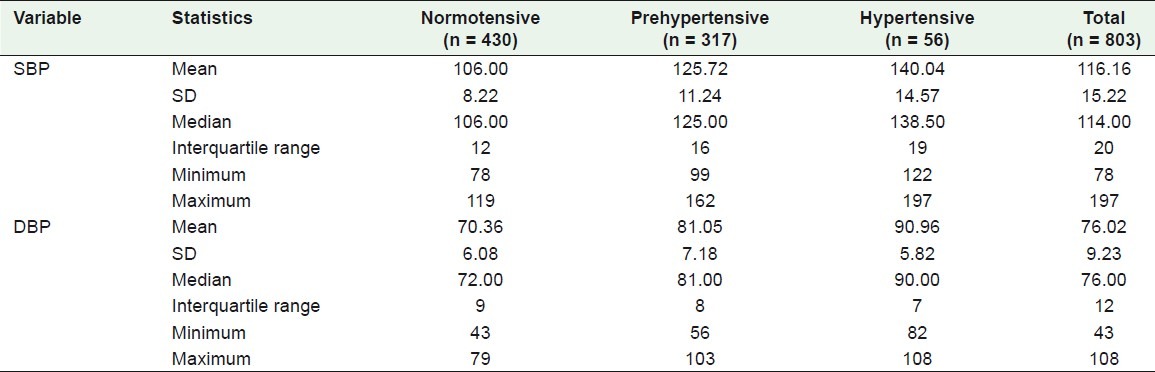
Table 2 shows that more male college students were found to be hypertensive than their female counterparts, 85.7 and 14.3%, respectively. The percentage of male normotensive students was 21.9%, while those pre-hypertensive and hypertensive were 64.4 and 85.7%, respectively.
Table 2.
Comparison among normotensive, pre-hypertensive, and hypertensive students according to some studied demographic variables
Regarding the smoking habit, the percentage of students who smoked in relation to the status of blood pressure, that is, normotensive, pre-hypertensive, and hypertensive were 7.7, 17.4, and 17.9%, respectively, where P < 0.001. The percentage of positive family history of HTN among the hypertensives (53.6%) was higher than among both the pre-hypertensive (42.6%) and normotensive students (40%), where P = 0.147. History of chronic diseases and family history of diabetes mellitus (DM) were not statistically significant, as shown by the P-values.
It was found that general overweight was less among normotensives (42.3%), and high among hypertensive students (75%), while 62.1% of the pre-hypertensive students were overweight as shown in Table 3. These differences were statistically significant, P < 0.001.
Table 3.
Comparison among normotensive, pre-hypertensive, and hypertensive students according to some studied anthropometric variables
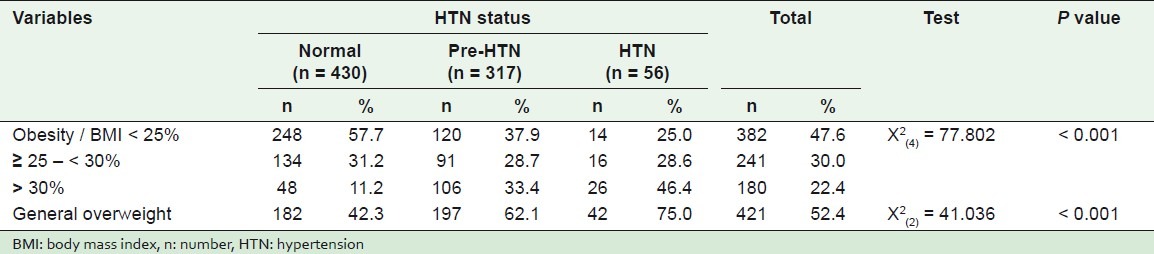
Categorization of BMI on the basis of normal (< 25%), overweight (≥ 25 - < 30%), and obese (≥ 30%), showed that the percentage of normotensive obese students was 11.2%, while the percentages of pre-hypertensives and hypertensives were 33.4 and 46.4%, respectively. These differences were statistically significant, where P < 0.001. Overweight according to waist circumference (WC) was not statistically significant, P = 0.164.
