Abstract
Background:
In earlier studies uninostril yoga breathing was shown to influence the activity of the cerebral hemispheres differently, based on (i) auditory evoked potentials recorded from bilateral scalp sites, and (ii) performance in hemisphere-specific tasks. But change in P300 (event-related potential generated when subjects attend to and discriminate between stimuli) from bilateral scalp sites when subjects were practicing uni- and alternate-nostril breathing are yet to be explored.
Aim:
The present study was designed to determine whether or not immediately after uninostril or alternate nostril yoga breathing there would be a change in the ability to pay attention to a given stimulus.
Materials and Methods:
Twenty-nine healthy male volunteers, with ages between 20 and 45 years were randomly allocated to five sessions, viz., (i) right-, (ii) left-, (iii) alternate-nostril yoga breathing, (iv) breath awareness and (v) no intervention, each for 45 min on separate days. The P300 event related potential was recorded using an auditory oddball paradigm from sites on the left (C3) and right (C4), referenced to linked earlobes, before and after each session.
Results:
Post-hoc analysis with Bonferroni adjustment showed that the P300 peak latency was significantly lower at C3 compared to that at C4, following right nostril yoga breathing (P<0.05).
Conclusion:
These results suggest that right nostril yoga breathing facilitates the activity of contralateral (left) hemisphere, in the performance of the P300 task.
Keywords: Contralateral changes, P300, uni-nostril yoga breathing
INTRODUCTION
Changes in the amount of blood flowing through the cavernous tissues of the nasal conchae’ was the way in which the nasal cycle was described.[1] This cycle was believed to be an ultradian rhythm seen in people with normal health, with alternating patency of the nostrils every one to six h. More recently this was re-examined using numerical measures of reciprocity and the division of airflow between the nasal passages over time was quantified.[2] Only 21% of volunteers studied had patterns of nasal airflow which met the numerical definition of the nasal cycle. In contrast to this, an investigation of the time periods of multiple systems in sleep and waking rest studied ten variables including the nasal cycle.[3] Across all ten variables time series analyses detected periods at 115-145, 70-100, and 40-65 min.
Hence there appears to be a possibility of spontaneous changes in nasal patency in humans, though the underlying mechanisms are not clear. Despite these questions about the occurrence of the nasal cycle, specific trends have been observed during spontaneous shifts in nostril dominance, as well as during unilateral forced nostril breathing. While observations have been made about the effect of uninostril breathing on autonomic functions, in the present study the trends being considered are related to the effects of uninostril breathing on the functions of the cerebral hemispheres. The effect of unilateral forced nostril breathing was studied on the integrated electroencephalogram (EEG) amplitudes recorded from both hemispheres separately.[4,5] In the five volunteers studied, forced breathing through one nostril produced a relative increase in the EEG amplitude recorded over the contralateral cerebral hemisphere. The relevance of an increase in the EEG power to mental activity usually is interpreted as a decrease in EEG amplitude being associated with increased mental activity. However the authors interpreted the increased EEG amplitudes recorded over the cerebral hemisphere contralateral to the dominant nostril as indicative of increased mental activity. This interpretation appears to be supported by studies on performance in hemisphere-specific tasks related to unilateral forced nostril breathing. For example, spontaneously occurring asymmetries in nasal airflow were correlated with performance in hemisphere specific tasks.[6] During a phase of spontaneously occurring left nostril breathing subjects performed better in a spatial (right-hemispheric) tasks, whereas during spontaneous right nostril breathing their performance was better in a verbal (left hemisphere specific) task. A similar trend of hemispheric activation contralateral to the dominant nostril was observed during unilateral forced nostril breathing. In one study the unilateral airflow wcorrelated with verbal-spatial task performance in 23 right-handed men.[7] In another study, the effect of thirty min of unilateral forced nostril breathing was studied in 51 right handed volunteers.[8] Spatial task performance (a right hemisphere specific task) was significantly increased during left nostril breathing, while verbal task performance (a left hemispheric task) showed a trend of increase during right nostril breathing.
