Abstract
Excess body weight is associated not only with an increased risk of type 2 diabetes and cardiovascular disease (CVD) but also with various types of malignancies. Adiponectin, the most abundant protein secreted by adipose tissue, exhibits insulin-sensitizing, antiinflammatory, antiatherogenic, proapoptotic, and antiproliferative properties. Circulating adiponectin levels, which are determined predominantly by genetic factors, diet, physical activity, and abdominal adiposity, are decreased in patients with diabetes, CVD, and several obesity-associated cancers. Also, adiponectin levels are inversely associated with the risk of developing diabetes, CVD, and several malignancies later in life. Many cancer cell lines express adiponectin receptors, and adiponectin in vitro limits cell proliferation and induces apoptosis. Recent in vitro studies demonstrate the antiangiogenic and tumor growth-limiting properties of adiponectin. Studies in both animals and humans have investigated adiponectin and adiponectin receptor regulation and expression in several cancers. Current evidence supports a role of adiponectin as a novel risk factor and potential diagnostic and prognostic biomarker in cancer. In addition, either adiponectin per se or medications that increase adiponectin levels or up-regulate signaling pathways downstream of adiponectin may prove to be useful anticancer agents.
This review presents the role of adiponectin in carcinogenesis and cancer progression and examines the pathophysiological mechanisms that underlie the association between adiponectin and malignancy in the context of a dysfunctional adipose tissue in obesity. Understanding of these mechanisms may be important for the development of preventive and therapeutic strategies against obesity-associated malignancies.
Introduction
-
Adiponectin Biology
Identification and history of discovery
Adiponectin gene and expression
Adiponectin structure
Adiponectin receptors
Adiponectin signaling pathways
-
Adiponectin Physiology and Pathophysiology
Adiponectin physiological functions
Circulating adiponectin and its determinants
Adiponectin levels in relation to disease states
-
Epidemiological Evidence That Links Adiponectin to Cancer
Adiponectin and colorectal cancer
Adiponectin and breast cancer
Adiponectin and endometrial cancer
Adiponectin and gastric cancer
Adiponectin and esophageal cancer
Adiponectin and pancreatic cancer
Adiponectin and liver cancer
Adiponectin and renal cancer
Adiponectin and prostate cancer
Adiponectin and hematological malignancies
Adiponectin and lung cancer
Adiponectin and other malignancies
-
Adiponectin and Carcinogenesis Mechanisms
Direct mechanisms of action
Indirect mechanisms of action
Conclusion and Future Perspectives
I. Introduction
Obesity and overweight constitute a worldwide problem reaching epidemic proportions and impacting on the risk and prognosis of several disease states including cardiovascular disease, type 2 diabetes mellitus, and common forms of cancer (1–5). The prevalence of obesity has increased substantially over the previous decades not only in industrialized countries but all over the world; recent data from several Western countries indicate that only one third of the population is normal weight, and approximately one third is obese (4–6). In 2003, it was estimated that in the United States excess weight was responsible for 14% of all cancer deaths in men and 20% of those in women (5, 7, 8). There is also accumulating evidence that excess body weight constitutes an established risk factor for colon cancer, postmenopausal breast cancer, endometrial cancer, renal cell cancer, and esophageal adenocarcinoma (EA) (6). Moreover, obesity has recently been implicated in the occurrence of hematological malignancies such as non-Hodgkin's lymphoma, leukemia, and multiple myeloma (MM) (6, 9); thyroid cancer; pancreatic cancer (PaC); gallbladder cancer; high-grade prostate cancer; and ovarian cancer (5, 7, 10, 11). For example, 56.8% of endometrial cancer cases in the United States and 45.2% in Europe may be attributable to obesity, whereas the respective numbers of esophageal cancer cases attributable to obesity are 52.4% in the United States and 42.7% in Europe (12). Across all malignancies, obesity has been responsible for 52 and 88% higher mortality rates in males and females, respectively (8, 13). It is believed that the metabolic changes associated with excess weight, and in particular central obesity, could lead to a dysfunctional adipose tissue causing insulin resistance, chronic inflammation, and abnormal secretion of adipocytokines (14–18). The main underlying mechanisms that link obesity to cancer development and progression include: 1) abnormalities of insulin resistance and the IGF-I system; 2) the impact of adiposity on the biosynthesis and bioavailability of endogenous sex hormones; 3) obesity-induced low-grade chronic systemic inflammation; and 4) alterations in the levels of adipocyte-derived factors (13, 14, 18). This review will specifically focus on adiponectin, the most abundant adipocyte-derived factor, which, according to accumulating evidence, lies upstream of most of the above factors in the etiology of obesity-associated malignancies.
In addition to its fat-storing capacity, adipose tissue is the largest endocrine organ regulating energy homeostasis, metabolism, inflammation, immunity, endocrine balance, and bone remodeling (15–17). Adipose tissue is responsible for the biosynthesis and secretion of more than 50 hormones and cytokines, known as adipocytokines or adipokines (19). Adiponectin represents the most abundant adipose-tissue protein with insulin-sensitizing, antiinflammatory, and antiatherogenic properties (15, 16). Hypoadiponectinemia is associated not only with insulin resistance, type 2 diabetes, atherosclerosis, and coronary heart disease (20–22), but also with malignancies (17). Very recently, studies have shown that adiponectin is a key mediator in the development and possible progression of several types of obesity-associated cancers (15, 23), yet the mechanism of association is still poorly understood.
The purpose of this review is to explore the role of adiponectin in carcinogenesis and cancer progression and to examine the mechanisms that underlie the association between adiponectin and malignancy. Understanding of the mechanisms connecting adiponectin with cancer is expected to be of importance in the development of preventive and therapeutic strategies against cancer. Significant advances in research that have been made over the past few years are reviewed in this article.
II. Adiponectin Biology
A. Identification and history of discovery
Adiponectin, a 244-amino acid protein secreted predominantly by white adipose tissue, is also known as AdipoQ (24), Acrp30 (adipocyte complement-related protein of 30 kDa) (25), apM1 (gene product of the adipose most abundant gene transcript-1) (26), and GBP28 (gelatin-binding protein-28) (27). It was discovered by four different research groups almost simultaneously in the mid-1990s. In 1995, Scherer et al. (25) first isolated adiponectin cDNA from the mouse adipocyte cell line 3T3-L1 and named it Acrp30 because of its similarity to the complement protein family. This was soon confirmed by Hu et al. (24), who isolated the protein from the 3T3-F442A adipocyte cell line and named it AdipoQ. Both Acrp30 and AdipoQ are still in use when referring to adiponectin in mice (28, 29). In parallel, Maeda et al. (26) identified human adiponectin cDNA, which was the most abundant transcript found in human adipose tissue, and called it apM1. Finally, using high-affinity chromatography, adiponectin was purified from plasma by Nakano et al. (27) as a gelatin-binding protein of 28 kDa (GBP28). In 1999, Arita et al. (30) decided to name it adiponectin because the gene product was predicted to be a matrix protein synthesized by adipose tissue. Nowadays, the most commonly used name is “adiponectin.” Adiponectin belongs to the expanding C1q/TNF family of proteins. Recently, a family of adiponectin paralogs designated as CTRP (C1q/TNF-related protein) 1–7, sharing a similar structure to adiponectin, was identified (31, 32). Interestingly, these paralogs are ubiquitously expressed in different mouse tissues and present similar biological activity to adiponectin (mCTRP2), indicating probably supplementary biological mechanisms for adiponectin (31, 32). In 2003, Yamauchi et al. (33) isolated and described for the first time mouse and human adiponectin receptors, transforming therefore the knowledge of several properties of this adipose tissue hormone. Finally, T-cadherin (also known as CDH13) was identified as a potential third adiponectin receptor (34).
B. Adiponectin gene and expression
The adiponectin gene, coding for a 244-amino acid polypeptide, is located on chromosome 3q27, a region associated with susceptibility for developing metabolic syndrome and type 2 diabetes in Caucasians (35, 36). In 2000, Takahashi et al. (35) described extensively the structure of adiponectin's gene, which consists of three exons and two introns. Polymorphisms of the adiponectin gene may be associated with alterations of adiponectin function and important clinical conditions. As a matter of fact, several single nucleotide polymorphisms (SNP) in the coding region and surrounding sequence were identified from different populations, with varying prevalence, degrees of association, and strength of effect on insulin resistance, type 2 diabetes, obesity, dyslipidemia, and cancer (35, 37–40).
Adiponectin is synthesized mainly in white adipose tissue and, at lower concentrations, in brown adipose tissue (16, 26). Other tissues likely express vastly lower quantities of adiponectin than adipose tissue. Several studies have reported expression in skeletal muscle (41), liver (42), colon (43), cardiac tissue (44), salivary glands (45), bone marrow (46), fetal tissue (46), placenta (47), cerebrospinal fluid (48), and breast milk (49). The expression of adiponectin in various tissues could indicate a possible paracrine/autocrine complementary role for adiponectin.
C. Adiponectin structure
Adiponectin shares homology with collagen VIII, X, complement factor C1q, and TNF-α, which presents an antagonistic action compared with adiponectin (50).
Structurally, adiponectin (a 244-amino acid protein) contains four distinct domains: an amino-terminal signal peptide, followed by a species-specific variable domain, a collagen-like region of 22 Gly-X-Y repeats, and a carboxyl-terminal globular domain that binds to the adiponectin receptors, and is similar to the complement factor C1q and resembles the trimeric topology of TNF-α (33, 50, 51).
The adiponectin collagen-like region of adiponectin allows oligomerization of the protein via disulfide bonds and through hydroxylation and glycosylation of four conserved lysine residues, which are important for the formation of its high molecular weight (HMW) complex (52, 53). Posttranslational modifications of adiponectin (i.e., glycosylation, sialylation, etc.) are critical determinants of its activity and binding to its receptors (50, 53, 54). For example, glycosylation and hydroxylation of the four lysines in the collagenous portion of adiponectin contribute to enhancing the ability of subphysiological concentrations of insulin to inhibit gluconeogenesis in hepatocytes (55).
Adiponectin is synthesized as a single subunit that undergoes oligomerization to form trimers, hexamers, and multimers before secretion. The monomeric form of adiponectin is thought to be present only in the adipocyte because it has not yet been detected in the circulation (28). In analogy to other collagen-domain proteins, the basic form of circulating adiponectin is a trimer (46). Adiponectin trimers are generated when a triple helix is formed by noncovalent interactions between the collagenous regions and hydrophobic interactions between the globular head domains (56). Trimer [low molecular weight (LMW)] complexes can associate through their collagenous regions into hexamers (medium molecular weight) and finally, into multimers of HMW (57).
At least three distinct and stable isoforms of adiponectin were isolated from Escherichia coli or cultured mammalian cells in both human (58) and mouse (54) plasma. Particularly in human plasma, adiponectin exists in its full-length (fAd) version or as a smaller fragment generated by proteolytic cleavage of fAd at amino acid 110 (59) that corresponds to the globular domain of the protein (gAd) with enhanced potency (60, 61). Although most active adiponectin appears to exist in the form of full-length or HMW adiponectin in plasma, LMW and gAd are also present in low concentrations probably due to their shorter half-life (58, 62, 63).
The HMW isoform may be the biologically active form of the hormone (52) and is strongly associated with insulin resistance, metabolic syndrome, and cardiovascular disease; however, the additional predictive value, above and beyond that conveyed by total adiponectin, in humans is minimal, as shown by our group (64, 65). HMW adiponectin may be more closely associated with postload glucose concentrations than total adiponectin (52), and variations in the ratio of HMW to total adiponectin correlated with amelioration in insulin sensitivity during thiazolidinedione treatment in humans, whereas variations in total adiponectin did not (66). The globular part of adiponectin appears to be as efficient as fAd at decreasing serum glucose and free fatty acid levels, at least in mice (67).
Recent studies have proposed that the various adiponectin multimers have different target tissues and/or different biological effects (50, 57). The HMW isoform may mediate the majority of adiponectin's effects in the liver (68), endothelial cells (69), and probably in skeletal muscle (70), whereas the trimers and full-length monomeric forms are responsible for other actions in various tissues (61). Moreover, the HMW isoform of adiponectin is considered to be responsible for its proinflammatory actions, whereas the LMW isoform is responsible for its antiinflammatory actions (71). Although the above underscore the need to consider adiponectin isoforms when studying its actions and functions, we have previously shown that in terms of in vivo whole body insulin sensitivity, total and HMW adiponectin are comparably good predictors without any major difference in their predictive value (64). The relative value of measuring HMW vs. total adiponectin in relation to other physiological functions of adiponectin remains to be fully elucidated.
The aforementioned also emphasize the need to develop reliable laboratory methods to estimate total adiponectin and its isoforms. This remains an active area of research.
D. Adiponectin receptors
Adiponectin binds to a number of receptors. So far, three adiponectin receptors have been identified: two main receptors—AdipoR1 and AdipoR2; and one receptor similar to the cadherin family (40, 46).
The two classical adiponectin receptors are structurally very related because their protein sequence shares 67% identity and they are also highly conserved, sharing 95% identity between humans and mice (46). The existence of these distinct adiponectin receptors serves the distinct adiponectin tissue specificities within the organism and the different affinities to various adiponectin isoforms.
The two different receptor isoforms, AdipoR1 and AdipoR2, are seven-transmembrane proteins with internal N-terminus and external C-terminus regions, contrary to the topology of G protein-coupled receptor family (33). AdipoR1 and AdipoR2 may form both homo- and heteromultimers, and it is well recognized that they mediate fatty acid oxidation and glucose uptake by adiponectin (40, 71). AdipoR1 presents high affinity for gAd and low affinity for fAd, and it is expressed ubiquitously but abundantly in skeletal muscle and endothelial cells. AdipoR2 has intermediate affinity for both forms of adiponectin and is predominantly expressed in the liver (40). Although both receptors are detected in almost every tissue, including pancreatic β-cells (72, 73) and cancerous cells, one or the other receptor usually prevails.
The classical adiponectin receptors affect the downstream target AMP-activated protein kinase (AMPK), an important cellular metabolic rate control point. Expression of the receptors is correlated with insulin levels and is reduced in mouse models of diabetes, particularly in skeletal muscle and adipose tissue (74). Thus, the expression of AdipoR1/R2 is inversely correlated with plasma insulin levels in vivo under physiological (i.e., increase with fasting, decrease with feeding) and pathological conditions (74). Obesity seems to decrease the expression of AdipoR1/R2, thereby diminishing adiponectin sensitivity, which in turn leads to a vicious cycle of insulin resistance (75). Physical activity up-regulates adiponectin receptors (76) in muscle and fat (AdipoR2 mRNA expression) and increases circulating adiponectin, suggesting that the adiponectin hormonal system may mediate exercise-associated improvements in insulin resistance (76, 77). We recently reported that aging and prolonged exposure to high-fat feeding down-regulate adiponectin levels and up-regulate the expression of adiponectin receptors (78). Observational findings in mice by our group showed that diet-induced obesity may be associated with an earlier decreased visceral expression of adiponectin, and this could be the main reason for diminished total adiponectin in response to high-fat diet and the development of the metabolic syndrome, including higher leptin and insulin levels (78). Increasing adiponectin and adiponectin receptor levels, paralleling the progressive increasing adiposity, may represent a compensatory mechanism by which mice attempt to prevent the development of insulin resistance in early stages of exposure to a high-fat diet, indicating an intermediate step in the biological process of the metabolic syndrome. Recently, we have also demonstrated an association of both adiponectin receptors with insulin resistance in humans, although only the association with AdipoR1 remained significant after multivariable adjustment (76).
We have also shown that both leptin and melanocortin agonists alter AdipoR1/R2 expression in mice (79). Reduced expression of adiponectin receptors has been reported in skeletal muscle and adipose tissue in leptin-deficient ob/ob (74) and db/db (80) mice, as well as in fa/fa Zucker rats, indicating that leptin could regulate adiponectin receptor expression. Moreover, we have shown that a medication with leptin-like effects, ciliary neurotrophic factor, results in significantly up-regulated serum adiponectin and AdipoR1-mRNA expression in muscle and liver (81). Altered levels of adiponectin and adiponectin receptors may underlie the effect of ciliary neurotrophic factor to enhance insulin sensitivity in diet-induced obese mice (81). Peroxisome proliferator-activated receptor-α (PPAR-α) and PPAR-γ are able to regulate adiponectin receptor expression in adipose tissue and cultured adipocytes, but not in cultured myocytes (74, 82, 83).
Finally, recent evidence has suggested that adiponectin receptors may play a pivotal role in the physiological regulation of ceramide and the antiapoptotic sphingosine 1 phosphate (S1P) balance, which are key mediators of inflammation, cell growth, and survival (84). AdipoR1 and AdipoR2, which belong to the progesterone and adiponectin Q receptor family, enhance ceramidase activity (85). Excessive accumulation of ceramide and glucosylceramide has been involved in a plethora of metabolic processes including insulin resistance, atherosclerosis, and lipotoxic heart failure (84). Adiponectin lowers ceramide levels via its classical receptors. On the contrary, the phosphorylated sphingoid base S1P represents a potent inducer of proliferation and inhibitor of apoptosis (86).
A nonclassical third potential adiponectin receptor is T-cadherin, which was isolated by Hug et al. (34). T-Cadherin is a cell-surface receptor in endothelial and smooth muscle cells playing an important role in cell adhesion and in calcium-mediated cell to cell interactions and signaling (34). T-Cadherin is also expressed on tumor-associated endothelial cells (87), indicating that adiponectin could possibly influence the endothelium directly. HMW and hexameric adiponectin is a proposed ligand for T-cadherin; however, the pathophysiological importance in humans is not yet clearly understood. Hug et al. (34) reported that T-cadherin was capable of binding adiponectin in C2C12 myoblasts, but not in hepatocytes. Because T-cadherin lacks an intracellular domain needed for signal transduction, it is believed that it may act as a coreceptor by competing with AdipoR1 and AdipoR2 receptors for adiponectin binding or interfering with adiponectin signal transduction (88). The significance of similar interactions in cancer pathophysiology remains to be fully elucidated.
Several tumor cell lines express adiponectin receptors, suggesting that adiponectin could possibly exert direct effects on these cells by signaling through its receptors. Adiponectin receptors are expressed in a plethora of malignant tissues including breast, prostate, hepatocellular, gastric, and colon carcinoma, pancreatic adenocarcinoma, and lung cancer (15, 89–95). Although the functional relevance of adiponectin receptors in cancerous cells has not yet been clarified, there is evidence that activation of adiponectin receptors limits the proliferation of cancer cell lines in vitro (15, 93, 96).
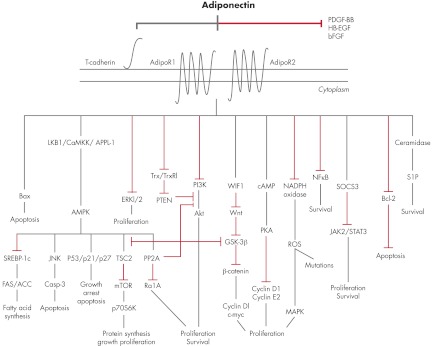
E. Adiponectin signaling pathways
Adiponectin activates several intracellular signaling pathways when binding to its receptors, mainly AMPK, but also mammalian target of rapamycin (mTOR), nuclear factor-κB (NF-κB), c-Jun N-terminal kinase (JNK), and signal transducer and activator of transcription (STAT3) (97, 98). However, a very recent study has shown that adiponectin may exert beneficial metabolic effects through a reduction of cellular ceramide levels mediated by AdipoR1 and AdipoR2 activation independently from AMPK, particularly in pancreatic β-cells, hepatocytes, and cardiomyocytes (84). Ceramide promotes a set of actions leading to metabolic disease opposite to adiponectin effects on metabolism (99). Ceramide was recently identified as an important factor for Toll-like receptor 4-mediated antagonism of insulin action (100), and thus it has been proposed that a therapeutic approach based on a targeted disruption of ceramide accumulation would improve insulin action and promote pancreatic β-cell survival, similar to the actions of adiponectin (101, 102). It has been recently proposed that adiponectin receptors may play a critical role in the physiological regulation of ceramide and S1P balance. Moreover, S1P can activate AMPK (103), and this AMPK activation observed in a subset of cell types could be a downstream event rather than an upstream activator of adiponectin action, mediated by a conversion of ceramides into S1P (84).
APPL-1 is the first identified binding protein that interacts directly with AdipoR1/R2 receptors (104). This adaptor protein, which includes a phosphotyrosine domain, a pleckstrin homology domain, and a leucine zipper motif, interacts with the N-terminal intracellular region of adiponectin receptors, thereby mediating adiponectin signaling and its effects on metabolism, antiinflammation, and cytoprotection (46, 105). APPL-1 also acts in the insulin-signaling pathway, playing a pivotal role in the cross talk between adiponectin/insulin-signaling pathways (105). Finally, APPL-1 functions as a mediator of other signaling pathways through direct interaction with membrane receptors and proteins (to date, 14 proteins are known to bind APPL-1), affecting cell proliferation and survival, apoptosis, endosomal trafficking, and chromatin remodeling (105–108).