The percentage of high levels of TG in hypertensive students (17.9%) was significantly higher than was evident in the pre-hypertensive and normotensive students 9.1 and 7.9%, respectively, as shown in Table 4.
Table 4.
Comparison among normotensive, pre-hypertensive, and hypertensive students according to some studied clinical variables
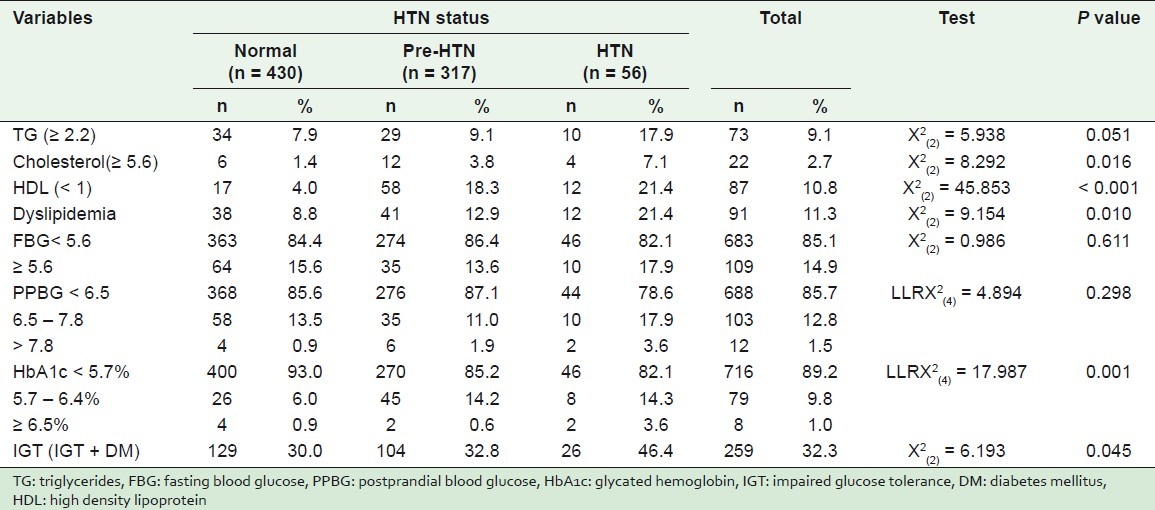
The percentage of dyslipidemia in hypertensive students (21.4%) was significantly higher than was found in both pre-hypertensives (12.9%) and normotensives (8.8%), where P = 0.010.
Hypertensive students showed significantly higher levels of cholesterol (7.1%) than pre-hypertensive (3.8%) and normotensive (1.4%) students, where P = 0.016.
The percentages of low levels of high HDL in hypertensive, pre-hypertensive, and normotensive students were 21.4, 18.3, and 4%, respectively, where P < 0.001.
There was an increased proportion of high fasting blood glucose (FBG), although not significant, in hypertensives (17.9%) than in pre-hypertensive (13.6%) and normotensive students (15.6%), where P = 0.611. Moreover, the highest percentage of postprandial blood glucose (PPBG), although not significant, was in hypertensive (3.6%) rather than in pre-hypertensive and normotensive students (1.9 and 0.9%, respectively), where P = 0.298.
High HbA1c levels (5.7 – 6.4%) were significantly higher in both hypertensive (14.3%) and pre-HTN (14.2%) as compared to normotensive (6.0%) students. Moreover, the percentage of hypertensive students (3.6%) with very high HbA1c levels (≥ 6.5%) was significantly higher than both pre-hypertensive (0.6%) and normotensive (0.9%) students, where P = 0.001.
It was found that the percentage of impaired glucose tolerance (IGT), as the sum of IGT and presence of DM, in hypertensives (46.4%) was significantly higher than in both pre-hypertensive (32.8%) and normotensive (30.0%) students. These differences were statistically significant, where P < 0.045.