There are voluntarily regulated yoga-breathing techniques (pranayamas) which involve inhalation and exhalation through one nostril exclusively, as well as through both nostrils alternately. These yoga breathing techniques have provided a way to study the effects of voluntarily regulated unilateral or alternate nostril breathing, practiced effortlessly for long periods.[9]
The effects of right nostril yoga breathing, left nostril yoga breathing, and alternate nostril yoga breathing, breath awareness and no intervention, were studied over —10-day period, using two hemisphere-specific memory tasks.[10] All four yoga breathing groups increased spatial, but not verbal memory task scores, while the no intervention group showed no change. Hence uninostril yoga breathing did not increase the functioning of the contralateral cerebral hemisphere, in that study.
A more recent study evaluated the effects of right uninostril yoga breathing, left uninostril yoga breathing, alternate nostril yoga breathing, breath awareness and no intervention, on the performance in a letter cancellation task which is left hemisphere specific.[11] The performance in the letter cancellation task improved following right uninostril yoga breathing, and alternate nostril yoga breathing, which was suggestive of a contralateral effect.
The present study was designed to determine whether immediately after uninostril or alternate nostril yoga breathing there would be a change in the ability to pay attention to a given stimulus. The P300 component of the event-related brain potentials (ERPs) is a cognitive neuro-electric phenomenon, generated when subjects attend to and discriminate between stimuli which differ in a single dimension, and which requires attention and immediate memory.[12] Hence in the present study the P300 was recorded from bilaterally symmetrical scalp sites before and after right-, left- and alternate-nostril yoga breathing, breath awareness and a no intervention period for comparison.
MATERIALS AND METHODS
Subjects
In this study, 39 healthy male volunteers participated and their ages ranged from 20 to 45 years (mean age±SD, 26.0 ± 5.5 years). The range of experience of practicing the yoga breathing techniques was 3 to 84 months (mean experience±SD, 25.5 ± 5.0 months). The participants were selected on a routine clinical examination and the study was restricted to males, as auditory evoked responses vary with the phases of the menstrual cycle.[13] Also, P300 event related potentials recorded with visual stimuli varied with gender.[14] The volunteers were informed about the study, and their signed consent was obtained.
Design
Each participant was assessed in five different sessions at the same time of the day. The participants were allocated randomly to the five possible sessions using a random number table.[15] The sessions were (i) right nostril yoga breathing (RNYB), (ii) left nostril yoga breathing (LNYB), (iii) alternate nostril yoga breathing (ANYB), (iv) breath awareness (BAW) and (iv) a control (CTL) session. Recordings of the P300 were made before and after each session. Each session was 20 min in duration. No recordings were made during a session as the practice of yoga breathing resulted in movement artifact. All participants gave their signed consent to participate in the study. The study was approved by the Institution's Ethics committee.
Assessment
Assessments of the P300 event related potentials were made using a Nicolet Bravo System (U.S.A.). For the recording an oddball paradigm was used, wherein two auditory stimuli were presented randomly. The participants were required to discriminate between infrequent target stimuli and frequent standard stimuli by mentally counting the target stimuli.[12]
Recording conditions
Participants were asked to avoid substances which influence cognitive performance (e.g., coffee for the caffeine content) on the day preceding and the day of the recording. Where this was unavoidable, sessions were recorded on another day. Participants were seated in a sound attenuated and dimly lit cabin and monitored using a closed circuit television. Instructions were given through a two-way intercom to minimize external disturbances during sessions.
Electrode positions
Ag/AgCl disk electrodes were placed on the scalp using ten-20 conducting paste (D.O. Weaver, USA). The electrodes sites were C3 and C4 scalp sites, referred to linked earlobes with a ground electrode placed at FPz. The electro-oculogram (EOG) activity was recorded from two sites placed 1 cm above and 1 cm below the outer canthus of the right eye. All electrode impedances were kept below 5 kΩ. The electrode positions were based on the International 10-20 system for electrode placement.[16]
Amplifier settings
The electroencephalographic (EEG) activity was amplified with a sensitivity of 100 μV. The pre-stimulus delay was set at 75 ms and the P300 ERPs were computer averaged in 500 trial sweeps, with a range between 75 and 750 ms. The rejection level for artifact was kept at 90%. The low cut filter was set at 0.01 Hz and the high cut filter was set at 30.0 Hz.