Primarily, adiponectin exerts its insulin-sensitizing effects through an interaction with APPL-1 via the sequential activation of AMPK, p38 MAPK, PPAR-α, and RAS-associated protein 5 (46, 105). It was shown that APPL-1 suppression by small interfering RNA abrogates the adiponectin-mediated stimulation of AMPK and MAPK in C2C12 myocytes (109). In the same study, it was further reported that adiponectin activates through interaction with APPL-1 the RAS-associated protein 5, a guanine triphosphatase involved in glucose transporter 4 translocation (109). Additionally, in APPL-1-deficient myocytes, decreases in adiponectin-activated fatty acid oxidation, glucose uptake, and AMPK, MAPK, and acetyl-coenzyme A carboxylase (ACC) phosphorylation further support the involvement of APPL-1 in the adiponectin signaling cascade (109). The binding of adiponectin to its receptors provokes the activation of AMPK via APPL-1, promoting glucose utilization that results in increased fatty-acid oxidation, increased glucose uptake at the level of skeletal muscle, and reduced gluconeogenesis in the liver. AMPK is considered a cellular energy sensor that is stimulated by an increase in the intracellular AMP/ATP ratio (110). In addition, adiponectin activates PPAR-α, thereby enhancing fatty acid combustion and energy consumption, leading to a tissue decrease content of triglycerides in the liver and skeletal muscle, and improving insulin sensitivity in vivo (111). Interestingly, activation of AMPK is mediated mainly by AdipoR1, whereas stimulation of PPAR-α could be attributed to adiponectin's binding to AdipoR2 (112).
Which adiponectin isoform triggers primarily the activation of AMPK remains an active area of research. fAd, but not gAd, down-regulates genes participating in hepatic gluconeogenesis via AMPK, lowering serum glucose through suppression of hepatic glucose production (113). Interestingly, a very recent study by Miller et al. (114) revealed that adiponectin could suppress gluconeogenic gene expression in mouse hepatocytes independently of liver kinase B1 (LKB1; an upstream regulator of AMPK)-AMPK signaling. In one study, trimeric adiponectin was the most potent configuration in terms of suppression of hepatocyte production reducing serum glucose levels compared with oligomeric isoforms (63). In other studies, it was reported that both fAd and gAd activate fatty acid oxidation, lactate production, and glucose uptake through AMPK in C2C12 myocytes (115). Nevertheless, further evidence suggests that trimeric and gAd, but not hexameric and HMW adiponectin, increase AMPK phosphorylation in skeletal muscle (53). AMPK activation also regulates several downstream targets such as enzymes that are involved in the regulation of protein, fatty acids, and triglyceride synthesis such as ACC and fatty acid synthase, transcription factors, and other regulatory proteins (16). In muscle cells, adiponectin can activate AMPK through two distinct pathways: a major pathway that involves APPL-1 and a minor one (the phospholipase C/Ca2+/ Ca2+ calmodulin-dependent protein kinase kinase-dependent pathway) that provokes Ca2+ release from intracellular stores (116). Additionally, AMPK is an upstream regulator (inhibitor) of mTOR through tuberous sclerosis complex 2 (TSC2), thus counteracting carcinogenesis (117). Direct inhibition of this pathway results in suppression of cell proliferation (118). Furthermore, activated AMPK plays a pivotal role in the regulation of growth arrest and apoptosis by stimulating p21 and p53 (119). Finally, a recent study showed that the cytoprotective and antiinflammatory effects of adiponectin are mediated, in part, via an APPL-1-dependent AMPK activation of the phosphatidylinositol 3-kinase (PI3K)-v-akt murine thymoma viral oncogene homolog (Akt) signaling pathway (120).
The proliferative effects of adiponectin could be explained by the activation through APPL-1 mediation of the ERK1/2-MAPK pathway, which is crucial for cell cycle initiation, cell growth, and survival (121) as well as by stimulation of ceramidase activity, associated with its two classical receptors, enhancing ceramide catabolism and biosynthesis of the antiapoptotic molecule S1P. S1P increases intracellular calcium and activates AMPK. All these actions promote cell survival, nutrient uptake and utilization, and mitochondrial proliferation (84). The JNK and STAT3 signaling pathways are also proposed as mediators of adiponectin's effects on the metabolic syndrome and cancer (98). Adiponectin suppresses the proinflammatory and antiapoptotic NF-κB pathway through the suppression of inhibitor of NF-κB phosphorylation (75). Suppression of NF-κB by adiponectin could represent an important molecular mechanism for the inhibition of monocyte adhesion to endothelial cells, conferring to this adipocytokine antiinflammatory and antiatherosclerotic properties. A study conducted by Tsao et al. (54) showed that adiponectin can activate NF-κB in C2C12 myocytes, but further studies are needed to explore the role of adiponectin and its different isoforms.
Finally, the interaction of adiponectin to T-cadherin, the third putative adiponectin receptor, seems to restrict AdipoR1/R2 signaling. A recent study in HEK293 cells showed that the decrease of T-cadherin mRNA by small interfering RNA led to a marked increase in adiponectin-activation of ERK1/2 phosphorylation (88). In Section V.A.2, we analyze the specific adiponectin signaling pathways implicated in cancerogenesis.
III. Adiponectin Physiology and Pathophysiology
A. Adiponectin physiological functions
Adponectin has pleiotropic effects on a plethora of tissues and organs, with the various isoforms presenting different biological effects on different target tissues (16). Adiponectin has insulin-sensitizing, antiinflammatory, antiatherogenic, cardioprotective effects as well as distinct effects on lipid metabolism (15–17). Key metabolic actions include regulation of glucose and lipid metabolism through stimulation of fatty acid oxidation, suppression of hepatic glucose output, and increased insulin sensitivity in liver and skeletal muscle. HMW adiponectin may be the major mediator in adiponectin's effects, especially in the liver (70). Adiponectin can redirect fatty acids to the muscles for their oxidation, decreasing the influx of fatty acids to the liver and the total triglyceride content and leading to an improved insulin signal transduction and a higher insulin sensitivity (29, 111). Interestingly, adiponectin reduces triglyceride content in skeletal muscle through mechanisms that involve increased expression of fatty acid transport molecules, combustion of fatty acid by ACC, and energy dissipation by uncoupling protein 2 (61, 111). Furthermore, adiponectin enhances insulin-induced phosphorylation of the insulin receptor (IR) and the ability of insulin to activate the phosphorylation of the adaptor protein insulin receptor substrate 1 (IRS-1) (122, 123). Adiponectin regulates pancreatic β-cell proliferation in conjunction with leptin, suggesting that adiponectin may present a direct effect on insulin secretion (72). Finally, adiponectin presents potent protective effects against insulin resistance and chronic inflammation due to its ability to improve systemic carbohydrate and lipid profiles (13, 67).
Adiponectin has been reported to present direct antiatherogenic actions by inhibiting atherosclerosis and plaque formation. It suppresses neointimal formation by strongly inhibiting the expressions of adhesion molecules such as intracellular adhesion molecule-1, vascular cellular adhesion molecule-1, E-selectin, and the TNF-α-induced NF-κB (46, 75, 124). It also suppresses the uptake of cholesterol by inhibiting the expression of scavenger receptors and the foam cell formation (125). Adiponectin activates endothelial nitric oxide synthase, enhancing nitric acid production (126), and modulates vascular remodeling by suppressing smooth cell migration (127). Collectively, the antiatherogenic actions of adiponectin can be condensed as follows: adiponectin acts in the injured vascular wall by reducing the ability of macrophages to transform into foamy cells, inhibits subendothelial lipid accumulation, and stimulates vasodilatation and increased blood flow.
Aside from its peripheral actions, adiponectin may act centrally to modulate food intake and energy expenditure (128). A growing body of evidence suggests that adiponectin is an important regulator of reproductive events, with beneficial actions in ovulation, implantation process, and fetal growth and development (47, 129). Adiponectin also plays an important role in bone homeostasis, although the precise mechanism of action remains unclear (71). Further innovative research is needed to identify and clarify the different and tissue-specific effects of this adipose tissue hormone.
B. Circulating adiponectin and its determinants
Normal plasma adiponectin levels range from 2 to 30 μg/ml (depending on the assay methodology) (16), thus accounting for 0.01% of total plasma proteins in humans, and increase slightly with age (15, 29). Adiponectin concentration values are 1000 times higher than leptin and cortisol levels (in the order of nanograms per milliliter) and 106 times greater than other cytokines such as IL-6 and TNF-α (in the order of picograms per milliliter) (15, 16).
Circulating adiponectin levels are determined by various genetic, anthropometric, hormonal, inflammatory, dietary, and pharmacological factors. Unlike most of the other adipose tissue-derived proteins, serum adiponectin is reduced in obesity and generally correlates negatively with body mass index (BMI), waist and hip circumference, waist-to-hip ratio, and visceral (intraabdominal) fat rather than sc, independently of age and menopausal status (15, 16, 115, 130). In addition to overall obesity, our group showed that central fat distribution is an independent negative predictor of circulating adiponectin, suggesting that adiponectin may represent a link between central obesity and insulin resistance (131). Therefore, a negative correlation between circulating adiponectin and obesity, especially central obesity, insulin resistance, and type 2 diabetes has been well established (28). One possible explanation of the reduced adiponectin levels in obesity may be due to cytokines increased in obesity that contribute to the decreased adiponectin production such as TNF-α (71). Another potential mechanism indicates a negative feedback of adiponectin on its own production during the development of obesity. It has also been hypothesized that the diminished expression of sirtuin 1 (a nicotinamide adenine dinucleotide+-dependent protein deacetylase involved in adipogenesis) and Forkhead box O1 in fat tissues of obese mouse models could be implicated in the decreased adiponectin expression associated with obesity (132). Indeed, adiponectin may control its own production and probably the expression of its receptors via a regulatory feedback loop (132, 133). Higher adiponectin is associated with weight loss (28) but predicts higher weight gain in healthy women, suggesting that elevated adiponectin production might be a sign of “healthy” adipose tissue with further capacity to accumulate fat (134). Weiss et al. (135) showed that a 10% or higher weight loss is associated with a significantly greater increase in adiponectin levels. In obese women, bariatric surgery, which leads to an average weight loss of 15 to 25%, is significantly associated with a higher increase in circulating adiponectin and a reduction in breast cancer risk (136, 137). Moderate aerobic exercise in the absence of fat mass reduction and significant weight loss has little effect on adiponectin levels (138, 139). Only high-intensity endurance training may improve plasma adiponectin concentrations (140).
Adiponectin levels in the circulation display a diurnal variation, reaching nadir at night and peak in the morning (130, 141), and are higher in women than men, independent of fat mass and/or fat distribution (130, 142), most likely due to differences in circulating estrogens or androgens (16, 143). It was demonstrated that testosterone suppresses serum total adiponectin levels in mice and men, whereas exogenous estrogen treatment or ovariectomy does not affect adiponectin concentration (144–147). In women without a history of diabetes, we have shown that serum adiponectin is also independently and negatively correlated with estradiol levels, but not free testosterone, cortisol, and leptin levels (131). Neither short-term fasting nor leptin administration alters serum adiponectin levels (131). In the same study, postmenopausal women presented higher adiponectin levels with lower estradiol levels compared with premenopausal women (131).
Plasma adiponectin is closely related to hormonal markers of insulin sensitivity and fasting insulinemia (21, 123, 148, 149), and hypoadiponectinemia at baseline precedes a decrease in insulin sensitivity (123). Regarding other hormonal factors, adiponectin production may be down-regulated by prolactin, GH, and glucocorticoids (150). Nutritional parameters can also modulate plasma adiponectin concentrations (151). Generally, chronic caloric restriction leading to weight loss increases plasma adiponectin (15, 22, 152). Beyond any effect of specific food item, we have shown that adherence to a Mediterranean-style dietary pattern or the Alternate Healthy Eating Index is positively associated with plasma adiponectin levels (153, 154). Adiponectin levels are inversely associated with glycemic load in a dose-response manner (152). Higher intakes of fiber and magnesium as well as coffee consumption have been associated with increased plasma adiponectin (152, 155, 156), whereas fruit consumption is related to HMW adiponectin levels (157). Also, short-term walnut consumption (4 d) may increase circulating total adiponectin in obese subjects with the metabolic syndrome (158). Circulating adiponectin levels positively correlate with high-density lipoprotein cholesterol and negatively associate with triglyceride and apolipoprotein-B (159, 160). Hypoadiponectinemia is usually associated with an atherosclerotic lipid profile (161). Adiponectin has also been linked to several inflammatory markers such as C-reactive protein (CRP) and fibrinogen (15, 159). Several lipid-lowering drugs, such as fibrates, hydrophilic statins, and omega-3 fatty acids as well as antihypertensive drugs including angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, etc., may increase circulating adiponectin or improve adiponectin signaling through its receptors (46, 162–164). In vitro and in vivo studies in mice and humans have indicated that adiponectin expression and secretion is up-regulated by some antidiabetic drugs such as thiazolidinediones and/or selective PPAR-γ, predominantly the HMW isoform (165–167). On the contrary, metformin, which significantly improves insulin sensitivity, is not capable of modulating adiponectin (168).
Finally, genetic factors such as certain polymorphisms of the ADIPOQ gene have been associated with hypoadiponectinemia in diabetic individuals (35, 38, 169, 170) and posttransplantation diabetes mellitus (ADIPOQ rs1501299) in male patients receiving kidney transplants without a history of diabetes (171). In a recent large-scale meta-analysis of three genome-wide association studies for circulating adiponectin levels from population-based cohorts (n = 14,733 participants), it was reported that five SNP were genome-wide significant, whereas SNP at the adiponectin-encoding ADIPOQ locus (rs266717) demonstrated the strongest association with adiponectin levels (172). A novel variant in the ARL-15 (ADP-ribosylation factor-like 15) gene (rs4311394-G) was associated with hypoadiponectinemia, cardiovascular disease, and diabetes type 2 (172) supporting a role of this novel protein ARL-15 in determining adiponectin levels. Whether these polymorphisms are also associated with risk for malignancies remains to be studied further.
C. Adiponectin levels in relation to disease states
Hypoadiponectinemia caused by genetic or environmental factors such as obesity and diet may play a crucial causal role in the pathogenesis of insulin resistance (20, 21), metabolic syndrome, type 2 diabetes (21, 22, 65), gestational diabetes (173), hypertension, cardiovascular disease (68), and several malignancies (15, 17). Interestingly, hypoadiponectinemia is the common denominator of the constellation of risk factors that constitute the metabolic syndrome such as hypertension, dyslipidemia, obesity, hyperglycemia, and insulin resistance. A recent meta-analysis of prospective studies including a total of 14,598 subjects and 2,623 cases of type 2 diabetes showed that higher adiponectin levels were associated with a lower risk for type 2 diabetes (174). Higher adiponectin levels were also associated with a moderate decrease in risk for coronary artery disease in diabetic men (175) and with improved glycemic control and lipid concentrations as well as reduced inflammation in diabetic women (176). The association of hypoadiponectinemia and liver disease is well documented, and a protective effect of adiponectin against fatty liver disease was proposed. Lower adiponectin levels were observed in patients suffering from chronic hepatitis with liver steatosis, correlating inversely with the grade of steatosis (177).
On the contrary, higher serum adiponectin levels were found in patients with anorexia nervosa (178) and chronic inflammatory joint diseases such as rheumatoid arthritis, whereas serum adiponectin correlated with the severity (179) of joint damage (180). Elevated adiponectin is associated with more severe proteinuria in chronic kidney disease patients, possibly underlying a protective response aimed at countering the high renal and cardiovascular risk of high proteinuria (181). Further studies are needed to elucidate the role of adiponectin in other diseases, such as ischemic cerebrovascular diseases (182, 183), preeclampsia (184, 185), and polycystic ovary syndrome (46), whereas there is no clear evidence of a relationship with adiponectin.
IV. Epidemiological Evidence That Links Adiponectin to Cancer
Our group and others have recently shown that hypoadiponectinemia in vivo is inversely linked to the risk of obesity-associated malignancies and insulin resistance (17, 186), that is, endometrial cancer (187, 188), postmenopausal breast cancer (96, 189, 190), colon cancer (191), renal cancer (192), leukemia (193), and other hematological malignancies (194–198). Furthermore, low adiponectin concentrations have been reported in gastric (199) and prostate cancer (200). Table 1 portrays recent comparative epidemiological studies that depict associations between serum adiponectin levels and risk of different types of cancer. Table 2 depicts epidemiological studies showing association between genetic variation (SNPs) in adiponectin (ADIPOQ) and adiponectin receptors (ADIPOR1/R2) and risk of different types of cancer.
Table 1.
