Abstract
Background & objectives:
Vibrio cholerae produces acute infection by liberating potent enterotoxin, called cholera toxin in human intestine. Cardiovascular drug lacidipine possessing powerful in vitro action against V. cholerae was tested to determine its possible activity against a toxigenic V. cholerae strain in an established animal model.
Methods:
In the rabbit intestine four loops were constructed, 3 of which were injected with over night grown V. cholerae 569B culture. Of these, two loops were simultaneously given graded doses (100, 200 μg) of lacidipine, one was left as such for a positive control. The first loop received sterile medium (negative control). After 18 h, contents of all the loops were examined for accumulation of fluid and number of viable cells.
Results:
Lacidipine when administrated with live V. cholerae 569B, caused a reduction in the number of viable bacteria along with amount of fluid in the loops. The amount of fluid and number of viable cells were much reduced in the loop that had 200 μg of lacidipine than the loop that received 100 μg of the drug.
Interpretation & conclusions:
Lacidipine has distinct inhibitory action against V. cholerae 569B with respect to both viability and production of cholera toxin in the rabbit ileum. Structural modifications of this compound may possibly lead to procurement of new potent antimicrobial drugs.
Keywords: Antibacterial, antitoxigenic, cholera toxin, lacidipine, non-antibiotic, Vibrio cholerae
The therapeutic efficacies of antibiotics and antibacterial agents to cure almost all major infections are gradually becoming limited due to the ever increasing problem of emergence of drug resistances among bacterial pathogens. Such escalating levels of drug resistances have rendered the possibilities to explore newer drugs with potent antibacterial action. During the last three decades a large number of compounds belonging to different pharmacological categories have been found to carry moderate to powerful antimicrobial action1–4. These studies have revealed that phenothiazines possessing tricyclic benzene rings and antihypertensive cardiovascular drugs possessing at least two benzene rings are also potent antimicrobials5–8.
The cardiovascular drug lacidipine was found to be highly inhibitory against a large number of organisms including Vibrio cholerae8. The antibacterial potential was further substantiated by determining its protective capacity in Swiss albino mice challenged with virulent Salmonella enterica serovar Typhimurium. The present study was aimed to describe the inhibitory activity of lacidipine against cholera toxin introduced in the rabbit ileum following the standard procedure9,10.
Material & Methods
The study was carried out at Department of Microbiology, Herbicure Healthcare Bio-Herbal Research Foundation and Bio-Equivalence Study Centre, Department of Pharmaceutical Technology, Jadavpur University, Kolkata.
The strain of Vibrio cholerae 569B was obtained from Vibrio Reference Centre, National Institute of Cholera and Enteric Diseases (NICED), Kolkata, as a freeze-dried ampoule. The authenticity of the culture was confirmed by different biochemical and serological tests11. Lacidipine was obtained as a pure dry powder from Sun Pharmaceuticals Ltd, India. Fluid media were peptone water containing 1.0 per cent bacteriological peptone (Oxoid, UK) plus 0.5 per cent Analar sodium chloride and nutrient broth (Oxoid); solid media were thiosulphate citrate bile-salts sucrose (TCBS) agar (Oxoid), MacConkey agar (Oxoid) and nutrient agar (Oxoid); pH of all these media ranged from 7.4-7.6.
Five healthy male New Zealand rabbits each weighing1.6 to 1.8 kg with an average age of 10 wk were used. The rabbits were kept in the animal house maintaining the standard condition of 21±1°C and 50-60 per cent relative humidity with a photo period of 14:10 h of light: darkness. Water was provided ad libitum.
Known rabbit virulent toxin producing Inaba strain of V. cholerae 569B was grown in TCBS agar for 24 h to obtain the challenge dose. One single colony was picked up and was given as an inoculum to 5 ml of peptone water and incubated at 37°C. After 24 h cfu (colony forming unit) count was determined, which was found to be 4.5 × 108; 1 ml of this culture was the challenge dose.