Stimulus characteristics
Auditory clicks were delivered through close fitting earphones (TDH-39, Amplivox, UK). Binaural tone stimuli of alternating polarity delivered at 0.9 ms with a frequency of 1 KHz (50 cycles for the plateau; 10 cycles for the ramp) for standard stimuli and 2 KHz (10 cycles for the plateau; 20 cycles for the ramp) for target stimuli were used to trigger online averaging of the EEG. The percentage of standard stimuli was set at 80 and for target stimuli at 20. Stimulus intensity was kept at 80 dB SPL.
Interventions
Each participant was tested in five sessions; this included four experimental sessions and one session with no intervention as a control session. Each session lasted for 20 min and sessions were on five separate days at the same time of the day. The five sessions were (1) right nostril yoga breathing (RNYB) or suryanuloma viloma pranayama practice which involves breathing exclusively in and out through the right nostril while the left nostril is occluded, (2) left nostril yoga breathing (LNYB) or chandra anuloma viloma pranayama practice, which involves breathing through the left nostril exclusively in and out while the right nostril is occluded, (3) alternate nostril breathing (AYNB) or nadisuddhi pranayama practice which involves breathing through left and right nostrils alternately, in the following sequence: Breathe out from the left, breathe in through the left, breathe out from the right, breathe in from the right, and breathe out from the left, to complete one round, and (4) the breath awareness session, during which participants maintained awareness of the breath without manipulation of the nostrils.[17] Throughout the practices, participants sat cross-legged posture and were aware of their breath. During the above four practices the subjects’ attention was directed to the flow of air as it moved through the nasal passages. (5) In the control session participants were also asked to sit cross-legged but without being aware of their breath. Though the participants were long term yoga practitioners trained to be aware of their breath, they were able to direct their attention away from their breath, with a conscious effort. This session served to assess the retest effect, i.e., weather recording the P300 once more after 20 min would have any effect, if there was no intervention during the 20 min period. The three pranayamas involved nostril manipulation. The thumb and the ring finger of the right hand were used to manipulate or occlude the nostrils. This is a characteristic yoga gesture (nasika mudra in Sanskrit) prescribed during pranayama practice.[18]
Data extraction
The peak amplitude and peak latency of the P300 were assessed from C3 and C4, electrode sites. The peak amplitude (μV) was defined as the voltage difference between a pre-stimulus baseline and the largest positive peak of the ERP waveform within a 250-450 ms latency window (Polich, 1999). The peak latency (ms) was defined as the time from stimulus onset to the point of maximum positive amplitude within the latency window.
Data analysis
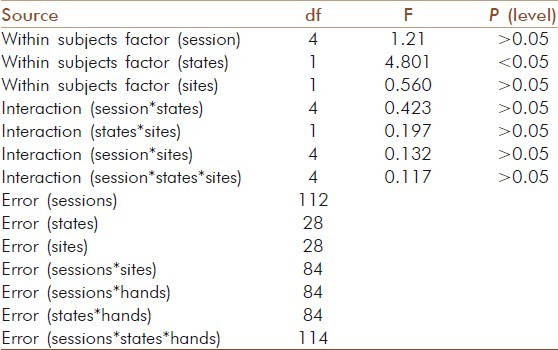
The values obtained before and after each practice were analyzed using SPSS (Version 16.0). Repeated measures analyses of variance (ANOVA) were performed with three within-subjects factors, i.e., (i) Sessions (5 levels, i.e., right nostril yoga breathing, left nostril yoga breathing, alternate nostril yoga breathing, breath awareness and no intervention); (ii) States (2 levels, i.e., pre, and post), and (iii) Sites (2 levels, i.e., C3, and C4). Separate ANOVAs were conducted for the peak latency and the peak amplitude values of the P300 component. Post-hoc analyses for multiple comparisons with Bonferroni adjustment were performed to detect significant differences. Multiple comparisons done simultaneously may increase the risk of getting false positives. The Bonferinni adjustment takes the number of comparisons into account and checks for significance with that basis.
RESULTS
Repeated measures analyses of variance (ANOVAs) showed a significant difference between States, [(F=4.801, df=1, 28), P<0.05, Huynh Feldt epsilon=1.000]. Post-hoc analysis with Bonferroni adjustment showed that the P300 peak latency was significantly lower at C3 compared to that at C4, following right nostril yoga breathing (P<0.05) [Tables 1 and 2].