Recent comparative epidemiological studies (2006 to the present) that depict association between serum adiponectin levels and risk of different types of cancer
| First author, year (Ref.) | Type of study | No. of cases/controls, population | OR (95% CI) or significance | Additional results, analysis, and comments |
|---|---|---|---|---|
| Hematological malignancies | ||||
| MDS | ||||
| Dalamaga, 2007 (197) | Retrospective case control | 101/101, Greek | 0.14 (0.06–0.34), highest vs. lowest quartile of adiponectin, controlling for age, gender, BMI, and leptin levels | Lower serum total adiponectin was independently associated with MDS risk, controlling for age, gender, BMI, and leptin. MDS subtypes showed different adiponectin levels, with refractive anemia presenting significantly higher adiponectin levels than more aggressive subtypes |
| Dalamaga, 2008 (196) | Retrospective case control | 101/101, Greek | 0.35 (0.13–0.915), highest vs. lowest quartile of adiponectin; 0.29 (0.11–0.78), highest vs. lowest quartile of HMW adiponectin | Lower serum total or HMW adiponectin was independently associated with MDS risk, controlling for age, gender, BMI, leptin, IGF-I, and IGFBP-3. HMW adiponectin did not offer any additional predictive value over total adiponectin |
| MPD | ||||
| Avcu, 2006 (302) | Retrospective case control | 30/29, Turkish | P < 0.001 | Significant lower adiponectin levels in MPD patients than controls. MPD patients who received interferon presented significantly higher levels of adiponectin (P < 0.001) |
| Childhood acute myeloblastic leukemia | ||||
| Petridou, 2006 (193) | Retrospective case control | 22/201, Greek | 0.56 (0.34–0.94) | Adiponectin was inversely associated with acute myelogenous leukemia risk, adjusting for age, gender, weight, and height centiles |
| MM | ||||
| Dalamaga, 2009 (195) | Retrospective case control | 73/73, Greek | 0.08 (0.02–0.42), highest vs. lowest quartile of adiponectin, adjusting for age, sex, BMI, leptin, and resistin levels | Lower adiponectin levels were associated with MM risk. No significantly different adiponectin levels were found amid different prognostic stages and paraprotein classes in MM |
| Reseland, 2009 (307) | Retrospective case control | 23/23, Norwegian | P > 0.05, P < 0.05 | Similar adiponectin levels in MM cases and controls but significant lower levels of adiponectin in male patients than male controls |
| Fowler, 2011 (309) | Retrospective case control | 20 patients with MGUS who progressed to MM; 20 patients with MGUS; 40 controls; American | P < 0.05 | Significant percentage decrease from matched control in serum HMW adiponectin concentrations in MGUS patients that either progress or do not progress to myeloma. Decreased serum adiponectin concentrations in female MGUS patients were associated with progression to myeloma |
| B-CLL | ||||
| Avcu, 2006 (302) | Retrospective case control | 19/36, Turkish | P < 0.001 | CLL patients presented lower adiponectin levels than controls. Adiponectin did not correlate with disease stage and clinical course (P > 0.05) in patients |
| Pamuk, 2006 (306) | Retrospective case control | 23/17, Turkish | P > 0.05 | No significant differences in adiponectin levels between patients and controls |
| Dalamaga, 2010 (198) | Retrospective case control | 95/95, Greek | 0.99 (0.31–3.15), highest vs. lowest tertile of adiponectin; p trend = 1; 2.21 (0.64–7.61), highest vs. lowest tertile of HMW adiponectin; p trend = 0.22 | No significant association between B-CLL and adiponectin, adjusting for age, gender, family history of cancer, BMI, and leptin levels. Adiponectin and HMW adiponectin levels correlated with LDH, a marker of disease severity in CLL |
| Adult NHL | ||||
| Pamuk, 2006 (306) | Retrospective case control | 28/17, Turkish | P < 0.05 | Higher adiponectin levels in NHL patients. Adiponectin correlated positively with IL-10 levels (P = 0.04) |
| Childhood ALL | ||||
| Petridou, 2006 (193) | Retrospective case control | 161 ALL-B and 18 ALL-T/201; Greek | 0.88 (0.71–1.10) for ALL-B; 1.08 (0.67–1.72) for ALL-T | Adiponectin was not significantly associated with either ALL-B or ALL-T, controlling for age, gender, weight and height |
| Moschovi, 2010 (314) | Retrospective case control and follow-up study (21 months) | 9/9, Greek | P < 0.001, P = 0.019 | Mean adiponectin levels were lower in cases than controls. During maintenance period, adiponectin increased significantly but remained at lower levels compared to controls. Adiponectin correlated negatively with leukemic burden |
| Childhood HL | ||||
| Petridou, 2010 (308) | Retrospective case control | 75/75, Greek | 1.25 (0.9–1.8); P = 0.09, adjusting for anthropometric, lifestyle, sociodemographic variables, and leptin | Elevated serum adiponectin might be a risk factor for childhood HL |
| Childhood NHL | ||||
| Petridou, 2009 (194) | Retrospective case control | 121/121, Greek | 1.82 (1.30–2.56), adjusting for anthropometric, lifestyle, sociodemographic variables, and leptin | Elevated adiponectin levels were independently associated with childhood NHL as well as with poor prognosis (relapse and poor survival) |
| Solid tumor malignancies | ||||
| PaC | ||||
| Chang, 2007 (271) | Retrospective case control | 72/290 and 39 patients with chronic pancreatitis; Taiwanese | P = 0.0035 | Median levels of adiponectin were significantly higher in patients suffering from PaC compared to those with chronic pancreatitis and controls. Adiponectin could be used as tumor-specific marker for PaC |
| Stolzenberg-Solomon, 2008 (273) | Prospective nested case control | 311/510, Finnish male smokers | 0.65 (0.39–1.07), for highest vs. lowest quintile adjusting for smoking, blood pressure, and C-peptide levels | Higher adiponectin concentrations were inversely associated with PaC risk |
| Dalamaga, 2009 (95) | Retrospective case control | 81/81, Greek | 2.81 (1.04–7.59), adjusting for age, gender, BMI, history of diabetes, family history of cancer, alcohol, smoking status, and leptin | Higher adiponectin levels were associated with higher odds of PaC. Adiponectin did not correlate with PaC stage |
| Pezzilli, 2010 (275) | Retrospective case control | 34 with PC, 75 with chronic pancreatic diseases, 12 with intraductal papillary mucinous tumors of the pancreas; Italian | P > 0.05 | Similar adiponectin levels amid the three groups of patients |
| Krechler, 2011 (272) | Retrospective case control | 64/64, Czech | P < 0.001 | Higher adiponectin/leptin ratio in PaC patients independently from age, BMI, and waist circumference |
| Grote, 2011 (274) | Prospective case control | 452/452, EPIC study; European | 0.44 (0.23–0.82); 1.59 (0.67–3.76) | Among never-smokers (in particular women), higher circulating levels of adiponectin were associated with a reduction in PaC risk. Among current smokers, there was no significant association between adiponectin and PaC risk |
| BC | ||||
| Chen, 2006 (234) | Retrospective case control | 100/100, Taiwanese | P = 0.003 | Lower adiponectin levels in premenopausal and postmenopausal cases. Higher leptin/adiponectin ratio in cases than controls (P = 0.009) |
| Korner, 2007 (96) | Retrospective case control | 74/76, Greek | 0.35 (0.14–0.87), adjusting for age, BMI, and known risk factors for BC; 0.30 (0.11–0.82), adjusting for age, BMI, and known risk factors for BC | Lower adiponectin levels were associated with BC risk. HMW adiponectin did not offer any predictive value |
| Tworoger, 2007 (148) | Prospective case control | 1477/2196, postmenopausal; 858/1309, premenopausal; (316/506) NHS and NHSII | 0.89 (0.71–1.11) for all women; 0.73 (0.55–0.98) for postmenopausal; 1.30 (0.80–2.10) for premenopausal | The first prospective study of adiponectin in BC. Adiponectin was inversely associated with postmenopausal BC risk only |
| Kang, 2007 (239) | Retrospective case control | 41/43, Korean | P = 0.37 | No significant difference in adiponectin between cases and controls. Lower adiponectin levels were associated with lymph node metastasis (P = 0.017) |
| Hou, 2007 (233) | Retrospective case control | 80/50, Chinese | 0.805 (0.704–0.921); 0.742 (0.504–0.921) | Lower adiponectin was associated with BC risk and lymph node invasion |
| Tian, 2007 (244) | Retrospective case control | 244/244; premenopausal, 141 cases; postmenopausal, 103 cases; Taiwanese | 0.55 (0.23–0.97), adjusting for age at enrollment, date at enrollment, fasting, menopausal status, BMI, and waist to height ratio; 0.53 (0.27–0.98), adjusting for age at enrollment, date at enrollment, fasting, menopausal status, BMI, and WHR | Adiponectin was inversely associated with postmenopausal BC risk only. Significant inverse association of adiponectin with ER-positive BC, but not ER-negative BC |
| Shahar, 2010 (235) | Retrospective case control | 70/138, Malaysian | P < 0.05 | Lower adiponectin levels in cases than controls. Greater reduction of BC risk with increasing levels of adiponectin in percentiles |
| Gaudet, 2010 (240) | Prospective case control | 234/234, postmenopausal; American | P for linear trend = 0.43, adjusting for BMI, reproductive factors, sex steroid hormones, and current hormonal use | Postmenopausal BC risk was not associated with circulating levels of adiponectin |
| Dalamaga, 2011 (190) | Retrospective case control | 102/102, postmenopausal; Greek | P = 0.04 | Lower mean adiponectin levels in cases than controls |
| EC | ||||
| Soliman, 2006 (253) | Retrospective case control | 117/238, American | 10.5 (4.49–24.57), for lowest vs. higher tertile of adiponectin (analysis for women younger than 65 yr) | Confirms previous published case-control studies from Europe that found lower adiponectin levels in EC cases than controls. The association is also strong in nonobese women |
| Cust, 2007 (256) | Prospective case control | 284/548, EPIC study; European | 0.56 (0.36–0.86) top vs. bottom quartile of adiponectin | First prospective study of adiponectin in EC, whereas the inverse association was independent of other obesity-related risk factors |
| Rzepka-Gorska, 2008 (254) | Retrospective case control | 105, obese women with EC, polyps, and normal epithelium; Polish | P < 0.001, P < 0.05 | Significant lower adiponectin in patients with EC than in patients with polyps or normal epithelium. Adiponectin did not present any association with cancer stage, but lower adiponectin levels were observed in patients with grade III (P < 0.05) |
| Ashizawa, 2010 (255) | Retrospective case control | 146/150, postmenopausal; Japanese | P < 0.0001 | Significant lower adiponectin levels in cases than controls. The leptin/adiponectin ratio was independently associated with postmenopausal EC risk |
| Soliman, 2011 (257) | Prospective case control | 146/377, NHS; American | 0.86 (0.53–1.39) for all women; 0.66 (0.29–1.5) for postmenopausal women, adjusting for BMI at blood draw, parity, age at last birth, and diabetes | Second prospective study, whereas prediagnostic adiponectin was not predictive of EC risk, independently from known risk factors |
| PC | ||||
| Baillargeon, 2006 (293) | Prospective case control | 125/125, San Antonio Center for PC Biomarkers | 0.87 (0.46–1.65), highest vs. lowest tertile of adiponectin | No statistically significant results; the method used is not the standard one because the sensitivity of the assay is questioned |
| Michalakis, 2007 (290) | Retrospective case control | 75/150, Greek | 0.29 (0.10–0.89), highest vs. lowest quartile of adiponectin | Association was independent of age, BMI, smoking, alcohol, insulin, and testosterone levels |
| Housa, 2008 (295) | Retrospective case control | 43/25 with BPH, Czech | P > 0.05 | Serum adiponectin levels did not differ in BC and BPH but were significantly higher in T3 stage of PC compared to T2 stage (P = 0.003). Serum adiponectin might serve as an auxiliary marker for discriminating PC stages |
| Arisan, 2009 (291) | Retrospective case control | 50/50 | P < 0.05 | Significant lower adiponectin levels in patients suffering from PC |
| Li, 2010 (292) | Prospective case control | 654/644, Physicians Health Study; American | 0.25 (0.07–0.87), highest vs. lowest quintile; P (trend) = 0.02 for presenting lethal PC | Serum adiponectin was not associated with risk of overall PC. Men with higher adiponectin concentrations had lower risk of developing high-grade or lethal cancer (metastatic or fatal disease) |
| CC | ||||
| Lukanova, 2006 (218) | Prospective case control | 381/381, Janus Project; Norwegian men | P > 0.05; 0.8 (0.5–1.4) for lowest vs. higher quartile of adiponectin, adjusted for leptin and C-peptide; P (trend) = 0.30 | Similar adiponectin levels in both cases and controls. No association between prediagnostic adiponectin and CC risk |
| Ferroni, 2007 (212) | Retrospective case control and follow-up study for CC cases for 3 yr | 60/30, Italian | P < 0.001, P = 0.037 | Lower median adiponectin levels in cases than controls. Median adiponectin concentration gradually decreased with an increase in tumor stage. Lower adiponectin was an adjunctive tool for CC recurrence |
| Kumor, 2008 (209) | Retrospective case control | 36/25 and 37 patients with colorectal adenomas; Polish | P < 0.05 | Patients with CC presented significantly lower adiponectin levels than patients with colorectal adenoma and controls |
| Stocks, 2008 (220) | Prospective case control | 306/595, Northern Sweden Health and Disease Cohort; Swedish | P > 0.05 | Adiponectin was not significantly associated with CC risk |
| Guadagni, 2009 (211) | Retrospective case control and follow-up study for CC cases for at least 3 yr | 90/30, Italian | P < 0.0001 | Lower adiponectin levels in cases than controls. The leptin/adiponectin ratio was 8-fold greater in patients than in controls and an independent predictor for adverse outcome in CC |
| Erarslan, 2009 (213) | Retrospective case control | 54 patients with CC and colorectal adenoma, 50 controls; Turkish | P < 0.05 | Lower adiponectin levels in cases than controls. Adiponectin did not correlate with visceral fat accumulation in both CC and colorectal adenoma groups |
| Gonullu, 2010 (210) | Retrospective case control | 36/37, Turkish | P < 0.05 | Significant lower adiponectin levels in cases than controls. Adiponectin was negatively correlated with cancer stage |
| Nakajima, 2010 (219) | Retrospective case control | 115/115 and 72 patients with colorectal adenoma, 72 controls; cases and controls were age-, sex-, and BMI-matched; Japanese | 0.802 (0.321–2.003) for CC risk; 0.422 (0.189–0.946) for colorectal adenoma risk | No association between adiponectin and CC risk as well as between adiponectin and CC stage (P = 0.94). Adiponectin might be a good biomarker for colorectal adenomas |
| Kemik, 2010 (215) | Retrospective case control | 126/38, Turkish | P < 0.001 | Lower serum adiponectin levels in cases than controls |
| Otake, 2010 (216) | Retrospective case control | 47 male patients with adenoma; 34 male patients with early CC; 17 with advanced CC; 26 male controls | 5.762 (1.683–19.739), 4.495 (1.090–18.528) | Lower adiponectin levels were associated with increased adenoma risk. Lower adiponectin levels were associated with early CC risk. No association of lower adiponectin levels with advanced CC risk |
| Catalan, 2011 (217) | Retrospective case control | 11/18 Spanish | P < 0.01 | Lower circulating adiponectin levels in cases than controls |
| Gialamas, 2011 (214) | Retrospective case control | 104 patients with CC; 208 age- and gender-matched controls | 0.72 (0.53–0.99) for CC risk; P = 0.05 | Lower adiponectin levels were associated with CC risk, controlling for demographic, anthropometric, lifestyle variables as well as diabetes mellitus. Adiponectin was negatively correlated with tumor grade |
| Gastric cancer | ||||
| Nakajima, 2009 (261) | Retrospective case control | 156/156, Japanese | P = 0.0004, P = 0.058 | Adiponectin levels were significantly lower in patients than controls. Patients with stage I showed decreased adiponectin than controls |
| Seker, 2010 (262) | Retrospective case control | 40/43, Turkish | P > 0.05 | Plasma adiponectin levels were similar in both cases and controls. No association was detected between serum adiponectin and tumor stage, localization, nodal status, lymphatic and vascular invasion (P > 0.05). Undifferentiated tumors showed significantly higher plasma adiponectin levels than well-differentiated grade tumors |
| Esophageal cancer (ESCC and EA) | ||||
| Yildirim, 2009 (266) | Retrospective case control | 62/30, Turkish | P < 0.05, P < 0.05 | Both patients with ESCC and EA presented lower adiponectin levels than controls. More specifically, patients with EA had significantly lower adiponectin than those with ESCC |
| Diao, 2009 (270) | Retrospective case control | 43 patients with ESCC; 47 patients with dysplasia; 37 patients with hyperplasia; 33 controls | P = 0.802 | Plasma adiponectin levels were similar amid the different esophageal pathologies |
| Nakajima, 2010 (269) | Retrospective case control | 117 patients with ESCC; 117 controls; Japanese | P = 0.01 | Serum adiponectin levels and BMI were significantly lower in subjects with ESCC compared to controls in a multivariate logistic regression model |
| Liver cancer | ||||
| Kotani, 2009 (276) | Prospective nested case control | 59/334, Japanese | 0.50 (0.22–1.15), higher tertile of circulating LMW adiponectin vs. lowest, adjusting for age, gender, area, BMI, smoking, alcohol, coffee, diabetes history, and hepatitis C antibody positivity (although not statistically significant at 0.05) | Higher percentage of circulating LMW adiponectin might lead to a reduction of liver cancer risk |
| Arano, 2010 (282) | Retrospective cohort study | 325 with CHC; of these, 122 developed liver cancer; 70 healthy controls; Japanese | P < 0.05 (P = 0.008 for males and P = 0.0003 for females); 2.07 (1.06–4.04), P = 0.031 in females, and 1.82 (1.00–3.33), P = 0.05 in males; 1.96 (1.06–3.60) for the MLMW adiponectin isoform | Patients with CHC had significantly higher adiponectin levels than controls. Patients with CHC (especially females) and higher serum adiponectin levels presented a higher risk of liver cancer development. In the assessment of adiponectin isoforms, an elevated serum MLMW adiponectin level was an important risk factor for liver cancer |
| Sumie, 2011 (284) | Retrospective case control | 97 with CHC who developed liver cancer, 97 controls with CHC; Japanese | P = 0.670, P = 0.752 | No significant differences of serum total adiponectin between cases and controls. No significant differences of serum HMW adiponectin between cases and controls. Low total and HMW adiponectin levels were independent risk factors for worse HCC histological grades |
| Renal cancer | ||||
| Spyridopoulos, 2007 (192) | Retrospective case control | 70/280, Greek | 0.76 (0.57–1.00), P = 0.05 | Serum adiponectin concentrations were inversely associated with renal cancer, controlling for BMI but not central obesity |
| Lung cancer | ||||
| Petridou, 2007 (94) | Retrospective case control | 85/170, Greek | 1.13 (0.64–2.02); 0.25 (0.10–0.78) | Circulating adiponectin levels were not significantly different between cases and controls. Circulating adiponectin levels were significantly lower amid patients with advanced disease stage compared to those with limited one |
| Karapanagiotou, 2008 (316) | Retrospective case control and follow-up study for patients | 101 patients with advanced NSCLC; 51 healthy controls; Greek | P > 0.05 | Serum adiponectin presented no difference between cases at diagnosis and controls. Adiponectin did not show any predictive value for overall survival and disease progression |
| Petridou, 2011 (315) | Retrospective case control | 81/162, Greek | 2.00 (0.80–4.97); P = 0.14 | No significant association between adiponectin and lung cancer risk, controlling for BMI, weight change, education, WHR, smoking, alcohol, coffee consumption, leptin, and HOMA-IR. Adiponectin was not a major predictor of lung cancer risk |
| Other malignancies | ||||
| Melanoma | ||||
| Mantzoros, 2007 (320) | Retrospective case control | 55/165, Greek | 0.75 (0.52–1.10), adjusting for education, age, gender, WHR, skin type, and eye color | Although there was a lack of statistical significance, adiponectin might present a protective role in the development of melanoma (25% reduction of melanoma risk per 1 sd value of adiponectin) |
| Thyroid cancer | ||||
| Mitsiades, 2011 (321) | Retrospective case control | 175/107, Greek | P < 0.001; 0.29 (0.16–0.55) for any type of thyroid carcinoma; 0.27 (0.14–0.55) for papillary thyroid carcinoma | Thyroid cancer patients presented significantly lower levels of serum adiponectin than controls. Circulating adiponectin was independently and inversely associated with the risk of thyroid cancer |
| Pheochromocytoma | ||||
| Isobe, 2009 (322) | Retrospective case control | 10/33, Japanese | P < 0.001 | Serum total and HMW adiponectin were three times higher in patients with noradrenaline-type tumors than in controls. Adiponectin levels normalized after adrenalectomy |
BC, Breast cancer; BPH, benign prostatic hyperplasia; EC, endometrial cancer; HOMA-IR, homeostatic model assessment-insulin resistance; PC, prostate cancer.
Table 2.
Recent epidemiological studies (2005 to present) that depict association between genetic variation (SNP gene polymorphisms) in adiponectin (ADIPOQ) and adiponectin receptors (ADIPOR1/R2) and risk of different types of cancer
| First author, year (Ref.) | Type of study | No. of cases/controls, population | OR (95% CI) or significance | Additional results, analysis, and comments |
|---|---|---|---|---|
| BC | ||||
| Kaklamani, 2008 (39) | Retrospective case control | 733/839, American | 0.61 (0.46–0.80) for rs2241766; 1.80 (1.14–2.85) for rs1501299, adjusting for age, race, and SNP from the same genes; 0.51 (0.28–0.92), adjusting for age, race, and SNP from the same genes | Two ADIPOQ SNP (rs2241766*TG and rs1501299*GG) related to circulating levels of adiponectin were associated with BC risk. One ADIPOR1 SNP (rs7539542) that modulates expression of AdipoR1 mRNA was associated with decreased BC risk |
| Teras, 2009 (249) | Prospective case control | 648/659, postmenopausal, American Cancer Society Prevention Study II, American | P > 0.05; OR ranged from 0.93 to 1.06, adjusting for age, sex, and date of blood draw | No significant associations between postmenopausal BC risk and any of ADIPOQ, ADIPOR1, and ADIPOR2 SNP. In particular, ADIPOR1 SNP (rs7539542) from the previous study was not associated with BC risk |
| Nyante, 2011 (250) | Population-based case control | 1972/1776, Carolina Breast Cancer Study, American Whites and African-American | P > 0.05 | No significant associations between BC risk (basal-like and luminal BC subtypes) and any ADIPOQ SNP |
| EC | ||||
| Chen, 2011 (258) | Retrospective case control | 1028/1932, Chinese | 0.68 (0.48–0.97) for women homozygous for the minor allele for rs3774262 | Three of the 10 SNP evaluated in the ADIPOQ gene were significantly associated with diminished endometrial cancer risk. No other SNP in the ADIPOR1/R2 genes were associated with cancer risk |
| CC | ||||
| Kaklamani, 2008 (224) | Retrospective case control (two studies) | Study 1: 441/658, Ashkenazi Jewish from New York; study 2: 199/199, American | Study 1: 0.72 (0.55–0.95) for rs266729, 0.37 (0.14–1.00) for rs822396, adjusting for age, race, and SNP from the same genes; 1.76 (1.09–2.84) for rs822395, 1.79 (1.18–2.72) for rs1342387, 0.52 (0.34–0.78) for rs266729, adjusting for age, race, and SNP from the same genes; 0.73 (0.53–0.99) for rs266729 | Three ADIPOQ and one ADIPOR1 SNP were associated with CC risk. ADIPOQ SNP (rs266729, rs822396) were associated with decreased CC risk. SNP (rs822395 and rs1342387) were associated with increased CC risk. ADIPOQ SNP (rs266729) was associated with a decreased CC risk. Combined analysis from the two studies showed an association of rs266729 with decreased CC risk |
| Pechlivanis, 2009 (225) | Retrospective case control | 702/752, Czech | 1.11 (0.94–1.13) for rs266729, adjusting for age; OR ranging from 0.16 to 0.98 adjusting for age; OR ranging from 0.12 to 1.06, adjusting for age, diabetes and educational level | No significant association between ADIPOQ SNP (rs266729) and CC risk. Combinations of the insulin INS SNP rs3842754 and the ADIPOQ SNP rs266729 (C-11374G) genotypes were associated with decreased CC risk. However, after further adjustment, no significant associations emerged |
| Carvajal-Carmona, 2009 (226) | Case control | Study 1: 931/929, White, British; study 2: 1216/1436, White, British | P > 0.05 | No significant association between 82 ADIPOQ SNP and CC risk |
| Partida-Perez, 2010 (227) | Retrospective case control | 68/102, Mexican | P > 0.05 | No significant association between CC risk and ADIPOQ SNP |
| He, 2011 (229) | Retrospective case control | 420/555, Chinese | 0.53 (0.35–0.81) for rs12733285C/T; 0.59 (0.45–0.78) for rs1342387A/G; 0.59 (0.39–0.89) for rs1342387 A/A; 0.59 (0.46–0.77) for rs1342387A/G; 1.50 (1.05–2.14) and 1.45 (1.03–2.05) for rs266729G/A and G allele of ADIPOQ, respectively | Four ADIPOR1 SNP were associated with decreased CC risk. ADIPOQ SNP were associated with increased risk for colon cancer but not rectal cancer |
| Gornick, 2011 (228) | Retrospective population-based case control | 1062/1062, Israeli (Ashkenazi Jewish and other ethnic groups in Israel) | 1.04 (0.88–1.23) for ADIPOQ SNP rs266729, adjusting for age, gender, and ethnicity (Jewish or not Jewish) | No evidence was found for an association between ADIPOQ and risk for CC (in particular rs266729). |
| Liu, 2011 (230) | Retrospective case control | 470/458, Chinese | 1.94 (1.48–2.54) for ADIPOQ rs1063538 | ADIPOQ SNP rs1063538 was associated with increased CC risk, presenting significant interactions with smoking status, family history of cancer, and alcohol consumption |
| PC | ||||
| Beebe-Dimmer, 2010 (298) | Retrospective case control | 131/344, Flint Men's Health Study, African- American | P > 0.05; 2.29 (1.12–4.72); P = 0.03 | No association between ADIPOQ and ADIPOR1 SNP and prostate cancer risk. ADIPOQ SNP (rs1501299) was associated with obesity |
| Kaklamani, 2011 (296) | Retrospective case control | 465/441, American | P < 0.05 | SNP of the ADIPOQ and ADIPOR1 (rs12733285, rs7539452, rs266729, rs822395, rs822396, and rs1501299) were significantly associated with PC risk |
| Dhillon, 2011 (297) | Prospective case control | 1286/1267, Physicians' Health Study, American | P < 0.05, P > 0.05 | SNP of the ADIPOQ (rs266729, rs182052, rs822391, and rs2082940) were significantly associated with overall PC risk. None of the 16 variants in ADIPOR1/R2 were related to PC risk |
| Lung cancer | ||||
| Cui, 2011 (317) | Retrospective case control | 344/264, Chinese | P < 0.05 for ADIPOQ SNP rs2241766, adjusting for age, sex, BMI, and smoking status | TT genotype of SNP rs2241766 of the ADIPOQ gene was significantly associated with susceptibility to NSCLC |
| NHL | ||||
| Willett, 2005 (301) | Retrospective population-based case control | 699/914, English | P > 0.05 | No differences in genotype distributions between cases and controls for the apM1 276G>T SNP |
BC, Breast cancer; EC, endometrial cancer; PC, prostate cancer.