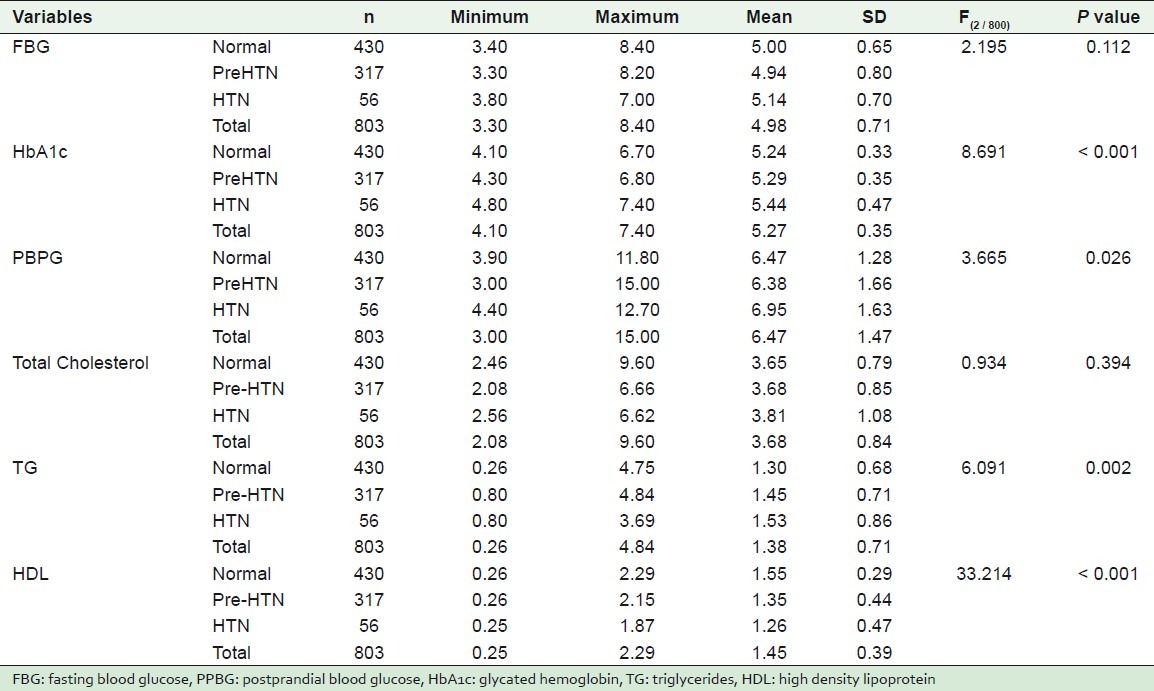
Table 5 shows the mean, standard deviation, minimum, and maximum values of some studied laboratory results according to the state of hypertension.
Table 5.
The mean, standard deviation, minimum, and maximum values of some quantitative variables, according to some studied laboratory findings
DISCUSSION
The present study found that the proportion of pre-hypertension (pre-HTN) and HTN in college students were 39.4 and 7%, respectively. These results were not much different from the recent results reported. Data from a study on university students, aged 18 – 24 years, indicated high rates of elevated systolic (47%) and diastolic blood pressures (39%).[21] A regional study on some Saudi military men aged 23 – 37 years, found that 17.3% were pre-HTN.[22] Another study revealed that the prevalence of HTN was 23.8% in Iranian college students.[23] A local study in Kuwait showed that the proportion of hypertensive patients was 11.8%.[15]
The data of the present study show differences in the proportion of HTN and pre-HTN between the genders. Generally, both HTN and pre-HTN are significantly more prevalent in males than in females. This is possibly due to the differences in hormonal activity between boys and girls in early life.[24,25]
These results are in agreement with the other studies. Erem et al., showed that the prevalence of HTN and pre-HTN were 44.0% (41.6% in women and 46.1% in men) and 14.5% (12.6% in women and 16.8% in men), respectively.[26] In addition, in a study of 2000 healthy adults Oladapo et al., found that 20.8% were hypertensive with a BP of ≥ 140 / 90 mm Hg. Among the men, 42.3%, and among the women, 36.8%, had BP of ≥ 130 / 85 mm Hg.[27] A study in Saudi Arabia showed the prevalence of hypertension in males as 28.6%, while in females it was 23.9% (P < 0.001).[28]
The present data showed that a family history of HTN was high among college students (42.0%). Although not statistically significant, the percentage of family history of HTN was the highest among hypertensive compared to pre-hypertensive and normotensive students. These results were in accordance with many studies which showed that family history of HTN was an important contributor to HTN and pre-HTN.[29–31] Our data also showed that the habit of smoking was an important risk factor for hypertension in students. These results were in agreement with the previous studies.[26,32] Al-Safi's regional study concluded that blood pressure was higher among smokers than non-smokers.[33] Smoking and high blood pressure were known to accelerate the development of the process of atherosclerosis and increase the risk of all other coronary lesions. Therefore, it was very important to monitor blood pressure in Kuwaiti college students, while they were still young.