Table 1.
Peak latency (ms) and peak amplitude (μV) at C3 and C4 of the P300 component before and after the five sessions. Values are groups mean±S.D
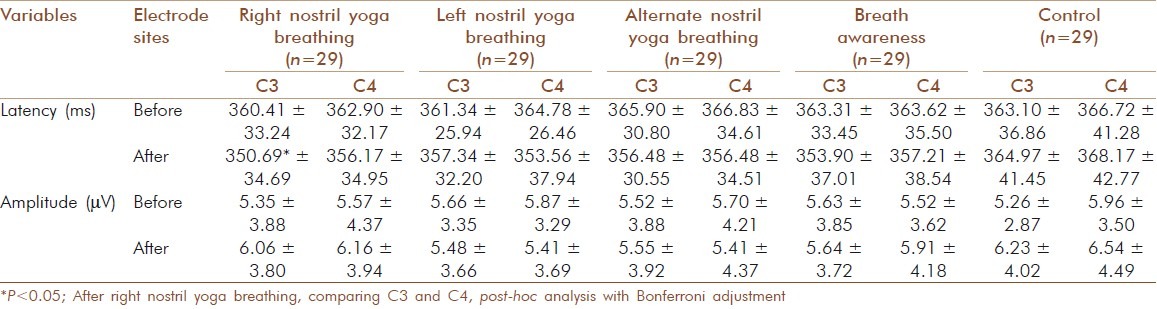
Table 2.
Analysis of variance
The groups mean values±SD for the peak latency and peak amplitude of P300 before and after yoga breathing practices are given in Table 1.
DISCUSSION
In total, 29 volunteers were each assessed in five sessions, viz., right-, left-, alternate-nostril yoga breathing, breath awareness and no intervention. After right nostril yoga breathing (RNYB), the P300 peak latency was significantly lower recorded from the left scalp site (C3), compared to the latency recorded from the right side (C4).
The P300 reflects the speed of stimulus classification, is generally not related to the overt response, and is independent of the behavioral reaction time.[19] Also, since the P300 latency is an index of stimulus processing rather than response generation, it is used as a motor-free measure of cognitive function. The P300 peak latency has been negatively correlated with mental function in normal persons as shorter latencies are associated with superior cognitive performance in tasks for attention and immediate memory.
Hence the present results suggest that RNYB facilitates the performance in the P300 task, possibly requiring less time to complete the task. These changes were recorded on the left side. A previous report on cytoarchitectonic data suggests that the primary auditory cortices of the right and left cerebral hemispheres are similarly organized.[20] This may explain why, under basal conditions, auditory evoked potentials with neural generators in the right and left auditory cortices have similar latency and amplitude characteristics.[21] Hence the asymmetric decrease in the P300 peak latency evoked by an auditory oddball paradigm following RNYB appears to be related to the breathing practice, rather than asymmetry in the underlying neural generators. However more complex processes are involved in this discrimination task, compared to the simple processing of auditory information as in auditory evoked potentials. The similarity in the cytoarchitectonic organization, of the primary auditory cortices has been the basis for drawing inferences about middle latency auditory evoked potentials related to uni- or alternate nostril yoga breathing.[22] The results hence suggest that RNYB facilitates the activity of the contralateral (left) hemisphere, in the performance of the P300 task. The fact that the change was seen only on the left side may indeed be related to the fact the speech and language dominance are in left hemisphere and the task involving counting and remembering. However since there is no proven link between the two, this remains a speculation.
Recently, similar trends (i.e., of uninostril breathing facilitating the activity of the opposite cerebral hemisphere) were also observed for uninostril yoga breathing in relation to a letter cancellation task.[11] The immediate effects of three yoga breathing practices (i.e., left-, right-, and alternate-nostril yoga breathing) were evaluated in relation to the performance in a letter-cancellation task, which is a left-hemisphere dominant task The letter-cancellation task scores were significantly improved, with fewer errors, following right and alternate nostril yoga breathing; the former (RNYB) being suggestive of a contralateral effect.