A. Adiponectin and colorectal cancer
Obesity, hyperinsulinemia, and insulin resistance have been considered important risk factors for the etiopathogenesis of colorectal cancer (CC) and adenoma, the precursor lesion of CC (201, 202). Similarly to other obesity-associated cancers, adiponectin has been proposed as a biological link between obesity and CC (15, 203). In two meta-analyses addressing the association between circulating adiponectin and the risk of CC and adenoma, patients, specifically men, with CC and adenoma demonstrated markedly lower adiponectin levels than healthy controls (204, 205). In particular, serum adiponectin levels are decreased in CC patients compared with controls, and adiponectin receptors, expressed in both adenocarcinoma and normal colorectal tissue, could mediate its effects on cellular proliferation and apoptosis (93, 206, 207). We found that expression of the adiponectin receptors is elevated in colorectal carcinoma than in nontumor tissues and in gastrointestinal stromal tumors, giving support to the hypothesis that adiponectin may be implicated in the pathogenesis of CC (208). An up-regulation of adiponectin receptors in CC tissues induced by hypoadiponectinemia may compensate and maintain adiponectin signaling pathways.
In a large, prospective study in the context of the Health Professionals Study, we have reported that the risk for CC was associated with lower plasma adiponectin (191). Specifically, men with the highest adiponectin concentrations presented a 60% reduced risk for CC, even after adjustment for body size, physical activity, and weight circumference. Several recent retrospective case-control studies have confirmed that lower adiponectin is associated with an increased risk for CC (209–217), with the exception of three studies (two prospective and one retrospective) without adjustment for major confounding factors, showing null results (218–220). Moreover, Nakajima et al. (219) found that serum adiponectin may be a promising biomarker for colorectal adenomas, considered as precancerous lesions. In this study, an inverse association between adiponectin levels and colorectal adenomas has been reported.
Adiponectin levels and tissue expression of adiponectin receptors seem to be associated not only with CC risk but also with components of CC clinicopathological characteristics, notably stage and grade. Lower adiponectin levels were correlated with CC stage progression in two studies (209, 210), but no statistically significant difference of serum adiponectin between early and metastatic CC stage was confirmed (221). These discrepancies may be due to power limitations. A negative association of serum adiponectin with CC grade, a positive association of AdipoR2 expression with tumor/nodes/metastasis stage, and an attenuated AdipoR1 expression amid patients with lymph node metastasis were noticed in a very recent retrospective case-control study (214). Furthermore, serum adiponectin could be used as an adjunctive diagnostic tool for cancer recurrence (212) as well as a predictor for adverse outcome in conjunction with leptin (leptin to adiponectin ratio) (211). All the above findings, along with the higher expression of adiponectin receptors in cancerous colorectal tissue (208, 222) and the increased immunoreactivity with advancing grade and stage (206), point to the role of adiponectin not only in the genesis of CC from colorectal adenomas but also in its progression and prognosis. However, larger prospective studies are warranted to confirm these observations and to explore the role of adiponectin and its receptors in CC progression and prognosis.
In summary, measuring serum adiponectin levels and/or assessing the expression of adiponectin receptors in CC tissue may be useful in predicting the risk of CC, establishing the prognosis and recurrence of CC. We also speculate that interventions to augment adiponectin levels could represent a preventive and therapeutic option for decreasing the occurrence of CC, improving its prognosis, and protecting against its recurrence. Candidate strategic interventions incorporate increased physical activity, weight control, and pharmacological approaches such as PPAR-γ agonists and others, including adiponectin itself (223).
In regard to polymorphisms of the genes encoding for adiponectin and its receptors, seven retrospective case-control studies have examined the association of ADIPOQ and/or ADIPOR1 SNP frequencies with CC risk (224–230) in different populations (Ashkenazi Jewish, Jewish, American, Chinese, Mexican, Czech, British) with contradictory results. Kaklamani et al. (224), He et al. (229), and Liu et al. (230) found associations between ADIPOQ and ADIPOR1 SNP with CC risk. In particular, Kaklamani et al. (224) found that, after combination of the two study populations (Ashkenazi Jewish from New York and American), ADIPOQ SNP (rs266729) was related to a decreased risk for CC; however, this SNP was not linked to CC risk in Ashkenazi Jews and other ethnic groups from Israel in a very recent study by Gornick et al. (228). The discrepancies of the results observed in the previous studies may be due to different ethnic groups in the studied populations, different sample sizes, SNP panels, and/or differential environmental effects. Larger studies are needed to determine the association of ADIPOQ and/or ADIPOR1/R2 SNP with CC risk.
B. Adiponectin and breast cancer
Adult weight gain and excess adiposity are positively associated with breast cancer in postmenopausal women and inversely associated in premenopausal women (5, 231).
Case-control studies conducted by our group linked lower total or HMW adiponectin levels to an increased risk for postmenopausal breast cancer independently of classical risk factors including leptin and the IGF-I system (96, 189, 190). A larger, prospective study in the context of the Nurses' Health Study (NHS) later confirmed these observations (148), which were independently confirmed by Miyoshi et al. (232) in both premenopausal and postmenopausal women. Other studies conducted in Chinese, Taiwanese, and Malaysian women found significantly low serum adiponectin levels in breast cancer patients compared with their matched controls (233–235), especially in postmenopausal women (190, 233). Moreover, a very recent study by Macis et al. (236) in premenopausal women identified lower plasma circulating adiponectin levels as a risk biomarker for progression from intraepithelial neoplasia to invasive cancer independently of age, BMI, and treatment group (236, 237). Because adipose tissue cells represent the predominant breast stromal element, adiponectin exerts a major paracrine influence in mammary epithelium. Adiponectin may play a role in breast cancer etiopathogenesis, particularly in the low-estrogen environment observed in postmenopausal women. In a study by our group, we have also shown that AdipoR1/R2 were expressed in breast cancer cell lines and tissue samples and that adiponectin may act not only via altering the hormonal milieu but directly through inhibition of breast cancer cell proliferation in vitro (96). Jeong et al. (238) found also that high adiponectin and AdipoR expression may be associated with breast cancer invasiveness. Nevertheless, one retrospective and one very recent prospective study failed to detect lower circulating adiponectin levels in breast cancer patients compared with their controls (239, 240). Specifically, a case-control study by Kang et al. (239) evaluated 41 newly diagnosed breast cancer female patients compared with 43 age- and BMI-matched controls and found no significant difference in adiponectin in either the pre- or postmenopausal group. The limitations of this study included its small sample size and the fact that risk factors for breast cancer were not controlled for. In a nested case-control study of 234 postmenopausal women and 234 controls within a cohort of U.S. women with prospectively collected serum samples, breast cancer risk was not associated with circulating adiponectin levels independently from other known risk factors (240). According to this study, the lack of association may be attributed to measurement errors of the laboratory assays. Karaduman et al. (241) measured adiponectin levels specifically in breast tissue samples of 27 breast cancer patients, which presented significantly increased adiponectin levels, compared with tissues of 33 controls with fibroadenoma. However, this group's findings of high adiponectin levels as a risk factor for breast cancer are in contrast with most other studies reported to date and could be due to measuring adiponectin levels in a very small group of tissue samples instead of serum and to not controlling for known confounding factors. Interestingly, a very recent study by the same group reported that serum adiponectin levels in 53 patients with breast cancer were significantly inversely correlated with tumor tissue adiponectin (242), pointing out that when tumor tissue adiponectin was increased, serum adiponectin levels were decreased. These data need to be replicated, and their implications remain to be elucidated.
Additionally, some but not all studies have suggested that breast tumors arising in women with hypoadiponectinemia may present a more aggressive phenotype [large size of tumor, higher histological grade, and estrogen receptor (ER) negativity] (189, 232, 234). It is unclear why hypoadiponectinemia is observed predominantly in ER/progesterone receptor (PR)-negative cancer. Probably, the effects of hypoadiponectinemia could be the deregulation of circulating sex steroids. Only five studies to date have addressed this issue. Two investigations found a significant association with receptor-negative breast cancer (232, 243), another with receptor-positive breast cancer (244), whereas two studies found no significant associations in regard to hormonal receptor status (148, 245). The interrelationship of estrogen and progesterone signaling pathway with insulin and adipose tissue hormones could be responsible for adiponectin secretion and action that in turn affects sex steroid levels and action in a true endocrine loop manner (246, 247).
Low adiponectin concentrations are associated with lymph node metastases (233, 239) and increased breast cancer mortality in breast cancer survivors after adjustment for covariates (248). In a very recent study by Oh et al. (243), serum adiponectin levels in ER/PR-negative breast cancer presented an inverse association with the risk of recurrence regardless of other factors, including obesity and insulin resistance.
Finally, in a large retrospective case-control study focusing on adiponectin genetic variants (ADIPOQ) and adiponectin receptor genes (ADIPOR1) and breast cancer risk, our group found that two ADIPOQ SNP, rs2241766 and rs1501299, related with circulating levels of adiponectin, and one ADIPOR1 SNP (rs7539542) was also associated with breast cancer risk (39). Based on the known function of rs2241766 and rs1501299, individuals with intermediate and low adiponectin signaling presented a greater breast cancer risk than high adiponectin signalers (39). However, two other American studies (one prospective study in postmenopausal women and one population-based case-control study among Whites and African-American women) showed no association between polymorphisms in ADIPOQ, ADIPOR1 (including rs7539542), and ADIPOR2 SNP and breast cancer risk (249, 250). The discrepancy of results may be attributed to the different panels of SNP, the different menopausal status and race of women, and the different pathological subtypes of breast cancer examined in these studies.
C. Adiponectin and endometrial cancer
Excess BMI and adiposity are associated with an increased risk of developing endometrial cancer (5), especially in premenopausal women (251), contrary to that of breast cancer risk. Our group has shown that lower adiponectin levels were associated with a higher risk of endometrial cancer, particularly in women younger than 65 yr, independently from BMI, leptin, the IGF system, and other known risk factors (187). This is in accordance with an in vitro study by our group reporting that adiponectin suppressed endometrial cancer cell proliferation acting through AdipoR increasing the adaptor molecule LKB1, responsible for the adiponectin-mediated activation of AMPK/S6 axis (252). It is worth noting that adiponectin receptors R1 and R2 are highly expressed in the endometrium during the midluteal period of the menstrual cycle (92). We have also found that AdipoR1 is higher than AdipoR2 in human endometrial cancer tissue, but the expression of AdipoR is similar to that from nonneoplastic tissues (252). Additionally, a combination of obesity and lower adiponectin constitutes a greater risk for endometrial cancer occurrence (187, 188, 232). Very recent retrospective case-control studies have confirmed the above findings (253–255) in both pre- and postmenopausal women. These findings were later confirmed by a larger, prospective, nested case-control study within the European Prospective Investigation into Cancer and Nutrition (EPIC) study showing that lower prediagnostic plasma adiponectin levels predispose to an elevated risk of endometrial cancer regardless of BMI status, measures of central obesity, and other obesity-related biological risk factors such as circulating levels of C-peptide (a marker of pancreatic insulin production), endogenous sex steroid hormones, and IGF binding protein 1 (IGFBP-1) and IGFBP-2, particularly among obese and peri-/postmenopausal women (256). However, a second prospective study from the NHS showed that prediagnostic adiponectin was not predictive of endometrial cancer risk, controlling for endometrial cancer risk factors such as BMI, parity, age at last birth, and diabetes (257). This discrepancy of results, especially between the two large prospective studies, might be attributed to the different interval of time from blood draw to endometrial cancer diagnosis [shorter in the study by Cust et al. (256)], to a higher proportion of premenopausal women in the study by Soliman et al. (257), and to the smaller sample size of cases in the NHS (146 cases and 377 controls) compared with the EPIC study (284 cases and 548 controls). Further studies are needed to define the association between adiponectin and endometrial cancer and to determine the duration of insulin resistance that must be present to increase the risk of endometrial cancer among women.
Finally, in a large Chinese retrospective case-control study focusing on adiponectin genetic variants (ADIPOQ) and adiponectin receptor genes and endometrial cancer risk, Chen et al. (258) found that three ADIPOQ SNP were associated with reduced endometrial cancer risk.
D. Adiponectin and gastric cancer
Lower plasma adiponectin levels have also been found in patients with gastric cancer, especially in upper gastric cancer, compared with healthy control subjects, and were inversely correlated with tumor size, depth of invasion, and tumor/nodes/metastasis stage, underscoring a potential role for adiponectin in gastric cancer progression (199). These results are consistent with the findings that adiponectin induced apoptosis and inhibited the proliferation of AZ521 and HCG27 gastric cancer cell lines through both adiponectin receptors and also suppressed the development of peritoneal metastasis of AZ521 when inoculated sc in nude mice (259). Moreover, adiponectin was involved in gastric cancer cell growth suppression via AdipoR1, whereas AdipoR1 expression was associated with good prognosis (260). A recent Japanese retrospective study confirmed that lower adiponectin levels were found in gastric cancer patients, particularly in stage I, compared with controls (261), whereas another small case-control study found similar adiponectin levels between cases and controls and no association of serum adiponectin with tumor stage, invasion (262), as well as tumor tissue adiponectin (263).
E. Adiponectin and esophageal cancer
Obesity, in particular visceral obesity, represents an important risk factor for both Barrett's esophagus and EA (264, 265). In a case-control study, Yildirim et al. (266) investigated the relationship between serum adiponectin and EA as well as esophageal squamous cell carcinoma (ESCC) in comparison to healthy controls. They found that hypoadiponectinemia characterized the two esophageal cancer types. More specifically, adiponectin levels were lower in patients with EA than in patients with ESCC and dropped as tumor stage progressed. These results are in line with a previous study showing that adiponectin caused a significant increase in apoptosis accompanied by augmented B-cell lymphoma-2 (Bcl-2) associated X protein (Bax) and decreased Bcl-2 expression in Barrett's adenocarcinoma cell line OE-19 and that the neoplastic tissue presented decreased expression of the adiponectin receptors (267). In a very recent study, Howard et al. (268) showed that up-regulated AdipoR2 expression was associated with obesity and tumor stage in EA, suggesting that pathways involving adiponectin could affect tumor biology. In agreement with the previous epidemiological study by Yildirim et al. (266), Nakajima et al. (269) found lower levels of adiponectin in Japanese patients with ESCC in comparison to their age- and sex-matched controls. On the contrary, Diao et al. (270) showed similar plasma adiponectin levels in patients belonging to different esophageal pathologies related to the multistage development of esophageal cancer. In this previous small study without adjustment for important covariates, controls with normal esophageal squamous epithelium cells (n = 33) presented similar adiponectin concentrations with patients suffering from basal cell hyperplasia (n = 37), esophageal squamous cell dysplasia (n = 47), and ESSC (n = 43). In retrospective studies for esophageal cancer, it is worth noting that one could expect higher adiponectin levels in patients than in controls due to the inflammation and weight loss observed in cachexia, which often accompanies the diagnosis of esophageal cancer. Further prospective and longitudinal studies are needed to elucidate the association of adiponectin with esophageal carcinoma.
F. Adiponectin and pancreatic cancer
Obesity, diabetes mellitus type 2, and insulin resistance, particularly in men, are associated with an increased risk for PaC (7). Evidence for the association between PaC and adiponectin levels is conflicting and depends mainly on the study design (i.e., prospective vs. retrospective). Serum adiponectin in PaC patients has been reported to be elevated in retrospective studies (95, 271, 272), decreased in a prospective study on male Finnish smokers (273) and in the EPIC prospective study among never smokers (274), and unchanged in the EPIC prospective study among current smokers (274) and in a study examining adiponectin amid patients suffering from different pancreatic pathologies (275). Specifically, our group investigated Greek subjects with PaC and hospital controls and found that hyperadiponectinemia was associated with PaC risk adjusting for age, gender, BMI, smoking status, alcohol consumption, history of diabetes, family history of gastrointestinal cancer or PaC, and leptin concentrations. In our data, further stratification by smoking status revealed that among never-smokers, the strength of the association between hyperadiponectinemia and PaC was more pronounced than among smokers [for never-smokers, odds ratio (OR), 1.154; 95% confidence interval (CI), 1.016–1.31; P = 0.027; and for current smokers, OR, 1.077; 95% CI, 1.002–1.158; P = 0.045).
Hyperadiponectinemia observed in case-control studies could be compensating for insulin resistance and/or inflammation and weight loss due to cancer-associated cachexia, a complex metabolic state characterized by loss of adipose and muscle tissue (95) that develops after cancer develops. Indeed, higher levels of adiponectin are seen in cases of anorexia nervosa and prolonged voluntary weight loss (15). An alternative, probably less likely explanation for the association between elevated adiponectin levels and PaC could be adiponectin resistance produced by a down-regulation of adiponectin receptors or signaling pathways downstream of the receptors leading to subsequent counter-regulatory increased adiponectin secretion. However, we have reported that adiponectin receptors were present in PaC and that both adiponectin receptors were strongly expressed in the vast majority of studied subjects (95). In disagreement with the previous study, a Finnish prospective nested case-control study by Stolzenberg-Solomon et al. (273) examining the association of prediagnostic adiponectin concentration and risk for PaC found that higher adiponectin levels were inversely related to PaC risk specifically in male smokers. Despite the prospective design and the large number of incident PaC cases, this study population represents a homogenous group of male smokers and cannot be generalized to nonsmokers and females. Finally, in a study by Chang et al. (271), serum adiponectin was able to differentiate between states of chronic pancreatitis and PaC, suggesting that this hormone may be used as a more specific tumor marker for PaC compared with the usual serum tumor marker CA 19-9. Further prospective studies are needed to clarify the role of adiponectin in PaC development, specifically taking into account the smoking status, insulin resistance, and the time of adiponectin measurement in relation to diagnosis. It is possible that lower adiponectin levels predispose to PaC, but it is also possible that adiponectin levels increase when the disease becomes overt in a compensatory manner.
G. Adiponectin and liver cancer
Evidence suggests an association between adiponectin and liver tumorigenesis (276). To date, very few studies have explored the link between serum adiponectin and hepatocellular carcinoma (HCC) risk. In a Japanese prospective nested case-control study, individuals with a higher percentage of circulating LMW adiponectin multimers tended to have a reduced liver cancer risk after controlling for age, gender, area, BMI, smoking, alcohol, coffee consumption, diabetes history, and hepatitis C virus-antibody positivity (276). This is in line with the finding that adiponectin has an antioncogenic potential in HCC cell lines HepG2 and Huh7, especially by inhibiting the oncogenic actions of leptin (277).