Experimental procedure: The ethical clearance was obtained from Bio-Equivalence Study Centre, Kolkata. The rabbits were kept on fasting for 48 h prior to the experiment and until they were sacrificed. Each rabbit was anaesthetized with anaesthetic ether (E. Merck, Germany) and the intestine was exteriorized through a midline incision maintaining a strict aseptic condition. Four experimental loops of 10 cm length each were constructed at the terminal ileum of a rabbit (Fig. 1)9,10,12. The loops were injected with the following fluids. The first loop (Fig. 2, 1) was injected with 1 ml of sterile peptone water (negative control), the second loop (2) received simultaneously challenge plus 200 μg of lacidipine as 0.1 ml fluid from a stock solution containing 2 mg/ml of the drug. The third loop (3) received challenge and 100 μg of lacidipine while only the challenge dose of 569B was administered to the fourth loop (4; positive control). Finally the ligated rabbit ileum was packed inside the abdomen and the skin layers were sutured perfectly. Each rabbit was taken back to its respective cage and was observed carefully. After 18 h the rabbits were sacrificed with the help of chloroform. The fluid from each loop was collected aseptically and the ratio of amount of fluid contained in a loop with respect to the length of the loop (fluid accumulation ratio, ml/cm) was calculated as a reflection of the activity of cholera toxin. The number of viable cells (cfu count) was determined separately from each loop to obtain the action of lacidipine on multiplication of V. cholerae 569B producing cholera toxin.
Fig. 1.

Preparation of experimental loops in the rabbit ileum to determine the effect of lacidipine on cholera toxin. The first loop (1) contained sterile peptone water (negative control); the second loop (2) received challenge plus 200 μg of lacidipine; the third loop (3) was given challenge plus 100 μg of lacidipine, while the fourth loop (4) received only the challenge (positive control). Experiment was replicated five times in five different rabbits.
Fig. 2.
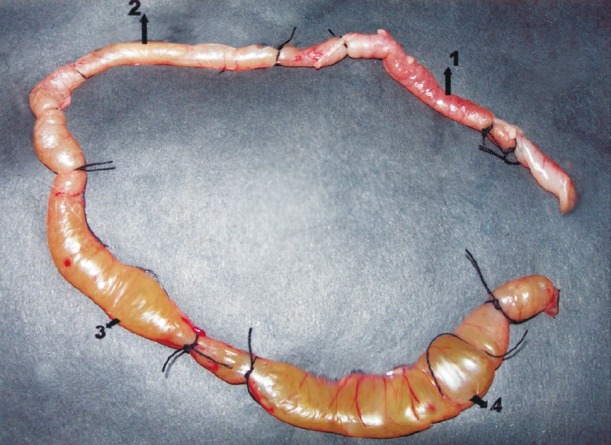
Inhibitory effect of lacidipine on cholera toxin-induced fluid accumulation in the rabbit ileal loop. There was no swelling in loop 1 (receiving only peptone water) and very little swelling in loop 2 (receiving challenge plus 200 μg lacidipine). Ballooning effect was observed in loop 3 which had challeng plus 100 μg of lacidipine. Ballooning effect was more pronounced in loop 4 which was given only the challenge.
Results
After 18 h of the actual test, the experimental loops were taken out. There was no apparent ballooning in the first loop and a minor ballooning in the second loop. However, the fourth loop was distinctly swollen, the third loop was also swollen but lesser than the fourth one (Fig. 2). Among the four loops, the fluid of the first loop that had received only peptone water was found to be absolutely sterile; while the fluid of the fourth loop which was given only the culture exhibited presence of V. cholerae, with a cfu count >108,11. The purity of the culture was confirmed again. The other two loops that were administered both the challenge and lacidipine exhibited significant reduction in the number of viable cells. Data on amount of fluid accumulation and cfu count in five test rabbits are given in the Table.
Table.
Effect of lacidipine on fluid accumulation and viability of V. cholerae 569 B cell in rabbit ileal loop

Discussion
Our results indicated that lacidipine could inhibit accumulation of fluid in the intestinal loops of experimental rabbits. The fluid was cholera toxin produced by multiplying cells of V. cholerae 569B. The effectiveness of the drug could be visualized further as the higher dose made significant reduction not only in the number of viable cells, but also in the amount of fluid accumulation. This finding further confirms our earlier observation that lacidipine is a bacteriostatic agent8. The cardiovascular drug lacidipine is a third generation calcium channel blocker used worldwide. It is now known for its antihypertensive action and also antioxidant property13. This class of pharmacological agents relaxes smooth muscles and dilates coronary and peripheral arteries. Lacidipine has more influence on vessels and less on the myocardium, and has no anti-arrythmic activity. It can rarely precipitate heart failure because its negative inotropic effect has minimal action on the left ventricular wall. The initial human dose of lacidipine for a patient with cardiovascular ailment is 4 mg daily, but, if necessary, it may be increased to 6 mg after 3-4 wk8. In this study, it was found that only 200 μg of lacidipine could significantly reduce the amount of cholera toxin formed in the rabbit ileal loops. It is known that the heat labile enterotoxin of Escherichia coli is structurally and functionally very similar to cholera toxin14. We had also noted earlier that enterotoxigenic E. coli K88, K99 were highly sensitive to lacidipine8. In conclusion, the findings of the present study elaborated significance of lacidipine as an antibacterial and antitoxic compound. The action of this non toxic agent may be enhanced further by chemical modifications in its structure to create new molecules with much more potent actions.