The immediate effects of right and left nostril yoga breathing were studied in forty-five adult participants randomly assigned to right nostril yoga breathing, left nostril yoga breathing, or breath awareness as a control intervention.[23] All participants were given a spatial and verbal memory task, which involved delayed recall, at the beginning and end of 45 min of practicing right and left nostril yoga breathing or breathe awareness. Following left nostril yoga breathing the participants had increased scores in the spatial memory task which was suggestive of a contralateral effect.
The exact mechanism by which uninostril breathing influences cerebral hemispheric activity is not known. However, previous experiments studying the effect of hyperventilation through the nose on EEG activity in the cortex suggests that this is produced by a neural reflex mechanism in the superior nasal meatus.[24]
The neuroelectric events that underlie the P300 generation arise from the interaction between the frontal lobe, hippocampal and temporoparietal function.[25] The primary neural generators for the P300 components are in the anterior cingulate, when new stimuli are processed into working memory, with subsequent activation of the hippocampal formation when frontal lobe mechanisms communicate with temporal or parietal lobe connections.[12]
In summary, the present results support previous results suggesting that uninostril yoga breathing (like spontaneous shifts in nostril dominance and unilateral forced nostril breathing) facilitates the activity of the contralateral cerebral hemisphere, in this case related to the P300 task.
The relevance of these findings is that certain psychiatric disorders are known to be associated with the selective disruption of the function of a specific hemisphere. Uninostril breathing practices have potential to be used in conditions like this. Left unilateral forced nostril breathing was tried with success in obsessive compulsive disorder, which was described by the authors as a disorder of the right hemisphere.[26] These therapeutic applications make it important to understand the effects in normal persons.
ACKNOWLEDGMENTS
The authors gratefully acknowledge a grant from the Central Council for Research in Yoga and Naturopathy, Ministry of Health and Family Welfare, Government of India, which funded this research.
Footnotes
Source of Support: Central Council for Research in Yoga and Naturopathy, Ministry of Health and Family Welfare, Government of India
Conflict of Interest: None declared
REFERENCES
- 1.Flanagan P, Eccles R. Spontaneous changes of unilateral nasal airflow in man: A re-examination of the nasal cycle. Acta Otolaryngol. 1997;117(Suppl 4):590–5. doi: 10.3109/00016489709113443. [DOI] [PubMed] [Google Scholar]
- 2.Shannahoff-Khalsa DS, Kennedy B, Yates FE, Ziegler MG. Low frequency ultradian insulin rhythms are coupled to cardiovascular, autonomic, and neuroendocrine rhythms. Am J Physiol. 1997;27:962–68. doi: 10.1152/ajpregu.1997.272.3.R962. [DOI] [PubMed] [Google Scholar]
- 3.Shannahoff-Khalsa DS, Yates FE. Ultradian sleep rhythms of lateral EEG, autonomic, and cardiovascular activity are coupled in humans. Int J Neurosci. 2000;101:21–43. doi: 10.3109/00207450008986490. [DOI] [PubMed] [Google Scholar]
- 4.Shannahoff-Khalsa DS. Lateralized rhythms of the central and autonomic nervous systems. Int J Psychophysiol. 1991;11:222–51. doi: 10.1016/0167-8760(91)90017-r. [DOI] [PubMed] [Google Scholar]
- 5.Shannahoff-Khalsa DS. San Diego, USA: Academic Press (Elsevier Scientific Publications); 2008. Psychophysiological states: The ultradian dynamics of mind-body interactions. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Pilon D, Prossner SD, Shannahoff-Khalsa DS. Nasal airflow asymmetries and human performance. Biol Psychol. 1986;23:127–37. doi: 10.1016/0301-0511(86)90077-3. [DOI] [PubMed] [Google Scholar]
- 7.Shannhaoff-Khalsa DS, Boyle MR, Buebel ME. The effects of Unilateral forced breathing on cognition. Int J Neurosci. 1991;57:239–49. doi: 10.3109/00207459109150697. [DOI] [PubMed] [Google Scholar]