Obesity and metabolic syndrome are recognized risk factors for hepatic steatosis (278), severe fibrosis (279), and HCC in patients with chronic hepatitis C (CHC) (280, 281). In a large-scale retrospective cohort study, Arano et al. (282) investigated the association of serum adiponectin levels and the risk of HCC in patients with CHC and found that higher adiponectin levels were an independent risk factor for HCC, particularly in female subjects, suggesting that adiponectin may possess oncogenic functions after accumulation in the fibrotic liver. Another possible explanation is the adiponectin resistance phenomenon caused by down-regulation of adiponectin receptors; however, further studies are needed to elucidate this mechanism. Moreover, in the aforementioned study, the middle and low molecular weight (MLMW) adiponectin isoform was an independent risk factor for HCC. In contrast to these results, Nkontchou et al. (283) reported that serum adiponectin was not associated with HCC occurrence in a cohort study of 248 Japanese patients with compensated hepatitis C cirrhosis. Sumie et al. (284) reported similar serum total and HMW adiponectin between 97 Japanese patients with CHC who developed HCC and 97 controls with only CHC. These discrepancies may be attributed to the different liver pathologies (liver cirrhosis vs. CHC) examined in relation to HCC occurrence in the previous studies. In the study by Sumie et al. (284), serum total and HMW adiponectin were predictors of liver fibrosis, and low total and HMW adiponectin levels were independent risk factors for worse HCC histological grades (284). Moreover, microarray analysis of tissue adiponectin expression levels in HCC patients revealed that adiponectin expression was inversely correlated with tumor size, supporting the hypothesis that adiponectin may inhibit proliferation and dedifferentiation (285). Finally, fasting hyperinsulinemia but not serum adiponectin was associated with a poorer prognosis of early stage HCC in a cohort of 140 Japanese patients with incident HCC (286).
H. Adiponectin and renal cancer
Our group showed that lower levels of adiponectin were positively associated with renal cell carcinoma risk controlling for BMI; however, after adjustment for central obesity [waist-to-hip ratio (WHR)], the association became not statistically significant. This finding suggests that altered concentrations of adiponectin may mediate the effect of visceral obesity (192, 287). Furthermore, lower plasma adiponectin levels were associated with larger tumor size and metastasis in clear-cell renal carcinoma (288). Both total and HMW adiponectin were decreased in patients with metastatic renal cancer compared with those with localized disease (289), suggesting that adiponectin may be a possible biomarker of renal cancer progression. Nonetheless, the authors speculated that antihypertensive and/or antilipidemic medications taken by the study group may be a confounding factor for these results. Both adiponectin receptors, particularly AdipoR1, are expressed in normal renal tissue as well as in renal cancer cells, whereas adiponectin receptors (especially AdipoR2 in metastatic tumors) may be down-regulated, reducing the potential protective effect of adiponectin on tumor cells (23, 288).
I. Adiponectin and prostate cancer
Although the association between prostate cancer and adiponectin concentrations has not been consistently shown, there is accumulating evidence that lower adiponectin levels are linked not only to prostate cancer risk (200, 290, 291) but also to the histological grade and disease stage (200, 290, 292). In a large retrospective study conducted by our group, we found almost a 70% reduced risk for prostate cancer in men with the highest adiponectin levels, independent of age, BMI, and other classic factors (290). Moreover, in this study, malignant prostate tissue samples presented weaker expression of adiponectin receptors compared with benign prostate tissue, supporting a role of adiponectin in the prostate cancer pathogenesis. However, two prospective studies failed to detect significant associations between adiponectin levels and prostate cancer risk (293), but the more recent study found that men with higher adiponectin concentrations presented lower risk for developing high-grade or metastatic cancer (292). Furthermore, in a prospective study, Sher et al. (294) found that, although no association existed between biopsy Gleason Score and adiponectin, in patients who had undergone radical prostatectomy, lower serum adiponectin was independently associated with high-grade prostate cancer. However, in a very small study, Housa et al. (295) reported that serum adiponectin levels were significantly higher in patients with T3 stage of prostate cancer compared with patients who had T2 stage, suggesting that cachexia at advanced stages of disease may be responsible for these findings.
Finally, three investigations have looked at ADIPOQ and ADIPOR1 SNP and prostate cancer risk and yielded different results. In a large clinic-based case-control study with 465 cases and 441 controls, Kaklamani et al. (296) found that several haplotype tagging SNP of ADIPOQ and ADIPOR1, previously related to diabetes, insulin resistance, coronary artery disease, and other cancers, were significantly associated to prostate cancer risk. In a large prospective study within the Physician's Health Study, Dhillon et al. (297) reported that four SNP of the ADIPOQ but not ADIPOR1/R2 genes were significantly associated with prostate cancer risk. Conversely, Beebe-Dimmer et al. (298) did not find any association between their studied SNP and prostate cancer risk in 131 African-American patients and 344 controls. The potential explanations for these differences include the different population (Caucasians vs. African-American men), the different panels of SNP used, as well as the smaller sample size in the study by Beebe-Dimmer et al. (298).
J. Adiponectin and hematological malignancies
Several epidemiological studies have underscored the significantly increased risk for hematological malignancies, i.e., leukemia, lymphoma, and myeloma, in individuals with a high BMI (9, 299–301). Adiponectin has been linked mainly to the risk of hematological malignancies of the “myeloid” cell line such as childhood acute myeloblastic leukemia (193), myelodysplastic syndromes (MDS) (196, 197), and myeloproliferative disorders including chronic myelogenous leukemia (CML) (302). These findings are in accordance with a previous hypothesis stating that adiponectin induces apoptosis and inhibits predominantly the proliferation of myeloid cell lineage (303). Furthermore, AdipoR1 expression level was higher in two CML cell lines (K562 and Meg-02) as well as in CML patients, whereas AdipoR2 expression level was unchanged in the previous cell lines and decreased in CML patients, suggesting different functions of adiponectin receptors in CML pathogenesis (304).
With regard to hematological malignancies of myeloid origin, we have shown that low serum adiponectin levels were associated with MDS, a preleukemic condition characterized by trilineage defects in hematopoiesis leading to fatal cytopenias and to a variable risk of progression toward acute myeloid leukemia (197). MDS subtypes exhibited different adiponectin levels, with refractive anemia presenting higher adiponectin levels compared with more aggressive subtypes (197). In an another retrospective case-control study from the same study population, our group found that higher adiponectin levels were associated with a lower risk of MDS, independently from the IGF-I system, serum leptin, resistin, age, gender, and BMI (196). Both total and HMW adiponectin may have a protective role in MDS. Significantly lower adiponectin levels have also been reported in a small study by Avcu et al. (302) in patients with myeloproliferative disorders (MPD) including CML, compared with controls. MPD patients who received interferon had higher adiponectin concentrations than untreated patients (302). It seems that interferon treatment has a positive influence in adiponectin secretion by inhibiting other inflammatory cytokines. Also, imatinib-treated CML patients presented a 3-fold increase of plasma adiponectin levels in the form of HMW and LMW complexes compared with pretreatment adiponectin levels, with a parallel increase of the intramedullary and peripheral adiposity after 6 months of imatinib (305). Elevated adiponectin in these patients may provide a potential mechanism for improved glucose and lipid metabolism as reported for some imatinib-treated patients (305).
Controversial data exist in the literature regarding serum adiponectin as a predictive marker of hematological malignancies from “lymphoid” origin. A decrease (195, 302), no change (193, 198, 306, 307), and even an increase in adiponectin levels have been reported (194, 306, 308). Moreover, no prospective epidemiological studies have been conducted due to the rarity of these malignancies in the general population.
With respect to serum adiponectin and MM risk, our group found that decreased levels of adiponectin were linked to a higher risk for MM, controlling for age, gender, BMI, serum leptin, and resistin (195) in accordance with a very recent study by Fowler et al. (309), which found a significant percentage decrease in serum HMW adiponectin concentrations in patients with monoclonal gammopathy of undetermined significance (MGUS) that either progress or do not progress to myeloma in comparison to age-, gender-, and BMI-matched controls. A recent study using human and murine systems identified a novel mechanism whereby reduced or absent adiponectin promotes myeloma progression, providing strong evidence for a tumor-suppressive role for adiponectin in MM etiopathogenesis (309). Adiponectin can directly induce apoptosis of myeloma cells through an activation of AMPK. Myeloma cell apoptosis is reduced in myeloma-bearing, adiponectin-deficient mice, and increasing adiponectin via L-4 F (an apolipoprotein peptide mimetic) increased apoptosis of myeloma cells in vivo and prevented myeloma bone disease (309). Reduced adiponectin levels observed in MM could be responsible in part for IL-6 (the key cytokine in MM pathogenesis) and TNF-α overproduction in the bone marrow milieu (310, 311). Additionally, we did not find either a significant association between serum adiponectin levels and MM prognostic stages and paraprotein classes or a significant relation between adiponectin and prognostic biological parameters of MM such as CRP, lactate dehydrogenase (LDH), and β-2 microglobulin (195). In contrast to the above findings, Reseland et al. (307) reported similar adiponectin levels in MM cases and controls but significant lower levels of adiponectin in male patients than male controls in a small study.
Regarding chronic lymphocytic leukemia (CLL), Avcu et al. (302) reported an association between lower levels of serum adiponectin in patients compared with controls in a small study with 19 patients and 36 controls. On the contrary, a very recent study by our group showed that there was no significant difference in serum total and HMW adiponectin levels between 95 cases with B-cell CLL (B-CLL) and 95 controls, controlling for age, gender, BMI, family history of hematological malignancies, and serum leptin (198). Similar to the study by Avcu et al. (302), no significant association was observed between both total and HMW adiponectin and B-CLL stage. Only a significant positive correlation was noted between LDH, a marker of disease severity, and adiponectin (198). However, in a cohort of 69 B-CLL patients with Binet stage A, Molica et al. (312, 313) were able to demonstrate that serum adiponectin was inversely correlated with CD38-positive CLL cells, absolute peripheral blood lymphocyte count, and the presence of ζ-chain-associated protein kinase 70, all markers of disease severity (312), and positively correlated with vascular endothelial growth factor (VEGF) (313). In the previous study, both AdipoR1/R2 receptors were highly expressed by CLL cells, but adiponectin gene expression was invariably low, suggesting a limited role of leukemic cells in the production of circulating adiponectin levels (312).
In both childhood B-acute lymphoblastic leukemia (B-ALL) and T-ALL, serum adiponectin was not significantly associated with disease risk (193). In contrast to the previous study, it has been reported in a very small study (nine patients) that lower adiponectin levels were present in childhood ALL cases at diagnosis compared with controls (314). Moreover, during the maintenance period, adiponectin increased gradually in cases but remained lower compared with controls. Adiponectin was also negatively correlated with leukemic burden (314).
Significantly higher adiponectin levels were found in patients with adult non-Hodgkin lymphoma (NHL), childhood NHL, and Hodgkin lymphoma (HL) compared with controls (194, 306, 308). In adult NHL, adiponectin correlated with IL-10 reflecting poor prognosis, and in childhood NHL, adiponectin was associated with poor prognosis (194). Adiponectin may act on NHL cells either directly or indirectly. It has been reported that both AdipoR1 and AdipoR2 were expressed in adult and childhood NHL tissue samples (194). Adiponectin receptors may be capable of direct downstream tumor-promoting signaling in NHL pathogenesis (194). The association of adiponectin with NHL risk may also mirror indirect effects of adiponectin via altered secretion of other cytokines, i.e., IL-10, TNF-α, etc., that play a pivotal role in NHL. Regarding adiponectin polymorphisms and NHL risk, a population-based case-control study found no differences in genotype distributions for the ADIPOQ 276G>T SNP between controls and cases suffering particularly from diffuse large B-cell and follicular lymphoma (301).
K. Adiponectin and lung cancer
Previous studies have evaluated the association between adiponectin and obesity-associated cancers, and little or no focus has been given to non-obesity-associated cancers, such as lung cancer. A case-control study by our group (94) found that serum adiponectin was not significantly different in patients with lung cancer compared with controls, but it was significantly lower in patients with advanced disease stage, suggesting that adiponectin could be a potential marker for lung cancer progression. When examining archival lung specimens, we found that both adiponectin receptors were expressed only in cancerous lung tissue, whereas AdipoR2 was mainly expressed in the non-small-cell lung carcinoma (NSCLC) tissues and in the advanced disease stage tissues (94). In a subsequent study, Petridou et al. (315) found that serum adiponectin was not a major predictor of lung cancer risk, with insulin resistance representing a meaningful risk factor for lung cancer taking into account anthropometric and lifestyle variables as well as metabolic parameters. Another study has reported that serum adiponectin presented no significant differences in 101 advanced NSCLC patients compared with 51 healthy controls and could not be used as a predictive parameter for overall survival (316). Finally, in a Chinese retrospective case-control study focusing on adiponectin genetic variants (ADIPOQ) and NSCLC risk, Cui et al. (317) found that an ADIPOQ SNP (rs2241766) was associated with susceptibility to NSCLC, adjusting for age, gender, BMI, and smoking status.
L. Adiponectin and other malignancies
New evidence associates obesity and insulin resistance with melanoma risk (318, 319). In a case-control study, our group (320) found that there was a sizeable inverse relationship between serum adiponectin levels and melanoma risk, although the results were not statistically significant.
A very recent case-control study conducted by our group found that circulating adiponectin was inversely associated with thyroid cancer risk, independently from other potential confounders attributed mainly as explained below to the metabolic effects of adiponectin (321). Human thyroid carcinomas and cell lines expressed both adiponectin receptors; however, recombinant adiponectin failed to manifest a clinically significant direct effect on cell cycle, proliferation, and apoptosis in thyroid cancer cell lines in vitro (321), indicating that adiponectin may primarily be acting through changing insulin resistance and/or inflammation at the whole body level.
With regard to endocrine tumors, Isobe et al. (322) reported that serum total and HMW adiponectin were three times higher in cases with pheochromocytoma than in controls and normalized after adrenalectomy. Moreover, ADIPO R1 gene expression was significantly associated with adrenaline content in the tumor tissue as well as with adrenaline levels secreted by the tumor, suggesting that adrenaline may induce adiponectin production and signaling (322).
Finally, it is worth noting that lower plasma adiponectin levels and insulin resistance increase malignancy risk, especially renal, thyroid, and gastric cancer incidence, in nondiabetic continuous ambulatory peritoneal dialysis patients (323).
To summarize, several epidemiological studies conducted to date (Table 1) link adiponectin to the risk of obesity-associated cancers, including but not limited to breast, endometrial, prostate, colon, gastric, pancreatic, and hematological malignancies. Additionally, many studies have reported adiponectin receptors and their expression in specific tumor tissues, and a few epidemiological studies have associated specific gene polymorphisms of adiponectin and adiponectin receptors with cancer risk (Table 2) with variable associations. Although adiponectin levels are highly heritable (37, 324, 325), the variability of associations between polymorphisms in the ADIPOQ, ADIPO R1/R2 genes and cancer risk in well-designed epidemiological studies may be due to small sample sizes, different panels of SNP, ethnicities, and clinical outcomes as well as differential environmental effects. Also, in the majority of these studies, serum adiponectin levels were not examined to elucidate associations between adiponectinemia and polymorphisms in the ADIPOQ, ADIPO R1/R2 genes, and cancer risk.
Hence, adiponectin may not only constitute a biomarker for cancer development in obesity, but may also act as a molecular mediator linking adipose tissue to carcinogenesis. The mechanisms underlying the actions of adiponectin as well as its potential diagnostic, prognostic, and/or therapeutic utility require further investigation.
V. Adiponectin and Carcinogenesis Mechanisms
Accumulating evidence suggests that adiponectin exerts antineoplastic effects via two mechanisms. First, it can act directly on cancer cells by stimulating receptor-mediated signaling pathways. Secondly, it may act indirectly by modulating insulin sensitivity at the target tissue site, regulating inflammatory responses and influencing tumor angiogenesis.
A. Direct mechanisms of action
1. In vitro studies
Expression of adiponectin's two main receptors, AdipoR1 and AdipoR2, has been reported in numerous cancer cell types in vitro and in vivo, as mentioned in Section IV. Thus, adiponectin can exert direct receptor-mediated action on cancer cells and tissues.
Adiponectin negatively influences growth of most obesity-related cancer types, although conflicting results have been published for some. Treatment of CC cell lines with adiponectin generally led to growth inhibition (93, 206, 207, 326) or had no effect on proliferation (208). An exception was noted in one study, wherein adiponectin promoted growth of colonic HT-29 cells and stimulated secretion of various proinflammatory cytokines (327). Interestingly, glucose availability was recently suggested to play an important role in the response of colon cancer cells to adiponectin. Under glucose deprivation, adiponectin positively influenced HT-29 and DLD-1 colorectal cell survival through enhancement of autophagic response in colon cancer cells, whereas it inhibited growth in glucose-containing medium (328). In the case of liver (285) and gastric carcinoma (259), EA (267), and endometrial (329) and prostate carcinoma (326, 330, 331), adiponectin exhibited clear anticarcinogenic effects, whereas it had no influence on melanoma cell proliferation (332). For clear-cell renal carcinoma, only indirect evidence, indicative of a negative effect of adiponectin on carcinogenesis, has been published so far (333). Studies on breast cancer have been conflicting and point toward cell line-dependent effects. A complete overview of the effect of adiponectin on breast cancer has recently been reviewed elsewhere (334). In general, adiponectin has been shown to inhibit with a varying degree of efficiency the growth of normal MCF-10A (335) human mammary epithelial cells as well as cancerous MCF7 (335–339), T-47D (96, 338, 340), MDA-MB-231 (340–345), and SK-BR-3 (338) cells, whereas it had no effect on MDA-MB-361 (338) and Hs 578T (345) cells. Differential expression of ERα might be one explanation because proliferation of ERα (+) cells was reduced at lower adiponectin concentrations than proliferation of ERα (−) cells (338). Indeed, the establishment of an ERα (+) MDA-MB-231 cell line exhibiting markedly increased sensitivity to adiponectin treatment supports this notion (338). On the other hand, ERα expression cannot be the only explanation, because variability also exists for the same cell line between different studies, as seen for example in the case of MCF7 (339, 345, 346) and T-47D (96, 344). Possible reasons may be biological variations between the several lines of the respective cells used in various laboratories, as well as differences in culture conditions (246). The latter can be exemplified in the usage of serum because serum-free media alone might favor an inhibition of cell growth (347). Indeed, the previously mentioned positive effect on HT29 colon cancer cell proliferation was seen with 1% fetal bovine serum (327), whereas another study reported no effect under serum-free conditions (208). Moreover, similar studies on HT29 cells proving inhibition of proliferation made use of gAd (207, 326). Thus, cellular responses might also be dependent on the specific adiponectin isoform used. This was also seen in the case of prostate carcinoma cells, where only HMW adiponectin was capable of inhibiting cell proliferation (331). Finally, incubation time or dosage might modulate the effect of adiponectin on cell growth (334). MCF7 breast cancer cells, for example, exhibited a reduction in proliferation when incubated for 48 h with 5 μg/ml adiponectin (338), whereas no effect was visible upon treatment with 10 μg/ml for up to 6 d (335).
Adiponectin can induce apoptosis in liver (277, 285), gastric (259), and endometrial (329) carcinoma as well as EA (267). This effect was shown to be mediated via up-regulation of the proapoptotic Bax and down-regulation of the antiapoptotic Bcl-2 protein in EA cells (267) as well as increased caspase 3 activity in HCC cells (285). Interestingly, the exact mechanism herein seems to depend on the cancer cell line used, as seen in the case of endometrial cancer (329). Additionally, adiponectin can enhance apoptosis in colon cancer cells (93, 206), although exceptions were noted (208, 327). In the case of breast cancer, the effect of adiponectin on apoptosis remains inconclusive. Whereas adiponectin does not affect the viability of T47-D (96, 340, 344, 345, 348), SK-BR-3 (345, 348), or Hs 578T (345) breast cancer cells, it was shown to stimulate apoptosis in MCF7 (336–339, 348) and MDA-MB-231 (343–345). Other studies, however, have suggested an opposite or no effect of adiponectin on both MCF7 (335, 345, 349) and MDA-MB-231 (340, 348) breast cancer cells.
Invasiveness, a common trait of many malignancies, is also influenced by adiponectin, and the outcome seems to depend on cancer cell type. Studies on breast (350, 351) and liver cancer (352) proved a negative effect of adiponectin on migration. Particularly in the latter, adiponectin was able to block signaling pathways related to invasion and down-regulated formation of lamellipodia, important for cell migration (352). On the other hand, a promigratory effect was seen in the case of prostate cancer (353) and chondrosarcoma (354), whereas adiponectin-treated cells exhibited increased expression of the integrins α5β1 and α2β1, respectively. In addition to cell line dependency, differences in the used adiponectin dosage might explain the divergent results. Studies noting a negative effect on migration used more than 10 μg/ml adiponectin (350–352), whereas studies indicating a positive influence used less than 1 μg/ml (353, 354).