The literature indicates that an increase in blood pressure is related to diverse causes, but most studies have highlighted obesity as an important factor. Based on the BMI categories, our results show that 22.4% of all the students are obese. It also demonstrates significantly that the highest proportion of HTN is in students with BMI ≥ 30 rather than their pre-hypertensive counterparts, and the lowest in normotensives. These results reveal the positive relation between HTN and obesity in college students.
Data from the Israeli Air Force revealed that 48% of pilots, aged 18, had pre-HTN and were more obese than the normotensive subjects.[34] Another study in China showed that prevalence of elevated blood pressure (pre-HTN and HTN) and mean levels of systolic and diastolic pressure had a positive relationship with BMI in both males and females.[35] Two studies, in Kuwait and United Arab Emirates, showed an apparent correlation between systolic and diastolic pressure and an increase in BMI, in both male and female students.[12,36]
The data presented in our study found that general overweight was least presented in normotensive students (42.3%), was higher in pre-hypertensive students (62.1%), and the highest among hypertensive students (75%). A recent study concluded that pre-HTN was significantly high among Chinese men and women who were overweight, 38.4 and 27.8%, respectively.[37] A population-based study showed that the degree of weight change in infancy was equally important for SBP in both boys and girls, but growth during puberty showed a stronger association between weight change and SBP, more in boys than in girls.[38] This is generally explained, by several genetic, sexual, and hormonal differences during the different phases of life.[24,25] In this region, a significant linear relationship has been shown between increasing overweight and prevalence of hypertension in the Saudi population.[28] Finally, Sabra et al., has demonstrated a relationship between the increased level of blood pressure and overweight in male students (aged 18 – 26 years) at King Faisal University (KFU) in Dammam, Saudi Arabia.[39]
One of the striking results in our study is the relationship between blood pressure and the proportions of lipids in the hypertensive students. It showed the significant increase in the percentage of the following components: Triglycerides, low levels of high HDL, cholesterol, and dyslipidemia among the hypertensive students. In support of these data, are several studies that have recognized the relation between higher levels of blood pressure and increased levels of lipids, weight, and BMI variables.[27,32] Gupta et al., have showed a positive relationship between hypertensive Indian adults, aged 20 to 29 years, and high levels of TG, low HDL cholesterol, LDL cholesterol, and BMI.[29]
Our data revealed that the pattern of metabolic variability, especially glycemic levels, were just as important as overweight and / or obesity and / or lipidic profile in affecting the severity of blood pressure in our students. Although not statistically significant, the highest proportion of fasting blood glucose (FBG) was among the hypertensive rather than the pre-hypertensive or normotensive students; also the highest percentage of postprandial blood glucose (PPBG) was found in hypertensive students. Previous researches have similarly indicated a strong relationship between elevated fasting blood glucose levels and increased blood pressure.[40,41]
Our data demonstrated that unstable metabolic conditions were most likely to have a strong effect on blood pressure. It showed an increased proportion of IGT / diabetes in students with hypertension than in pre-hypertensive and normotensive students. Many studies had reported that metabolic abnormalities could be a major risk factor in hypertensive subjects.[42–44] Our study also showed that levels of glycated hemoglobin were significantly higher in hypertensive students. The reason was that metabolic abnormalities were likely to play a major role in the development of HTN in the pre-HNT students. This was because, with time, pre-HTN students would not be able to handle the oral glucose challenge, resulting in damaged endothelium-dependent vasodilation, and consequently, an increase in blood pressure.