- 8.Jella SA, Shannahoff-Khalsa DS. The effects of unilateral forced nostril breathing on cognitive performance. Int J Neurosci. 1993;73:61–8. doi: 10.3109/00207459308987211. [DOI] [PubMed] [Google Scholar]
- 9.Raghuraj P, Telles S. Immediate effect of specific nostril manipulating yoga breathing on autonomic and respiratory variables. Appl Psychophysiol Biofeedback. 2008;33:65–75. doi: 10.1007/s10484-008-9055-0. [DOI] [PubMed] [Google Scholar]
- 10.Naveen KV, Nagarathna R, Nagendra HR, Telles S. Yoga breathing through a particular nostril increases spatial memory scores without lateralized effect. Psychol Rep. 1997;81:555–61. doi: 10.2466/pr0.1997.81.2.555. [DOI] [PubMed] [Google Scholar]
- 11.Telles S, Raghuraj P, Maharana S, Nagendra HR. Immediate effect of three yoga breathing techniques on performance on a letter cancellation task. Percept Mot Skills. 2007;104:1289–96. doi: 10.2466/pms.104.4.1289-1296. [DOI] [PubMed] [Google Scholar]
- 12.Polich J. P300 in clinical applications. In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalography: Basic Principles, Clinical Applications and Related Fields. Baltimore, Munich: Urban and Schwarzenberg; 1999. pp. 1073–91. [Google Scholar]
- 13.Yadav A, Tandon OP, Vaney N. Auditory evoked responses during different phases of menstrual cycle. Indian J Physiol Pharmacol. 2002;46:449–56. [PubMed] [Google Scholar]
- 14.Polich J, Conroy M. P3a and P3b from visual stimuli: Gender effects and normative variability. In: Reinvang I, Greenlee MW, Herrmann M, editors. The Cognitive Neuroscience of Individual differences. Delmenhorst, Germany: Hanse Institute for Advanced Study; 2003. pp. 293–06. [Google Scholar]
- 15.Zar JH. London: Prentice Hall; 1999. Biostatistical analysis. [Google Scholar]
- 16.Jasper HH. The ten-twenty electrode system of the International federation. Electroencephalogr Clin Neurophysiol. 1958;10:371–5. [PubMed] [Google Scholar]
- 17.Nagendra HR, Mohan T, Shriram A. Bangalore, India: Vivekananda Kendra Yoga Anusandhana Samsthan; 1988. Yoga in education. [Google Scholar]
- 18.Munger, Bihar: Bihar School of Yoga; 1994. Swami Niranajanananda Saraswati. Prana, Pranayamas, Pranavidya. [Google Scholar]
- 19.Polich J. Clinical applications of the P300 event-related brain potential. Phys Med Rehabil Clin N Am. 2004;15:133–61. doi: 10.1016/s1047-9651(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 20.Seldon HL. Structure of human cortex. I. Cytoarchitectonic and dendritc distribution. Brain Res. 1981;229:227–94. doi: 10.1016/0006-8993(81)90994-x. [DOI] [PubMed] [Google Scholar]
- 21.Liégeois-Chauvel C, Musolino A, Badier JM, Marquis P, Chauvel P. Evoked potentials recorded from auditory cortex in man: Evaluation and topography of the middle latency components. Electroencephalogr Clin Neurophysiol. 1994;92:204–14. doi: 10.1016/0168-5597(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 22.Raghuraj P, Telles S. Right uninostril yoga breathing influences ipsilateral components of middle latency auditory evoked potentials. J Neurol Sci. 2004;25:274–80. doi: 10.1007/s10072-004-0354-9. [DOI] [PubMed] [Google Scholar]
- 23.Joshi M, Telles S. Immediate effects of right and left nostril yoga breathing on verbal and spatial scores. Indian J Physiol Pharmacol. 2008;52:197–200. [PubMed] [Google Scholar]
- 24.Kristof M, Sevit Z, Manas K. Activating effect of nasal airflow on epileptic electrographic abnormalities in the human EEG.Evidence for the reflect origin of the phenomenon. Physiol Bohemoslov. 1981;30:73–7. [PubMed] [Google Scholar]
- 25.Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in the auditory and visual oddball tasks. Electroencephalogr Clin Neurophysiol. 1998;106:156–64. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- 26.Shannahoff-Khalsa DS, Beckett LR. Clinical case report: Efficacy of yogic techniques in the treatment of obsessive compulsive disorder. Int J Neurosci. 1996;85:1–17. doi: 10.3109/00207459608986347. [DOI] [PubMed] [Google Scholar]