In addition to the above receptor-dependent actions, adiponectin can exert receptor-independent, antiproliferative effects through controlling the bioavailability of certain growth and inflammatory factors, both of which have been associated with carcinogenesis (355). By selectively binding and sequestrating platelet-derived growth factor BB, heparin-binding epidermal growth factor, and basic fibroblast growth factor, adiponectin was able to attenuate DNA synthesis, proliferation, and migration of cultured smooth muscle cells (127, 356). These interactions were shown to depend on different oligomeric forms of adiponectin as well as distinct binding sites in the protein (356). Moreover, adiponectin was shown to decrease IL-1β-induced secretion of proinflammatory factors from stromal endometrial cells, thus exerting protective actions on the endometrium (92).
Finally, in vitro evidence points toward interactions between adiponectin and other hormonal signaling pathways, highlighting the complex mechanisms that regulate carcinogenesis in vivo. Generally, adiponectin was able to inhibit leptin-induced proliferation and cell growth, as shown for breast (337, 341), colon (357), liver (277), and prostate cancer (331), as well as EA (358). Furthermore, a cross talk exists between adiponectin and sex steroids. Pfeiler et al. (346) showed that whereas adiponectin alone had no effect on growth of breast cancer cells, a slight reduction was induced in hormone-independent MDA-MB-231 and SK-BR-3 cell lines, with an additional apoptosis stimulation in the former. On the other hand, hormone-dependent MCF7 cells exhibited an increased proliferation rate (346). Moreover, in LNCaP androgen-dependent prostate cancer cells, HMW adiponectin inhibited dihydrotestosterone-induced cell growth (331). IGF-I (336) and IL-6 (359) signaling can also be influenced by adiponectin. An example for the complex interactions can be seen in the case of colon cancer, where the outcome was shown to also depend on tumor stage. Fenton et al. (357) demonstrated that adiponectin reduced cell proliferation and blocked leptin-induced autocrine IL-6 production as well as trans-IL-6 signaling in a model of preneoplastic [adenomatous polyposis coli (Apc) −/+] colon epithelial cells. Normal colon epithelial cells (Apc +/+) on the other hand, were leptin insensitive and also less responsive to adiponectin alone (357). Moreover, in MC38 late-stage colon carcinoma cells, adiponectin treatment was not able to reduce insulin-induced proliferation (359, 360), whereas it could inhibit IL-6-dependent signaling. Leptin treatment alone had no effect on MC38 cell proliferation (359). Thus, along the continuum to carcinoma, adiponectin may influence cell homeostasis and proliferation via distinct mechanisms and interactions.
2. Summary of signaling pathways activated by adiponectin in malignancies
Physiological responses to adiponectin involve several intracellular signaling pathways including AMPK, mTOR, PI3K/Akt, MAPK, STAT3, NF-κB, and the sphingolipid metabolic pathway (Section II.E). Evidence suggests an involvement of these pathways in adiponectin-mediated inhibition of carcinogenesis. Activation of cAMP/protein kinase A (PKA) (336), inhibition of β-catenin (342, 344), as well as reduction of reactive oxygen species (ROS) (60) have also been implicated in the response of cancer cells to adiponectin. The signaling pathways described herein are depicted in Fig. 1. Conversely, new compelling molecular data suggest that adiponectin and its receptors show potent proangiogenic effects that could promote tumor growth particularly in murine mammary cancer models (84, 87, 361). Interestingly, a new array of observations provides insight in the ceramidase activity contained by the classical adiponectin receptors. Upon activation, AdipoR1/R2 catabolize ceramides to downstream degradation products such as sphingosines and the S1P. Elevated S1P is associated with increased cell survival and higher local proangiogenic activity seen in mammary tumor mouse models in which adiponectin levels have been manipulated (84, 361, 362). Based on these data, the sphingolipid metabolic pathway that is a critical mediator of inflammation, cell survival, and growth could represent an important component of adiponectin signaling in malignancies.
Figure 1.
Signaling pathways connecting adiponectin to carcinogenesis. Adiponectin may act on cancer tissues either by sequestrating growth factors at the prereceptor level or by binding to AdipoR1, AdipoR2, and T-cadherin. T-Cadherin has not been associated with downstream effector molecules and may serve as a coreceptor for adiponectin. Binding to AdipoR1 and AdipoR2, initiates a cascade of signaling molecules comprising, among others, activation of AMPK by the cofactors LKB1, APPL-1, and/or CaMKK; induction of Bax and cAMP/PKA; as well as inhibition of ERK1/2, PI3K/Akt, Wnt/β-catenin, nicotinamide adenine dinucleotide phosphate-oxidase/ROS/MAPK, NF-κB, Bcl-2, and JAK2/STAT3 signaling. Activated AMPK subsequently stimulates JNK, PP2A, and the cell cycle regulators p53/p21/p27, while negatively influencing fatty acid synthase (FAS)/ACC and the mTOR/S6K axis. Collectively, these effects result in reduced fatty acid and protein synthesis; decreased cellular growth, proliferation, and DNA-mutagenesis; and increased cell cycle arrest and apoptosis, thus negatively influencing carcinogenesis. Cross talk between the mentioned pathways adds further complexity to the adiponectin-induced signaling network. Finally, recent advances show that adiponectin can enhance ceramidase activity independently from AMPK via AdipoR1/R2, contributing to increased amounts of prosurvival S1P. The black and red lines indicate stimulatory and inhibitory effects, respectively. Trx, Thioredoxin.
Most of the effects of adiponectin on cancer are mediated through AMPK. AMPK can be stimulated by adiponectin through an increase of AMP levels, the adaptor protein APPL-1, calcium-dependent kinases (Section II.E), as well as the Ser/Thr kinase LKB1 (363). Importantly, in the case of breast cancer cells, LKB1 has recently been proven necessary for adiponectin-induced AMPK activation and subsequent inhibition of adhesion, migration, and invasion. LKB1 expression was also shown to be directly stimulated by adiponectin (350). AMPK negatively influences carcinogenesis through affecting cell growth mechanisms. It down-regulates the TSC2/mTOR/S6 axis, thus reducing protein expression, and inhibits de novo fatty acid synthesis via blocking sterol regulatory element binding protein-1c-mediated induction of ACC and fatty acid synthase (118). Additionally, it induces p53 and p21 expression, important regulators of growth arrest and apoptosis (118). Short-term adiponectin treatment was shown to activate AMPK in colon (93, 207, 326), breast (96, 336, 339, 350, 351), liver (285), prostate (326, 364), and endometrial (329) cancer as well as EA (358), mediating growth inhibition. Down-regulation of mTOR/S6 signaling was implicated in many of these studies (207, 285, 326, 350). Activation of protein phosphatase 2A (PP2A), a tumor suppressor involved in the inhibition of Akt and small GTP hydrolase Ras-like A (365), was also shown to depend on adiponectin/AMPK signaling in MDA-MB-231 breast cancer cells (351). Moreover, in colon cancer cells, adiponectin-induced AMPK activation was directly involved in increased expression of cyclin-dependent kinase inhibitors p21 and p27, leading to cell cycle arrest as well as reduced levels of sterol regulatory element binding protein-1c (93).
Additionally, studies point toward a direct negative effect of adiponectin on the PI3K/Akt pathway. In response to various growth factors, PI3K becomes activated, initiates phosphorylation of Akt, and stimulates a plethora of effector molecules positively regulating cell survival, growth, and proliferation. Interestingly, Akt can also phosphorylate and inhibit TSC2, thus counteracting the effects of activated AMPK. Phosphatase and tensin homolog (PTEN) acts as an inhibitor of PI3K signaling (366), and cell line-specific PTEN deficiency may determine whether AMPK or Akt prevails in regulating mTOR activation (364). Treatment of colon (328) and breast cancer cells (336) with adiponectin was shown to significantly reduce the phosphorylation and activation of PI3K and Akt. Moreover, adiponectin alleviated thioredoxin- and thioredoxin reductase-mediated inhibition of PTEN, thus indirectly influencing PI3K/Akt signaling (367).
Further mediators of adiponectin signaling can be found in the MAPK cascade, comprising among others JNK, p38, and ERK1/2, or p42/p44. The effects of stress-activated kinases JNK and p38 on proliferation and apoptosis depend on the cellular context, whereas ERK1/2 largely have mitogenic influences (368). In prostate (98) and hepatocellular (98, 285) carcinoma cells, adiponectin treatment led to increased JNK activity resulting in caspase 3-mediated apoptosis (285). Interestingly, AMPK activity seems to be pivotal for the phosphorylation of JNK, as shown in the case of liver cancer cells (285). Adiponectin-induced inhibition of ERK1/2 signaling has been noted in endometrial (329) and breast (96, 339) cancer cell lines contributing to a reduction in viability. Importantly, in MCF7 breast cancer cells, prolonged adiponectin treatment resulted in decreased levels of c-myc, cyclin D (over a period of 2 to 6 h), and Bcl-2 (8 h) while increasing expression of p53 and Bax (24 h), thus leading to cell cycle arrest and apoptosis (339). In contrast, adiponectin has been associated with increased p38 activity in several studies positively influencing carcinogenesis, as mentioned previously (327, 353, 354).
Adiponectin also modulates STAT3 signaling. STAT3 is activated by adipokine-induced (e.g., leptin) or cytokine-induced JAK phosphorylation and regulates many cancer-related processes such as cell survival and differentiation. Consequently, dysregulation of the JAK/STAT3 pathway favors carcinogenesis (369). Adiponectin treatment of liver cancer cells down-regulated leptin-induced STAT3 phosphorylation and subsequently reduced cell growth and migration. Importantly, suppressor of cytokine signaling 3, a JAK2/STAT3 inhibitor, was found elevated and may explain the adiponectin-leptin cross talk (277). Adiponectin was also able to suppress constitutively active STAT3-signaling in prostate (DU145) and hepatocellular (HepG2) carcinoma cell lines (98).
Overactivation of wingless-type protein (Wnt)-signaling along with increased β-catenin-induced expression of positive cell cycle regulators has been observed in many human cancer types (370). Wnt signals through frizzled to inactivate glycogen synthase kinase-3 (GSK-3β), which forms part of the β-catenin degradation complex, allowing accumulation of β-catenin in the nucleus (370). In MDA-MB-231 breast cancer cells, prolonged adiponectin treatment (24 h) inhibited phosphorylation of GSK-3β, destabilizing β-catenin and thus significantly reducing cyclin D1 expression. This effect was probably mediated by decreased Akt phosphorylation (344) as well as direct induction of Wnt inhibitory factor 1 expression, a molecule involved in down-regulating β-catenin signaling (342). Interestingly, cross talk between the canonical Wnt/β-catenin and PI3K/Akt pathways has been reported previously (371), supporting the former assumption.
In addition, adiponectin was shown to mediate cell cycle arrest and apoptosis in MCF7 breast cancer cells through modulating the cAMP/PKA pathway. Particularly, adiponectin treatment increased intracellular cAMP levels (at 15 min or 6 h), thus activating PKA (at 30 min or 6 h) and inhibiting expression of cyclin D1 (24 h) and E2 (48 h) (336). Phosphorylation of AMPK (30 min) was also found increased, but it depended on PKA activity (336). Inhibition of NF-κB, a transcriptional regulator involved in inflammation and cancer (372), has also been implicated in adiponectin signaling (357). Finally, adiponectin is able to decrease ROS production (60), which can promote growth factor signaling and induce DNA mutations (373). The reduction in intracellular ROS levels was shown to be mediated via down-regulation of nicotinamide adenine dinucleotide phosphate-oxidase activity and ultimately led to inactivation of MAPK, thus counteracting cell proliferation (60).
Of note, adiponectin's influence in cancer cells may not only be of a direct nature but may also depend on endocrine (tumor microenvironment) and paracrine (distal) interactions of adipocytes with tumor tissues (13). An example can be seen in the case of breast cancer, where tumor cells and adipocytes reside in close proximity to each other (13). In adipocytes, adiponectin induces LKB1/AMPK-dependent inhibition of aromatase activity, subsequently lowering estrogen production (374). Reduced ERα-stimulation in hormone-dependent cancer cells negatively impacts prosurvival pathways. This can be seen for epidermal growth factor receptor (EGFR) signaling (375), which induces proliferation through activating kinases such as ERK, PI3K and the STAT family of transcription factors (375, 376). In fact, EGFR signaling seems to be a point of convergence for direct and indirect effects of adipokines on cancer cells because adiponectin may also influence EGFR pathways directly through AdipoR1/R2-stimulation (13). However, the interaction between adipocytes and cancer cells is far more complex because it also involves other adipocyte-secreted molecules like, for example, leptin, inflammatory cytokines (TNFα, IL-6), extracellular matrix constituents, and proangiogenic factors (VEGF), as well as metabolic regulators like insulin (13).
3. Animal studies
The physiological relevance of the in vitro findings has recently been evaluated in animal experiments. Various models of carcinogenesis, combined with adiponectin deficiency and/or administration, have been able to confirm an antitumorigenic role for adiponectin. Examination of influences mediated by diet further contributed toward elucidating the complex mechanisms that underlie the action of adiponectin in vivo.
Inhibition of tumor growth has been demonstrated for colon (377–379), gastric (259), liver (285, 352), breast (342, 344, 351, 367), and lung cancer as well as melanoma (332). Adiponectin deficiency promoted azoxymethane (AOM)-induced colorectal and liver tumor formation and correlated with higher colon tumor stage (379). Also, cell proliferation was increased and associated with a concomitant increase of cyclooxygenase-2 (COX-2) expression, predominantly in tumor myofibroblasts (379). Thus, adiponectin might negatively regulate tumor growth by influencing COX-2 expression in stromal myofibroblasts (379), which have been shown to secrete prostaglandin E2 upon COX-2 expression and consequently stimulate cell proliferation (380). Along the same lines, Mutoh et al. (377) detected a gradual increase in polyp formation in Apc (multiple intestinal neoplasia) (/+) mice with adiponectin haplodeficiency and complete deficiency. Diminished AMPK phosphorylation resulting in elevated serum levels of plasminogen activator inhibitor-1, an adipocytokine positively involved in colon carcinogenesis, was associated with loss of adiponectin (377). Growth of intestinal adenomas in Apc (multiple intestinal neoplasia) (/+) mice was further shown to be suppressed by ip administration of adiponectin. This effect was probably mediated by binding to AdipoR1 and AdipoR2 because both receptors were present in cancerous tissues and their expression levels remained unaltered after treatment (378). Similar results were obtained in a nude mice-xenograft model of gastric cancer. Local and systemic adiponectin administration markedly inhibited growth and metastasis, and in vitro studies pointed toward an involvement of both receptors in the antitumorigenic response to adiponectin (259). To date, a relative paucity of studies exists regarding the beneficial effect of adiponectin on colon and gastric carcinogenesis. The main focus has been adiponectin-deficiency models or models utilizing adiponectin as a treatment of already established malignancies. Chemoprevention studies, on the other hand, have not been performed. These would be useful for fully elucidating the action of adiponectin on colon and gastric carcinogenesis from the beginning and for evaluating whether adiponectin could be used as a preventative agent.
In the case of liver cancer, mouse xenograft experiments provided evidence that adiponectin can reduce tumor growth and lung metastasis via increasing JNK phosphorylation (285) and inhibiting angiogenesis (352). Moreover, adiponectin has been shown to counteract leptin-induced stimulation of tumor growth (277). Finally, adiponectin deficiency in the context of a mouse model of nonalcoholic steatohepatitis accelerated liver cirrhosis and tumor formation through elevated oxidative stress (381). Thus, hypoadiponectinemia, as seen in the context of obesity, may be a risk factor for nonalcoholic steatohepatitis-related liver tumorigenesis (381).
Studies on breast cancer have so far published conflicting results. First of all, adiponectin haploinsufficiency was shown to promote tumor onset and aggressiveness through increased PI3K/Akt/β-catenin signaling (367). Furthermore, in a MDA-MB-231 xenograft model, pretreatment of cells with adiponectin as well as intratumoral or systemic administration of adiponectin negatively influenced mammary tumorigenesis. Inhibition of PI3K/Akt and GSK-3β/β-catenin signaling (344), possibly through elevated expression of Wnt inhibitory factor 1 (342), were found to be implicated. Additionally, direct adiponectin administration resulted in an AMPK-mediated increase of PP2A activity (351). On the other hand, complete adiponectin deficiency was shown to suppress mammary carcinogenesis in a mouse mammary tumor virus-polyoma middle T antigen (MMTV-PyVmT) tumor model, accompanied by decreased tumor angiogenesis (361, 362). Possible reasons for the observed contradictions are discussed in Section V.B.3.
Adiponectin has also been implicated in negatively regulating the growth of lung cancer and melanoma xenografts. The underlying mechanism may involve macrophage infiltration into tumor tissue because tumors from adiponectin knockout mice exhibited reduced macrophage numbers (332).
A number of studies have examined the importance of dietary fat intake and obesity on tumorigenesis. A high-fat diet, corresponding to the common Western-style diet, has been shown to positively and directly influence pancreas (382), colon (383), and Lewis lung (384) cancer as well as ALL (385). Furthermore, genetically induced obesity, as seen in ob/ob and db/db mice, has been associated with PaC promotion and dissemination, particularly in the context of insulin resistance and decreased levels of circulating adiponectin (386). On the other hand, intermittent caloric restriction was shown to reduce tumorigenesis in a mouse mammary tumor model. This effect was associated with an elevated adiponectin/leptin ratio, increased expression of AdipoR1 in mammary tissue, and higher levels of adiponectin in mammary fat pads (387). A direct role of adiponectin in the context of a high-fat diet has been studied in the case of AOM-induced colon cancer formation. By generating adiponectin-, AdipoR1-, and AdipoR2-deficient mice, Fujisawa et al. (388) provided evidence that adiponectin suppresses colonic epithelial proliferation exclusively under the high-fat diet condition. The observed effect involved AdipoR1-mediated activation of AMPK and subsequent inhibition of mTOR (388).
Interestingly, supraphysiological serum levels of adiponectin do not seem to protect against cancer development on the basis of adiponectin transgenic mouse models. A recent study utilizing adiponectin transgenic animals showed that growth of AOM-induced colon cancer was not attenuated by adiponectin overexpression (389). Insulin resistance, on the other hand, induced by high-fat diet treatment 1 wk after the final AOM injection, was efficiently prevented (389). These results seem to contradict previously mentioned studies on the beneficial effect of adiponectin on obesity-related colon tumorigenesis. However, a high-fat diet may not play a key role in the development of colon cancer in the model of Ealey et al. (389) because it was used during the final stages of the disease. It is known that obesity takes time to develop after feeding mice a high-fat diet (78). In fact, adiponectin levels decrease only after 10 wk of high-fat diet treatment or later (78). In addition, other factors may change during later stages of high-fat feeding, as shown for insulin and leptin (390). During these stages, AOM-induced colon cancer is already well established. Thus, the study by Ealey et al. (389) can only provide evidence for chemically induced and not obesity-induced colon carcinogenesis. Further chemoprevention studies administering different levels of adiponectin in combination with a high-fat diet are needed to examine the potential preventative effect of adiponectin on obesity-related colon cancer.
In summary, accumulating evidence provides support for an antitumorigenic effect of adiponectin on various cancer types, most of which are associated with obesity. Importantly, adiponectin seems to exert the strongest effect under the high-fat diet condition (388), i.e., a condition directly related with insulin resistance and proinflammatory state, a fact with important implications for its potential use in anticancer therapy.
B. Indirect mechanisms of action
Adiponectin exerts indirect antineoplastic action through an insulin-sensitizing and antiinflammatory effect. Additionally, it has been proposed to play a role in angiogenesis regulation, although conflicting evidence has been published.
1. Insulin-sensitizing effects
Insulin resistance and hyperinsulinemia have been proposed to play an important role in cancer development, particularly in the context of obesity (14). High insulin levels increase the bioavailability of IGF-I by stimulating its expression and simultaneously down-regulating IGFBP-1 and IGFBP-2. Insulin and IGF-I then signal through the IR and IGF-I receptor to inhibit apoptosis and enhance cellular proliferation. The resulting mitogenic and antiapoptotic environment favors the accumulation of genetic mutations and thus carcinogenesis (391). Many clinical studies have found an association between high levels of IGF-I and/or insulin with an increased risk for several malignancies, including colorectal and postmenopausal breast cancer (391–399).
An indirect link between the insulin pathway, adiponectin, and thus carcinogenesis has been established in many studies. Serum adiponectin levels are inversely associated with fasting insulin concentrations (22) and reduced in the context of obesity and insulin resistance (21, 30). Moreover, adiponectin exhibits potent insulin-sensitizing actions. Intraperitoneal injection of adiponectin lowers glucose levels in wild-type mice and transiently reverses hyperglycemia in ob/ob, nonobese diabetic, or streptozotocin-treated mice (67). These effects were shown to be mediated via increased ability of insulin to suppress glucose production in adiponectin-treated primary rat hepatocytes (67). Adiponectin deficiency, on the other hand, results in severe diet-induced insulin resistance (400).