Often, in college students, pre-hypertension may not be discovered until late. This is because college students are generally healthy and will only see a physician when they are very ill, but will not normally see a physician for a routine health check-up. The data from this study draws attention to the importance of examining the blood pressure of young persons.
CONCLUSION
We concluded that the increase in the proportion of HTN and pre-HTN in college students, a relatively understudied group, was due to two major factors: high levels of lipids and BMI-based obesity. TG, HDL, HbA1c, and cholesterol appeared to greatly influence pre-HTN. These results provided preliminary data on the high proportion of both HTN and pre-HTN, and its related risk factors in college students, in Kuwait.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
REFERENCES
- 1.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303:2043–50. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 2.Gu D, Reynolds K, Wu X, Chen J, Duan X, Muntner P, et al. Prevalence, awareness, treatment, and control of hypertension in china. Hypertension. 2002;40:920–7. doi: 10.1161/01.hyp.0000040263.94619.d5. [DOI] [PubMed] [Google Scholar]
- 3.The World Health Report 2002: Reducing risks, promoting healthy life. Geneva, Switzerland: 2002. World Health Organization. [Google Scholar]
- 4.Kaerney PM, Whelton M, Reynolds SK, Munter P, Whelton PK, He J. Global burden of hypertension: An analysis of world wide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 5.Ejike CE, Ugwu CE, Ezeanyika LU. Variations in the prevalence of point (pre) hypertension in a Nigerian school-going adolescent population living in a semi-urban and an urban area. BMC Pediatr. 2010;10:13–7. doi: 10.1186/1471-2431-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Culhane-Pera KA, Moua M, DeFor TA, Desai J. Cardiovascular disease risks in Hmong refugees from Wat Tham Krabok, Thailand. J Immigr Minor Health. 2009;11:372–9. doi: 10.1007/s10903-008-9211-x. [DOI] [PubMed] [Google Scholar]
- 7.Kitai E, Vinker S, Halperin L, Meidan A, Grossman E. Pre-hypertension is a common phenomenon: National database study. Isr Med Assoc. 2007;9:8–11. [PubMed] [Google Scholar]
- 8.Al-Salloum AA, El-Mouzan MI, Al Herbish AS, Al Omar AA, Qurashi MM. Blood pressure standards for Saudi children and adolescents. Ann Saudi Med. 1009;29:173–8. doi: 10.4103/0256-4947.51787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibhazehiebo K, Dimkpa UI, Iyawe VI. Hypertension and blood pressure response to graded exercise in young obese and non-athletic Nigerian university students. Niger J Physiol Sci. 2007;22:37–42. doi: 10.4314/njps.v22i1-2.54886. [DOI] [PubMed] [Google Scholar]
- 10.Moore WE, Eichner JE, Cohn EM, Thompson DM, Kobza CE, Abbott KE. Blood pressure screening of school children in a multiracial school district: The Healthy Kids Project. Am J Hypertens. 2009;22:351–6. doi: 10.1038/ajh.2009.13. [DOI] [PubMed] [Google Scholar]
- 11.Ng SW, Zaghloul S, Ali HI, Harrison G, Popkin BM. The prevalence and trends of overweight, obesity and nutrition-related non-communicable diseases in the Arabian Gulf States. Obes Rev. 2011;12:1–13. doi: 10.1111/j.1467-789X.2010.00750.x. [DOI] [PubMed] [Google Scholar]
- 12.Moussa MA, Skaik MB, Selwanes SB, Yaghy OY, Bin-Othman SA. Contribution of body fat and fat pattern to blood pressure level in school children. Eur J Clin Nutr. 1994;48:587–90. [PubMed] [Google Scholar]