References
- 1.Kristiansen JE. The antimicrobial activity of non-antibiotics. Acta Pathol Microbiol Scand Section A. 1992;100(Suppl):7–19. [Google Scholar]
- 2.Dasgupta A, Dastidar SG, Shirataki Y, Motohashi N. Antibacterial activity of artificial phenothiazines and isoflavones from plants. In: Motohashi N, editor. Bioactive heterocycles VI: Flavonoids and anthocyanins in plants, and latest bioactive heterocycles I. 1st ed. Vol. 1. Berlin, Heidelberg: Springer Verlag; 2008. pp. 67–132. [Google Scholar]
- 3.Molnar J, Mandi Y, Kiraly J. Antibacterial effect of some phenothiazine compounds and R-factor elimination by chlorpromazine. Acta Microbiol Acad Sci Hung. 1976;23:45–54. [PubMed] [Google Scholar]
- 4.Martins M, Dastidar SG, Fnning S, Kristiansen JE, Molnar J, Pages JM, et al. Potential role of non-antibiotics (helper compounds) in the treatment of multidrug-resistant Gram-negative infections : mechanisms of their direct and indirect activities. Int J Antimicro Agents. 2008;31:198–208. doi: 10.1016/j.ijantimicag.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Asok Kumar K, Ganguly K, Mazumdar K, Dutta NK, Dastidar SG, Chakrabarty AN. Amlodipine: a cardiovascular drug with powerful antimicrobial property. Acta Microbiol Polonica. 2003;52:285–92. [PubMed] [Google Scholar]
- 6.Mazumdar K, Ganguly K, Asok Kumar K, Dutta NK, Chakrabarty AN, Dastidar SG. Antimicrobial potentiality of a new non-antibiotic: the cardiovascular drug oxyfedrine hydrochloride. Microbiol Res. 2003;158:259–64. doi: 10.1078/0944-5013-00204. [DOI] [PubMed] [Google Scholar]
- 7.Pal T, Dutta NK, Mazumdar K, Dasgupta A, Jeyaseeli L, Dastidar SG. Assessment bacterial activity of the cardiodascular drug nifedipine. Orient Pharm Exp Med. 2006;6:126–33. [Google Scholar]
- 8.Dasgupta A, Jeyaseeli L, Dutta NK, Mazumdar K, Karak P, Dastidar SG, et al. Studies on the antimicrobial potential of the cardiovascular drug lacidipine. In Vivo. 2007;21:847–50. [PubMed] [Google Scholar]
- 9.De SN, Chatterjee DN. An experimental study of the mechanism of action of Vibrio cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953;66:559–62. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- 10.Gorbach SL, Banwell JG, Chatterjee BD, Jacobs B, Sack RB. Acute undifferentiated diarrhoea in the topics, 1. Alteration in the intestinal microflora. Clin Invest. 1971;50:881–9. doi: 10.1172/JCI106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collee FG, Miles RS, Watt B. Tests for identification of bacteria. In: Collee JG, Frase AG, Marmion BP, Simmons A, editors. Mackie and McCartney's practical medical microbiology. NewYork: Churchill Livingstone; 1996. pp. 131–50. [Google Scholar]
- 12.Hitotsubashi S, Fujii Y, Yamanoka H, Okamoto K. Some properties of purified Escherichia coli heat-stable enterotoxin II. Infect Immun. 1992;60:4468–74. doi: 10.1128/iai.60.11.4468-4474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cristofori P, Lanzoni A, Quartaroli M, Pastorino AM, Zancanaro C, Cominacini L, et al. The calcium-channel blocker lacidipine reduces the development of atherosclerotic lesions in the apoE-deficient mouse. J Hypertension. 2008;18:1429–36. doi: 10.1097/00004872-200018100-00010. [DOI] [PubMed] [Google Scholar]
- 14.Tsuji T, Inoue A, Miyama A, Noda M. Glutamic acid- 112 of the A subunit of heat-labile enterotoxin from enterotoxic Escherichia coli is important for ADP-ribosyltransferase activity. FEBS Lett. 1991;291:319–21. doi: 10.1016/0014-5793(91)81311-u. [DOI] [PubMed] [Google Scholar]