The mechanism underlying the observed adiponectin-mediated reversal of insulin resistance is slowly being unraveled. In Section III.A, direct AMPK-mediated effects on glucose and fatty acid metabolism are described in more detail. In addition to these direct effects, adiponectin may exert indirect influence on the insulin-signaling pathway. Administration of adiponectin increased insulin-induced phosphorylation of the IR and whole-body insulin sensitivity in rodents. Additionally, plasma adiponectin levels were found positively associated with skeletal muscle IR phosphorylation in humans (123). Moreover, adiponectin enhanced the ability of insulin to stimulate phosphorylation of the adaptor protein IRS-1 and Akt by reversing the S6 kinase-mediated negative regulation of IRS-1. Particularly, treatment of muscle cells with adiponectin activated the LKB1/AMPK/TSC1/2 pathway, ultimately leading to a reduction of S6 kinase phosphorylation (122).
Recently, new additions have been made to the established pathways linking adiponectin with insulin sensitivity. Ceramides, a class of sphingolipids, have been associated with impaired insulin sensitivity in muscle (84). Adiponectin was shown to stimulate ceramidase activity through AdipoR1 and AdipoR2 independently of AMPK. Thus, ceramide catabolism was induced and subsequently led to increased levels of the antiapoptotic S1P (84). This could present important implications for cells expressing adiponectin receptors, whereas in cells lacking adiponectin receptors ceramidase activity is impaired, resulting in an enhanced susceptibility to cell death. Moreover, adiponectin has been implicated in the inhibition of autophagy, which fuels gluconeogenesis (401). Cowerd et al. (401) provided evidence that activation of the PI3K pathway through APPL-1 in adiponectin-treated hepatoma cells increased expression of suppressor of glucose by autophagy, a novel negative regulator of autophagy (401). Finally, adiponectin was able to reduce cholesteryl ester formation in cultured macrophages via inhibition of acyl coenzyme A:cholesterol acyltransferase 1 (402), which has been implicated in positive regulation of tumor cell growth and invasion (403).
2. Antiinflammatory effects
Inflammation is a well-established pathway leading to cancer promotion and progression. In response to tissue injury or infection, a cascade of events is initiated. These lead to increased cell proliferation and production of reactive oxygen and nitrogen species from infiltrating immune cells. Thus, an environment is created that favors mutagenesis in rapidly dividing cells, a prerequisite for cancer development (404). Epidemiological studies have associated chronic inflammation with increased risk of several types of cancer. Persistent infections can lead to chronic inflammation, and thus cancer, as seen for example in the case of gastric cancer due to Helicobacter pylori infection (404). Additionally, there are strong associations between inflammatory bowel diseases, characterized by chronic inflammation, and CC (404).
Obesity reflects a low-grade systemic inflammatory state and is associated with increased proinflammatory markers such as IL-6, TNF-α, and CRP (405). CRP confers increased cancer risk, and both systemic IL-6 and TNF-α have been correlated with higher cancer rates, as examined in the case of colorectal neoplasia (405). Potential mechanisms might involve prosurvival effects of TNF-α on cancer cells and overstimulation of the JAK/STAT3 pathway by IL-6 (14). Matrix metalloproteinases (MMP) involved in cancer-cell invasion and metastasis and inflammation-induced oxidative stress are additional factors that have been implicated in the link between obesity, inflammation, and cancer development (14).
Adiponectin has been shown to exert antiinflammatory actions and may thus counteract the increased cancer risk seen in obesity-induced inflammation. Particularly, adiponectin influences the function of myelomonocytic cells, important mediators of innate immunity. Adiponectin inhibited proliferation of myelomonocytic precursor cells (303) and reduced secretion of proinflammatory cytokines TNF-α and IL-6 from activated macrophages (303, 406) while increasing the production of antiinflammatory cytokine IL-10 (406) and tissue inhibitor of metalloproteinase-1, an inhibitor of MMP and tissue remodeling (407). Moreover, adiponectin negatively influenced macrophage phagocytic activity (303), promoted the antiinflammatory M2 phenotype in vitro and in vivo (408), attenuated leukocyte-endothelium interactions in vivo (409), and prevented systemic inflammation by enhancing calreticulin-dependent opsonization of apoptotic bodies and their subsequent macrophage-mediated clearance (410). Adiponectin was also found to suppress IL-2-stimulated natural killer cell cytotoxicity by reducing the expression of interferon-γ and apoptotic effector molecules (411). Recently, adiponectin was implicated in the modulation of dendritic cell activity. Tsang et al. (412) showed that adiponectin-treated dendritic cells decreased T-cell proliferation and increased the percentage of CD4(+)CD25(+)FOXP3(+) regulatory T cells via up-regulation of the inhibitory molecule programmed cell death-1. Finally, adiponectin can influence T- and B-cell function. Through down-regulating T-cell chemoattractants from activated macrophages, adiponectin was able to reduce T-cell recruitment in atherogenesis (413). Adiponectin was also found to suppress B-cell lymphopoiesis, but only when stromal cells were present and when cultures were initiated with the earliest category of lymphocyte precursors (414).
Inhibition of NF-κB and ERK1/2 signaling has been implicated in some of the observed effects (406) as well as increased activation of PPAR-α and PPAR-γ (408). Additionally, adiponectin was shown to induce the expression of multiple genes with antiinflammatory properties, e.g., suppressor of cytokine signaling 3, in cultured macrophages (415).
3. Angiogenesis
Angiogenesis is very important for the growth of most primary tumors and their metastases. Up to a size of 1–2 mm, tumors can absorb sufficient nutrients and oxygen by diffusion. After that point, their further growth requires attachment to the vasculature (416). Inhibition of angiogenesis has been shown to suppress tumor growth (97, 417) and may represent a promising therapeutic target. On the other hand, antiangiogenic therapy should be used with caution because the resulting hypoxic environment and selective pressure can favor evasive mechanisms that reinitiate tumorigenesis in certain cancer types (417). The effect of adiponectin on neovascularization based on published results remains contradictory in that both pro- and antiangiogenic effects have been reported.
Regarding the proangiogenic action of adiponectin, Ouchi et al. (69) showed that it can induce capillary-like structure formation, enhance the migration of human umbilical endothelial cells, and stimulate blood vessel growth in vivo (69). Activation of AMPK, Akt, and endothelial nitric oxide synthase was found to be implicated (69). Also, in a separate study, adiponectin was able to rescue endothelial cells from serum starvation-induced apoptosis by suppressing caspase 3 activity (418). These findings seem to be particularly relevant in the case of obesity and metabolic syndrome, where patients exhibit, among other factors, impaired collateral artery formation (419). Hypoadiponectinemia has been suggested to causally link obesity with the observed cardiovascular dysfunction (419). Indeed, in a hind limb ischemia model, adiponectin-mediated AMPK signaling enhanced angiogenesis and improved limb reperfusion (419). Furthermore, in the context of atherosclerotic cardiovascular disease, adiponectin was shown to reverse the effects of IL-18. IL-18, a potent proinflammatory cytokine, is increasingly expressed in atherosclerotic lesions (120). In the study of Chandrasekar et al. (120), pretreatment of endothelial cells with adiponectin blocked IL-18-mediated apoptosis via APPL-1-dependent AMPK phosphorylation and subsequent PI3K/Akt signaling. Adiponectin also has positive effects on endothelial progenitor cells, which may contribute toward the formation of new blood vessels. Shibata et al. (420) and Yang et al. (421) showed that adiponectin can stimulate endothelial cell differentiation from human peripheral blood mononuclear cells (420) and human peripheral blood CD14 (+) monocytes (421), respectively. In addition, adiponectin was able to increase endothelial cell migratory activity through Akt-dependent cell division control protein 42 homolog/Ras-related C3 botulinum toxin substrate 1 stimulation (422) and enhance the formation of network structures (420). The clinical relevance of the above findings has been validated recently in the case of diabetes. Leicht et al. (423) proved that adiponectin-pretreated endothelial progenitors isolated from diabetic patients exhibited markedly improved neovascularization capabilities in nude mice xenografts. Thus, the proangiogenic role of adiponectin may contribute to its potent antidiabetic effects.
In the context of carcinogenesis, in vivo studies employing the MMTV-PyMT mouse mammary tumor model have found a positive influence of adiponectin on tumor vasculature. Denzel et al. (362) showed that tumors from adiponectin null mice exhibited reduced growth and angiogenesis along with increased hypoxia and apoptosis. These results were confirmed by Landskroner-Eiger et al. (361) for early stages of mouse mammary tumorigenesis (9 wk). Surprisingly, at 12 to 14 wk of age, when tumors characteristically progress to late carcinomas, adiponectin deficiency led to opposite effects, enhancing tumor growth and metabolic activity (361). Increased mobilization of circulating endothelial progenitors, along with up-regulated VEGF-A expression in tumor tissues, possibly fueled tumor growth (361). Additionally, the tumors exhibited greater aggressiveness, as seen by transcriptional profiling (361) and increased pulmonary metastases (362). Landskroner-Eiger et al. (361) hypothesized that the observed antiangiogenic stress and reduced nutrient/oxygen flow triggered an adaptive mechanism enabling the tumor cells at later stages to circumvent impairments caused by adiponectin deficiency.
Accumulating evidence suggests that T-cadherin may mediate the effects of adiponectin on tumor neovascularization. T-Cadherin was found to be selectively expressed in intratumoral capillaries of human HCC, whereas no expression was detected in nonneoplastic hepatocytes (424). Furthermore, adiponectin and T-cadherin were shown to colocalize in tumor vasculature (87, 362), and the association was lost upon T-cadherin deficiency, accompanied by increased serum adiponectin levels (87). Moreover, adiponectin (362) and T-cadherin (87) null mice exhibited striking parallels in mammary tumor growth and phenotype. Thus, T-cadherin seems to be necessary for adiponectin-mediated signaling at the level of endothelial cells, possibly by regulating the bioavailability of circulating adiponectin or by interacting with other receptors. The coincident reduction of T-cadherin and AdipoR2 in mouse mammary tumors supports the latter notion (362).
On the other hand, there are studies arguing for an antiangiogenic role of adiponectin. Adiponectin directly binds to proangiogenic growth factors (356) and inhibits both the production of TNF-α from macrophages and its action on endothelial cells (75). Considering the fact that TNF-α can stimulate angiogenesis (425), these results provide evidence for an indirect effect of adiponectin on the vasculature.
Importantly, mouse xenograft experiments directly contradict the above-mentioned in vivo proangiogenic findings. Bråkenhielm et al. (97) demonstrated that adiponectin treatment inhibits growth of murine fibrosarcoma cells sc injected into mice. The underlying mechanism involved loss of growth-enhancing angiogenesis (97). In vitro experiments revealed that adiponectin specifically reduced endothelial cell proliferation and migration and induced caspase 8-mediated apoptosis (97). Similar results were obtained in a mouse model of liver carcinogenesis. Adiponectin treatment reduced tumor growth and invasiveness and decreased microvessel density while inhibiting endothelial tube formation in vitro. Down-regulation of Rho-associated kinase, interferon-γ inducible protein 10, MMP9, angiopoietin 1, and VEGF was found to be implicated (352). Furthermore, adiponectin inhibited neovascularization in peritoneal metastases of gastric cancer cells injected into nude mice (259).
One possible explanation for the observed controversies lies in the mouse models used. Evidence for the antiangiogenic role of adiponectin was obtained from xenografts, whereas support for a proangiogenic effect of adiponectin came from MMTV-PyMT mouse mammary tumor models. Autochthonous tumors may replicate human disease more accurately than transplants (426). Importantly, contradictions between the two models have been noted, especially in the case of angiogenesis, suggesting that variability in the respective microenvironments might exert different influences on vascularization (427). Usage of bacterially produced adiponectin as seen in the antiangiogenic studies, and variations in the preparation of the recombinant protein could also be problematic in recapitulating the human condition (361).
In summary, the role of adiponectin in tumor angiogenesis remains to be defined. One possibility is that adiponectin differentially affects physiological and pathological angiogenesis, as has been reported for statins (428). Additionally, the influence of adiponectin might depend on cancer cell type, as can be seen in the previously discussed effects of adiponectin on proliferation. In the case of mammary tumors, however, direct comparison between the used models argues for a general proangiogenic contribution of adiponectin. The MMTV-PyMT model has been suggested to closely recapitulate human disease (361, 362). Moreover, the decline in adiponectin levels during tumor progression, along with the accelerated tumor growth under adiponectin deficiency in later stages of the disease (361), is consistent with epidemiological findings associating low adiponectin levels in women with increased breast cancer risk (189), higher histological grade (232), and metastases (239). Thus, not angiogenesis but hypoxia induced by hypoadiponectinemia along with increased selective pressure may be more detrimental for human cancer progression, allowing tumors to assume a highly aggressive phenotype (361). The general antitumorigenic role of adiponectin therefore remains consistent with a possible proangiogenic function.
VI. Conclusion and Future Perspectives
Adiponectin, the most abundant adipokine produced by the human adipose tissue, is linked to obesity, metabolic syndrome, insulin resistance, type 2 diabetes, coronary heart disease, inflammation, and several types of cancer. Genetic (polymorphisms of adiponectin and adiponectin receptors genes) and environmental (high-fat diet, physical inactivity) factors are associated with hypoadiponectinemia and may contribute to the development of insulin resistance, type 2 diabetes, atherosclerosis, and cancer.
The ability of adiponectin to enhance insulin sensitivity synergistically with its antiproliferative properties has rendered this adipokine a promising potential diagnostic and prognostic biomarker as well as a novel therapeutic tool in the pharmacological armamentarium for treating malignancies. In the future, based on serum adiponectin determinations and specific combinations of adiponectin pathway SNP, we could identify a high-risk population for developing cancer that could benefit from adiponectin replacement therapy. However, further more intensive basic research studies, in vivo animal studies, observational human studies, and larger prospective and longitudinal studies are needed to fully elucidate the mechanisms underlying the effects of adiponectin on cancer. Additional studies are needed for the development of reliable and “user-friendly” laboratory techniques (e.g., ELISA) to assess total adiponectin and its isoforms as well as their physiological relevance. Which adiponectin levels should be considered abnormal needs also to be determined because serum adiponectin levels vary by gender, race, and assay methodology. Finally, similar to many new assays, international standardization of levels and assay procedures is also needed in the future before full commercialization of adiponectin as a potential diagnostic for obesity-related malignancies.
Because it is extremely difficult to synthesize adiponectin and to convert its full-size protein into a viable drug to be used in humans, research efforts should be directed toward identifying ways to increase endogenous circulating adiponectin levels, to possibly moderate the obesity-cancer link. Interestingly, a new adiponectin-based short peptide, ADP 355, mimicking adiponectin action, restricted proliferation in a dose-dependent manner in several adiponectin receptor-positive cancer cell lines, modulated several key adiponectin signaling pathways (AMPK, Akt, STAT3, ERK1/2), and suppressed the growth of orthotopic human breast cancer xenografts by 31% in vivo (429). Agonists of AdipoR1/R2 but also strategies to increase adiponectin receptors and to modulate their sensitivity to adiponectin may provide novel therapeutic approaches for insulin resistance, diabetes type 2, and obesity-associated malignancies. Pharmacological agents such as full and selective PPAR-γ agonists (SPPARM), which either augment circulating adiponectin levels or stimulate adiponectin signaling through its receptors, are at the forefront of future therapeutic modalities for obesity-linked diseases and malignancies (430, 431). Although full PPAR-γ agonists such as rosiglitazone, pioglitazone, and ciglitazone have shown potent and durable glucose-lowering activity in patients with type 2 diabetes, they are linked to safety and tolerability issues (432–434). Interestingly, SPPARM are effective insulin sensitizers demonstrating a superior safety profile to that of full PPAR-γ agonists (435). The SPPARM INT131 (also known as T131 and AMG131), representing a novel class of non-thiazolidinedione PPAR ligands, increases the HMW/total adiponectin concentrations (430, 435).
Very recently, the apolipoprotein peptide mimetic L-4 F, used for the pharmacological enhancement of adiponectin, reduced tumor burden through induction of myeloma cell apoptosis, increased survival of myeloma-bearing mice, and provided protection against myeloma-destructive osteolytic bone disease, an important clinical feature of MM (309).
Although understanding the link of adiponectin with cancer might provide potential therapeutic targets, lifestyle amelioration remains the most important component in preventing obesity-related malignancies. Physical exercise, reduction of body weight, a Mediterranean-based diet with consumption of fruits, nuts, coffee, and/or moderate amounts of alcohol present a well-established association with increased plasma adiponectin concentrations and a reduced risk of developing insulin resistance, diabetes, cardiovascular disease, and cancer.
In summary, advances in adiponectin research have contributed toward making the association between cancer, obesity, and adiponectin clearer. At the same time, several issues remain to be clarified, and yet other areas need further investigation. Progress made creates the hope, however, that advances in this field of translational investigation may lead to tangible benefits to obese humans who are at increased risk from several cancers.
Acknowledgments
This work was supported by a discretionary grant from Beth Israel Deaconess Medical Center (to C.S.M.). The Mantzoros laboratory is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (Grants DK058785, DK079929, and DK081913), the National Institute on Aging (Grant AG032030), a VA Merit Award, and the California Walnut Commission.
Disclosure Summary: The authors have no conflict of interest to disclose.
Footnotes
- ACC
- Acetyl-coenzyme A carboxylase
- Acrp30
- adipocyte complement-related protein of 30 kDa
- ADIPOQ
- adiponectin
- AdipoR1/R2
- adiponectin receptor 1/2
- ALL
- acute lymphoblastic leukemia
- AMPK
- AMP-activated protein kinase
- AOM
- azoxymethane
- Apc
- adenomatous polyposis coli
- apM1
- gene product of the most abundant gene transcript-1
- Bax
- Bcl-2-associated X protein
- Bcl-2
- B-cell lymphoma-2
- B-CLL
- B-cell CLL
- BMI
- body mass index
- CC
- colorectal cancer
- CHC
- chronic hepatitis C
- CI
- confidence interval
- CLL
- chronic lymphocytic leukemia
- CML
- chronic myelogenous leukemia
- COX-2
- cyclooxygenase-2
- CRP
- C-reactive protein
- CTRP
- complement C1q/TNF-related protein
- EA
- esophageal adenocarcinoma
- EGFR
- epidermal growth factor receptor
- ER
- estrogen receptor
- ESCC
- esophageal squamous cell carcinoma
- fAd
- full-length adiponectin
- gAd
- globular adiponectin
- GBP28
- gelatin-binding protein 28
- GSK-3β
- glycogen synthase kinase-3β
- HCC
- hepatocellular carcinoma
- HL
- Hodgkin lymphoma
- HMW
- high molecular weight
- IGFBP
- IGF binding protein
- IR
- insulin receptor
- IRS-1
- insulin receptor substrate-1
- JAK
- Janus kinase
- JNK
- c-Jun N-terminal kinase
- LDH
- lactate dehydrogenase
- LKB1
- liver kinase B1
- LMW
- low molecular weight
- MDS
- myelodysplastic syndrome
- MGUS
- monoclonal gammopathy of undetermined significance
- MLMW
- middle and low molecular weight
- MM
- multiple myeloma
- MMP
- matrix metalloproteinase
- MMTV-PyVmT
- mouse mammary tumor virus-polyoma middle T antigen
- MPD
- myeloproliferative disorders
- mTOR
- mammalian target of rapamycin
- NF-κB
- nuclear factor-κB
- NHL
- non-Hodgkin lymphoma
- NSCLC
- non-small-cell lung carcinoma
- OR
- odds ratio
- PaC
- pancreatic cancer
- PI3K
- phosphatidylinositol 3-kinase
- PKA
- protein kinase A
- PP2A
- protein phosphatase 2A
- PPAR-α/-γ
- peroxisome proliferator-activator receptor-α/-γ
- PR
- progesterone receptor
- PTEN
- phosphatase and tensin homolog
- ROS
- reactive oxygen species
- S1P
- sphingosine 1 phosphate
- SNP
- single nucleotide polymorphism
- SPPARM
- selective PPAR-γ agonist
- STAT3
- signal transducer and activator of transcription
- TSC2
- tuberous sclerosis complex 2
- VEGF
- vascular endothelial growth factor
- WHR
- waist-to-hip ratio
- Wnt
- wingless-type protein.