- 13.Fuentes RM, Notkola IL, Shemeikka S, Tuomilehto J, Nissinen A. Tracking of systolic blood pressure during childhood: A 15 year follow up population based family study in Eastern Finland. J Hypertens. 2002;20:195–202. doi: 10.1097/00004872-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Kuklina EV, Yoon PW, Keenan NL. Prevalence of coronary heart disease risk factors and screening for high cholesterol levels among young adults, United States, 1999-2006. Ann Fam Med. 2010;8:327–33. doi: 10.1370/afm.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Shaibani H, El-Batish M, Sorkhoul I, Al-Shamali N, Al-Namash H, Habiba S, et al. Prevalence of Insulin Resistance Syndrome in a Primary Health Care Center in Kuwait. Fam Med. 2004;36:540. [PubMed] [Google Scholar]
- 16.Kalkners B. Hypertension in children and adolescents: Epidemiology and natural history. Pediatr Nephrol. 2010;25:1219–24. doi: 10.1007/s00467-009-1200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obesity: Preventing and managing the global epidemic: Report of a World Health Organization Consultation. Geneva, Switzerland: Presented at the World Health Organization; 1997. Jun, World Health Organization. [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 19.Disease and conditions Index- Hypotension. [Last accessed on 2008 Sep 16];National Heart Lung and Blood Institute. 2008 http://www.nhlbi.nih.gov/health/dci/Diseases/hyp/hyp_whatis.html . [Google Scholar]
- 20.American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;(Supplement 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke JD, Reilly RA, Morrell JS, Lofgren IE. The university of New Hampshire's young adult health risk screening initiative. Am Diet Assoc. 2009;109:1751–8. doi: 10.1016/j.jada.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Al-Asmary SM, Al-Shehri AA, Farahat FM, Abdel-Fattah MM, Al-Shahrani MM, Al-Omari FK, et al. Community-based screening for pre-hypertension among military active duty personnel. Saudi Med J. 2008;29:1779–84. [PubMed] [Google Scholar]
- 23.Alikhani S, Delayari A, Alaedini F, Kelishadi R, Rohbani S, Safaei A. A province-based surveillance system for the risk factors of non-communicable diseases: A prototype for integration of risk factor surveillance into primary health care systems of developing countries. Public Health. 2009;123:358–64. doi: 10.1016/j.puhe.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Oleda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offsprings. Hypertension. 2007;50:679–85. doi: 10.1161/HYPERTENSIONAHA.107.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hulanicka B, Lipowicz A, Koziel S, Kowalisko A. Relationship between early puberty and the risk of hypertension / overweight at age 50: Evidence for a modified Barker hypothesis among Polish youth. Econ Hum Biol. 2007;5:48–60. doi: 10.1016/j.ehb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Erem C, Hacihasanoglu A, Kocak M, Deger O, Topbas M. Prevalence of prehypertension and hypertension and associated risk factors among Turkish adults: Trabzon Hypertension Study. J Public Health (Oxf) 2009;31:47–58. doi: 10.1093/pubmed/fdn078. [DOI] [PubMed] [Google Scholar]
- 27.Oladapo OO, Salako L, Sodig O, Shoyinka K, Adedapo K, Falase AO. A prevalence of cardiometabolic risk factors among a rural Yoruba south-western Nigerian population: A population-based survey. Cardiovasc J Afr. 2010;21:26–31. [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Nozha MM, Abdullah M, Arafah MR, Khalil MZ, Khan NB, Al-Mazrou YY, et al. Hypertension in Saudi Arabia. Saudi Med J. 2007;28:77–84. [PubMed] [Google Scholar]
- 29.Gupta R, Misra A, Vikram NK, Kondal D, Gupta SS, Agrawal A, et al. Younger age of escalation of cardiovascular risk factors in Asian Indian subjects. BMC Cardiovasc Disord. 2009;9:28–31. doi: 10.1186/1471-2261-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chockalingam A, Ganesan N, Venkatesan S, Gnanavelu G, Subramaniam T, Jaganathan V, et al. Patterns and predictors of pre-hypertension among “healthy” urban adults in India. Angiology. 2005;56:557–63. doi: 10.1177/000331970505600506. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein IB, Shapiro D, Guthrie D. Ambulatory blood pressure and family history of hypertension in healthy men and women. Am J Hypertens. 2006;19:486–91. doi: 10.1016/j.amjhyper.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson TS, Younger NO, Tulloch-Reid MK, Wright MB, Ward EM, Ashley DE, et al. Prevalence of prehypertension and its relationship to risk factors for cardiovascular disease in Jamaica: Analysis from a cross-sectional survey. BMC Cardiovasc Disorder. 2008;8:20. doi: 10.1186/1471-2261-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Safi SA. Does smoking affect blood pressure and heart rate? Eur J Cardiovasc Nurs. 2005;4:286–9. doi: 10.1016/j.ejcnurse.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Grossman A, Grossman C, Barenboim E, Azaria B, Goldstein L, Grossman E. Pre-hypertension as a predictor of hypertension in military aviators: A longitudinal study of 367 men. Aviat Space Environ Med. 2006;77:1162–5. [PubMed] [Google Scholar]
- 35.Pang W, Sun Z, Zheng L, Li J, Zhang X, Liu S, et al. Body mass index and the prevalence of prehypertension and hypertension in a Chinese rural population. Intern Med. 2008;47:893–7. doi: 10.2169/internalmedicine.47.0528. [DOI] [PubMed] [Google Scholar]
- 36.Saleh EA, Mahfouz AA, Tavel KY, Naquib MK, Bin-al-Shaikh NM. Hypertension and its determinants among primary-school children in Kuwait: An epidemiological study. East Mediterr Health J. 2000;6:333–7. [PubMed] [Google Scholar]
- 37.Yu D, Huang J, Hu D, Chen J, Cao J, Li J, et al. Prevalence and risk factors of prehypertension among Chinese adults. J Cardiovasc Pharmacol. 2008;52:363–8. doi: 10.1097/FJC.0b013e31818953ac. [DOI] [PubMed] [Google Scholar]
- 38.Kark M, Tynelius P, Rasmussen F. Associations between birthweight and weight change during infancy and later childhood, and systolic blood pressure at age 15 years: The COMPASS study. Paediatr Perinat Epidemiol. 2009;23:245–53. doi: 10.1111/j.1365-3016.2008.01007.x. [DOI] [PubMed] [Google Scholar]
- 39.Sabra AA, Taha Az, Al-Sebiany AM, Al-Kurashi NY, Al-Zubier AG. Coronary heart disease risk factors: Prevalence and behavior among male university students in Dammam City, Saudi Arabia. J Egypt Public Health Assoc. 2007;82:21–32. [PubMed] [Google Scholar]
- 40.Yan W, Gu D, Yang X, Wu J, Kang L, Zhang L. High-density lipoprotein cholesterol levels increase with age, body mass index, blood pressure and fasting blood glucose in a rural Uygur population in China. J Hypertension. 2005;23:1985–9. doi: 10.1097/01.hjh.0000187254.12375.b6. [DOI] [PubMed] [Google Scholar]
- 41.Aboul Ella NA, Shehab DI, Ismail MA, Maksoud AA. Prevalence of metabolic syndrome and insulin resistance among Egyptian adolescents 10 to 18 years of age. J Clin Lipidol. 2010;4:185–95. doi: 10.1016/j.jacl.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Akintunde AA. Epidemiology of conventional cardiovascular risk factors among hypertensive subjects with normal and impaired fasting glucose. S Afr Med J. 2010;100:594–7. doi: 10.7196/samj.3180. [DOI] [PubMed] [Google Scholar]
- 43.Sathiyapriya V, Nanseesha H, Bobby Z, Pavithran P, Selvarai N, Rattina Dasse N. Insulin resistance and enhanced protein glycation in men with prehypertension. Clin Chem Lab Med. 2006;44:1457–61. doi: 10.1515/CCLM.2006.264. [DOI] [PubMed] [Google Scholar]
- 44.Gupta AK, Johnson WD. Prediabetes and prehypertension in disease free obese adults correlate with an exacerbated systemic proinflammatory milieu. J Inflamm (Lond) 2010;7:36. doi: 10.1186/1476-9255-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]