References
- 1. Hubert HB, Feinleib M, McNamara PM, Castelli WP. 1983. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 67:968–977 [DOI] [PubMed] [Google Scholar]
- 2. Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. 2003. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289:76–79 [DOI] [PubMed] [Google Scholar]
- 3. Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. 2006. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555 [DOI] [PubMed] [Google Scholar]
- 4. Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. 2007. The epidemiology of obesity. Gastroenterology 132:2087–2102 [DOI] [PubMed] [Google Scholar]
- 5. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. 2008. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371:569–578 [DOI] [PubMed] [Google Scholar]
- 6. Pischon T, Nöthlings U, Boeing H. 2008. Obesity and cancer. Proc Nutr Soc 67:128–145 [DOI] [PubMed] [Google Scholar]
- 7. Larsson SC, Orsini N, Wolk A. 2007. Body mass index and pancreatic cancer risk: a meta-analysis of prospective studies. Int J Cancer 120:1993–1998 [DOI] [PubMed] [Google Scholar]
- 8. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. 2003. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348:1625–1638 [DOI] [PubMed] [Google Scholar]
- 9. Lichtman MA. 2010. Obesity and the risk for a hematological malignancy: leukemia, lymphoma, or myeloma. Oncologist 15:1083–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wiseman M. 2008. The Second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc 67:253–256 [DOI] [PubMed] [Google Scholar]
- 11. Hsing AW, Sakoda LC, Chua S., Jr 2007. Obesity, metabolic syndrome, and prostate cancer. Am J Clin Nutr 86:s843–s857 [DOI] [PubMed] [Google Scholar]
- 12. Calle EE, Kaaks R. 2004. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4:579–591 [DOI] [PubMed] [Google Scholar]
- 13. Park J, Euhus DM, Scherer PE. 2011. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr Rev 32:550–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Kruijsdijk RC, van der Wall E, Visseren FL. 2009. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev 18:2569–2578 [DOI] [PubMed] [Google Scholar]
- 15. Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. 2007. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr 86:s858–s866 [DOI] [PubMed] [Google Scholar]
- 16. Ziemke F, Mantzoros CS. 2010. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr 91:258S–261S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kelesidis I, Kelesidis T, Mantzoros CS. 2006. Adiponectin and cancer: a systematic review. Br J Cancer 94:1221–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gallagher EJ, LeRoith D. 2011. Minireview: IGF, insulin, and cancer. Endocrinology 152:2546–2551 [DOI] [PubMed] [Google Scholar]
- 19. MacDougald OA, Burant CF. 2007. The rapidly expanding family of adipokines. Cell Metab 6:159–161 [DOI] [PubMed] [Google Scholar]
- 20. Hivert MF, Sullivan LM, Fox CS, Nathan DM, D'Agostino RB, Sr, Wilson PW, Meigs JB. 2008. Associations of adiponectin, resistin, and tumor necrosis factor-α with insulin resistance. J Clin Endocrinol Metab 93:3165–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. 2001. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86:1930–1935 [DOI] [PubMed] [Google Scholar]
- 22. Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. 2000. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20:1595–1599 [DOI] [PubMed] [Google Scholar]
- 23. Paz-Filho G, Lim EL, Wong ML, Licinio J. 2011. Associations between adipokines and obesity-related cancer. Front Biosci 16:1634–1650 [DOI] [PubMed] [Google Scholar]
- 24. Hu E, Liang P, Spiegelman BM. 1996. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271:10697–10703 [DOI] [PubMed] [Google Scholar]
- 25. Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. 1995. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270:26746–26749 [DOI] [PubMed] [Google Scholar]
- 26. Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. 1996. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun 221:286–289 [DOI] [PubMed] [Google Scholar]
- 27. Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. 1996. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem 120:803–812 [DOI] [PubMed] [Google Scholar]
- 28. Chandran M, Phillips SA, Ciaraldi T, Henry RR. 2003. Adiponectin: more than just another fat cell hormone? Diabetes Care 26:2442–2450 [DOI] [PubMed] [Google Scholar]
- 29. Garaulet M, Hernández-Morante JJ, de Heredia FP, Tébar FJ. 2007. Adiponectin, the controversial hormone. Public Health Nutr 10:1145–1150 [DOI] [PubMed] [Google Scholar]
- 30. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. 1999. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257:79–83 [DOI] [PubMed] [Google Scholar]
- 31. Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. 2004. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci USA 101:10302–10307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. 2008. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-γ agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J 416:161–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. 2003. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423:762–769 [DOI] [PubMed] [Google Scholar]
- 34. Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. 2004. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA 101:10308–10313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takahashi M, Arita Y, Yamagata K, Matsukawa Y, Okutomi K, Horie M, Shimomura I, Hotta K, Kuriyama H, Kihara S, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. 2000. Genomic structure and mutations in adipose-specific gene, adiponectin. Int J Obes Relat Metab Disord 24:861–868 [DOI] [PubMed] [Google Scholar]
- 36. Kissebah AH, Sonnenberg GE, Myklebust J, Goldstein M, Broman K, James RG, Marks JA, Krakower GR, Jacob HJ, Weber J, Martin L, Blangero J, Comuzzie AG. 2000. Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proc Natl Acad Sci USA 97:14478–14483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Menzaghi C, Ercolino T, Di Paola R, Berg AH, Warram JH, Scherer PE, Trischitta V, Doria A. 2002. A haplotype at the adiponectin locus is associated with obesity and other features of the insulin resistance syndrome. Diabetes 51:2306–2312 [DOI] [PubMed] [Google Scholar]
- 38. Kondo H, Shimomura I, Matsukawa Y, Kumada M, Takahashi M, Matsuda M, Ouchi N, Kihara S, Kawamoto T, Sumitsuji S, Funahashi T, Matsuzawa Y. 2002. Association of adiponectin mutation with type 2 diabetes: a candidate gene for the insulin resistance syndrome. Diabetes 51:2325–2328 [DOI] [PubMed] [Google Scholar]
- 39. Kaklamani VG, Sadim M, Hsi A, Offit K, Oddoux C, Ostrer H, Ahsan H, Pasche B, Mantzoros C. 2008. Variants of the adiponectin and adiponectin receptor 1 genes and breast cancer risk. Cancer Res 68:3178–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kadowaki T, Yamauchi T. 2005. Adiponectin and adiponectin receptors. Endocr Rev 26:439–451 [DOI] [PubMed] [Google Scholar]
- 41. Delaigle AM, Jonas JC, Bauche IB, Cornu O, Brichard SM. 2004. Induction of adiponectin in skeletal muscle by inflammatory cytokines: in vivo and in vitro studies. Endocrinology 145:5589–5597 [DOI] [PubMed] [Google Scholar]
- 42. Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, Ebenbichler CF, Patsch JR, Tilg H. 2005. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut 54:117–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fayad R, Pini M, Sennello JA, Cabay RJ, Chan L, Xu A, Fantuzzi G. 2007. Adiponectin deficiency protects mice from chemically induced colonic inflammation. Gastroenterology 132:601–614 [DOI] [PubMed] [Google Scholar]
- 44. Piñeiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, Diéguez C, Gualillo O, González-Juanatey JR, Lago F. 2005. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett 579:5163–5169 [DOI] [PubMed] [Google Scholar]
- 45. Katsiougiannis S, Kapsogeorgou EK, Manoussakis MN, Skopouli FN. 2006. Salivary gland epithelial cells: a new source of the immunoregulatory hormone adiponectin. Arthritis Rheum 54:2295–2299 [DOI] [PubMed] [Google Scholar]
- 46. Brochu-Gaudreau K, Rehfeldt C, Blouin R, Bordignon V, Murphy BD, Palin MF. 2010. Adiponectin action from head to toe. Endocrine 37:11–32 [DOI] [PubMed] [Google Scholar]
- 47. Chen J, Tan B, Karteris E, Zervou S, Digby J, Hillhouse EW, Vatish M, Randeva HS. 2006. Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines. Diabetologia 49:1292–1302 [DOI] [PubMed] [Google Scholar]
- 48. Kusminski CM, McTernan PG, Schraw T, Kos K, O'Hare JP, Ahima R, Kumar S, Scherer PE. 2007. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia 50:634–642 [DOI] [PubMed] [Google Scholar]
- 49. Bronsky J, Karpísek M, Bronská E, Pechová M, Jancíková B, Kotolová H, Stejskal D, Prusa R, Nevoral J. 2006. Adiponectin, adipocyte fatty acid binding protein, and epidermal fatty acid binding protein: proteins newly identified in human breast milk. Clin Chem 52:1763–1770 [DOI] [PubMed] [Google Scholar]
- 50. Simpson F, Whitehead JP. 2010. Adiponectin—it's all about the modifications. Int J Biochem Cell Biol 42:785–788 [DOI] [PubMed] [Google Scholar]
- 51. Ruan H, Lodish HF. 2003. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-α. Cytokine Growth Factor Rev 14:447–455 [DOI] [PubMed] [Google Scholar]
- 52. Fisher FM, Trujillo ME, Hanif W, Barnett AH, McTernan PG, Scherer PE, Kumar S. 2005. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia 48:1084–1087 [DOI] [PubMed] [Google Scholar]
- 53. Tsao TS, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, Heuser JE, Lodish HF. 2003. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem 278:50810–50817 [DOI] [PubMed] [Google Scholar]
- 54. Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. 2002. Oligomerization state-dependent activation of NF-κB signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30). J Biol Chem 277:29359–29362 [DOI] [PubMed] [Google Scholar]
- 55. Wang Y, Xu A, Knight C, Xu LY, Cooper GJ. 2002. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem 277:19521–19529 [DOI] [PubMed] [Google Scholar]
- 56. Shapiro L, Scherer PE. 1998. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr Biol 8:335–338 [DOI] [PubMed] [Google Scholar]
- 57. Wang Y, Lam KS, Chan L, Chan KW, Lam JB, Lam MC, Hoo RC, Mak WW, Cooper GJ, Xu A. 2006. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem 281:16391–16400 [DOI] [PubMed] [Google Scholar]
- 58. Peake PW, Kriketos AD, Campbell LV, Shen Y, Charlesworth JA. 2005. The metabolism of isoforms of human adiponectin: studies in human subjects and in experimental animals. Eur J Endocrinol 153:409–417 [DOI] [PubMed] [Google Scholar]
- 59. Waki H, Yamauchi T, Kamon J, Kita S, Ito Y, Hada Y, Uchida S, Tsuchida A, Takekawa S, Kadowaki T. 2005. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology 146:790–796 [DOI] [PubMed] [Google Scholar]
- 60. Goldstein BJ, Scalia R. 2004. Adiponectin: a novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab 89:2563–2568 [DOI] [PubMed] [Google Scholar]
- 61. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. 2006. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116:1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. 2001. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA 98:2005–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. 2003. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem 278:9073–9085 [DOI] [PubMed] [Google Scholar]
- 64. Blüher M, Brennan AM, Kelesidis T, Kratzsch J, Fasshauer M, Kralisch S, Williams CJ, Mantzoros CS. 2007. Total and high-molecular weight adiponectin in relation to metabolic variables at baseline and in response to an exercise treatment program: comparative evaluation of three assays. Diabetes Care 30:280–285 [DOI] [PubMed] [Google Scholar]
- 65. Heidemann C, Sun Q, van Dam RM, Meigs JB, Zhang C, Tworoger SS, Mantzoros CS, Hu FB. 2008. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med 149:307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. 2004. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279:12152–12162 [DOI] [PubMed] [Google Scholar]
- 67. Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. 2001. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7:947–953 [DOI] [PubMed] [Google Scholar]
- 68. Trujillo ME, Scherer PE. 2005. Adiponectin—journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med 257:167–175 [DOI] [PubMed] [Google Scholar]
- 69. Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. 2004. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem 279:1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hada Y, Yamauchi T, Waki H, Tsuchida A, Hara K, Yago H, Miyazaki O, Ebinuma H, Kadowaki T. 2007. Selective purification and characterization of adiponectin multimer species from human plasma. Biochem Biophys Res Commun 356:487–493 [DOI] [PubMed] [Google Scholar]
- 71. Tilg H, Moschen AR. 2006. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6:772–783 [DOI] [PubMed] [Google Scholar]
- 72. Kharroubi I, Rasschaert J, Eizirik DL, Cnop M. 2003. Expression of adiponectin receptors in pancreatic β cells. Biochem Biophys Res Commun 312:1118–1122 [DOI] [PubMed] [Google Scholar]
- 73. Chinetti G, Zawadski C, Fruchart JC, Staels B. 2004. Expression of adiponectin receptors in human macrophages and regulation by agonists of the nuclear receptors PPARα, PPARγ, and LXR. Biochem Biophys Res Commun 314:151–158 [DOI] [PubMed] [Google Scholar]
- 74. Tsuchida A, Yamauchi T, Ito Y, Hada Y, Maki T, Takekawa S, Kamon J, Kobayashi M, Suzuki R, Hara K, Kubota N, Terauchi Y, Froguel P, Nakae J, Kasuga M, Accili D, Tobe K, Ueki K, Nagai R, Kadowaki T. 2004. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem 279:30817–30822 [DOI] [PubMed] [Google Scholar]
- 75. Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. 2000. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation 102:1296–1301 [DOI] [PubMed] [Google Scholar]
- 76. Blüher M, Bullen JW, Jr, Lee JH, Kralisch S, Fasshauer M, Klöting N, Niebauer J, Schön MR, Williams CJ, Mantzoros CS. 2006. Circulating adiponectin and expression of adiponectin receptors in human skeletal muscle: associations with metabolic parameters and insulin resistance and regulation by physical training. J Clin Endocrinol Metab 91:2310–2316 [DOI] [PubMed] [Google Scholar]
- 77. Blüher M, Williams CJ, Klöting N, Hsi A, Ruschke K, Oberbach A, Fasshauer M, Berndt J, Schön MR, Wolk A, Stumvoll M, Mantzoros CS. 2007. Gene expression of adiponectin receptors in human visceral and subcutaneous adipose tissue is related to insulin resistance and metabolic parameters and is altered in response to physical training. Diabetes Care 30:3110–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bullen JW, Jr, Bluher S, Kelesidis T, Mantzoros CS. 2007. Regulation of adiponectin and its receptors in response to development of diet-induced obesity in mice. Am J Physiol Endocrinol Metab 292:E1079–E1086 [DOI] [PubMed] [Google Scholar]
- 79. Blüher S, Ziotopoulou M, Bullen JW, Jr, Moschos SJ, Ungsunan L, Kokkotou E, Maratos-Flier E, Mantzoros CS. 2004. Responsiveness to peripherally administered melanocortins in lean and obese mice. Diabetes 53:82–90 [DOI] [PubMed] [Google Scholar]
- 80. Inukai K, Nakashima Y, Watanabe M, Takata N, Sawa T, Kurihara S, Awata T, Katayama S. 2005. Regulation of adiponectin receptor gene expression in diabetic mice. Am J Physiol Endocrinol Metab 288:E876–E882 [DOI] [PubMed] [Google Scholar]
- 81. Blüher S, Bullen J, Mantzoros CS. 2008. Altered levels of adiponectin and adiponectin receptors may underlie the effect of ciliary neurotrophic factor (CNTF) to enhance insulin sensitivity in diet-induced obese mice. Horm Metab Res 40:225–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tsuchida A, Yamauchi T, Takekawa S, Hada Y, Ito Y, Maki T, Kadowaki T. 2005. Peroxisome proliferator-activated receptor (PPAR)α activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARα, PPARγ, and their combination. Diabetes 54:3358–3370 [DOI] [PubMed] [Google Scholar]
- 83. Kaltenbach S, Staiger H, Weisser M, Haas C, Stumvoll M, Machicao F, Häring HU. 2005. Adiponectin receptor gene expression in human skeletal muscle cells is not regulated by fibrates and thiazolidinediones. Int J Obes (Lond) 29:760–765 [DOI] [PubMed] [Google Scholar]
- 84. Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. 2011. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 17:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Villa NY, Kupchak BR, Garitaonandia I, Smith JL, Alonso E, Alford C, Cowart LA, Hannun YA, Lyons TJ. 2009. Sphingolipids function as downstream effectors of a fungal PAQR. Mol Pharmacol 75:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Takabe K, Paugh SW, Milstien S, Spiegel S. 2008. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev 60:181–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hebbard LW, Garlatti M, Young LJ, Cardiff RD, Oshima RG, Ranscht B. 2008. T-Cadherin supports angiogenesis and adiponectin association with the vasculature in a mouse mammary tumor model. Cancer Res 68:1407–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lee MH, Klein RL, El-Shewy HM, Luttrell DK, Luttrell LM. 2008. The adiponectin receptors AdipoR1 and AdipoR2 activate ERK1/2 through a Src/Ras-dependent pathway and stimulate cell growth. Biochemistry 47:11682–11692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mistry T, Digby JE, Chen J, Desai KM, Randeva HS. 2006. The regulation of adiponectin receptors in human prostate cancer cell lines. Biochem Biophys Res Commun 348:832–838 [DOI] [PubMed] [Google Scholar]
- 90. Wu X, Yan Q, Zhang Z, Du G, Wan X. 2012. Acrp30 inhibits leptin-induced metastasis by downregulating the JAK/STAT3 pathway via AMPK activation in aggressive SPEC-2 endometrial cancer cells. Oncol Rep 27:1488–1496 [DOI] [PubMed] [Google Scholar]
- 91. Takahata C, Miyoshi Y, Irahara N, Taguchi T, Tamaki Y, Noguchi S. 2007. Demonstration of adiponectin receptors 1 and 2 mRNA expression in human breast cancer cells. Cancer Lett 250:229–236 [DOI] [PubMed] [Google Scholar]
- 92. Takemura Y, Osuga Y, Yamauchi T, Kobayashi M, Harada M, Hirata T, Morimoto C, Hirota Y, Yoshino O, Koga K, Yano T, Kadowaki T, Taketani Y. 2006. Expression of adiponectin receptors and its possible implication in the human endometrium. Endocrinology 147:3203–3210 [DOI] [PubMed] [Google Scholar]
- 93. Kim AY, Lee YS, Kim KH, Lee JH, Lee HK, Jang SH, Kim SE, Lee GY, Lee JW, Jung SA, Chung HY, Jeong S, Kim JB. 2010. Adiponectin represses colon cancer cell proliferation via AdipoR1- and -R2-mediated AMPK activation. Mol Endocrinol 24:1441–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Petridou ET, Mitsiades N, Gialamas S, Angelopoulos M, Skalkidou A, Dessypris N, Hsi A, Lazaris N, Polyzos A, Syrigos C, Brennan AM, Tseleni-Balafouta S, Mantzoros CS. 2007. Circulating adiponectin levels and expression of adiponectin receptors in relation to lung cancer: two case-control studies. Oncology 73:261–269 [DOI] [PubMed] [Google Scholar]
- 95. Dalamaga M, Migdalis I, Fargnoli JL, Papadavid E, Bloom E, Mitsiades N, Karmaniolas K, Pelecanos N, Tseleni-Balafouta S, Dionyssiou-Asteriou A, Mantzoros CS. 2009. Pancreatic cancer expresses adiponectin receptors and is associated with hypoleptinemia and hyperadiponectinemia: a case-control study. Cancer Causes Control 20:625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Körner A, Pazaitou-Panayiotou K, Kelesidis T, Kelesidis I, Williams CJ, Kaprara A, Bullen J, Neuwirth A, Tseleni S, Mitsiades N, Kiess W, Mantzoros CS. 2007. Total and high-molecular-weight adiponectin in breast cancer: in vitro and in vivo studies. J Clin Endocrinol Metab 92:1041–1048 [DOI] [PubMed] [Google Scholar]
- 97. Bråkenhielm E, Veitonmäki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, Funahashi T, Cao Y. 2004. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci USA 101:2476–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Miyazaki T, Bub JD, Uzuki M, Iwamoto Y. 2005. Adiponectin activates c-Jun NH2-terminal kinase and inhibits signal transducer and activator of transcription 3. Biochem Biophys Res Commun 333:79–87 [DOI] [PubMed] [Google Scholar]
- 99. Holland WL, Scherer PE. 2009. PAQRs: a counteracting force to ceramides? Mol Pharmacol 75:740–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA. 2011. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest 121:1858–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. 2007. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5:167–179 [DOI] [PubMed] [Google Scholar]
- 102. Zhao H, Przybylska M, Wu IH, Zhang J, Siegel C, Komarnitsky S, Yew NS, Cheng SH. 2007. Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. Diabetes 56:1210–1218 [DOI] [PubMed] [Google Scholar]
- 103. Levine YC, Li GK, Michel T. 2007. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK→Rac1→Akt→endothelial nitric-oxide synthase pathway. J Biol Chem 282:20351–20364 [DOI] [PubMed] [Google Scholar]
- 104. Buechler C, Wanninger J, Neumeier M. 2010. Adiponectin receptor binding proteins—recent advances in elucidating adiponectin signalling pathways. FEBS Lett 584:4280–4286 [DOI] [PubMed] [Google Scholar]
- 105. Deepa SS, Dong LQ. 2009. APPL1: role in adiponectin signaling and beyond. Am J Physiol Endocrinol Metab 296:E22–E36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Schenck A, Goto-Silva L, Collinet C, Rhinn M, Giner A, Habermann B, Brand M, Zerial M. 2008. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell 133:486–497 [DOI] [PubMed] [Google Scholar]
- 107. Liu J, Yao F, Wu R, Morgan M, Thorburn A, Finley RL, Jr, Chen YQ. 2002. Mediation of the DCC apoptotic signal by DIP13 α. J Biol Chem 277:26281–26285 [DOI] [PubMed] [Google Scholar]
- 108. Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, Zerial M. 2004. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell 116:445–456 [DOI] [PubMed] [Google Scholar]
- 109. Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ. 2006. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 8:516–523 [DOI] [PubMed] [Google Scholar]
- 110. Hardie DG, Scott JW, Pan DA, Hudson ER. 2003. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett 546:113–120 [DOI] [PubMed] [Google Scholar]
- 111. Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. 2001. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941–946 [DOI] [PubMed] [Google Scholar]
- 112. Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. 2007. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 13:332–339 [DOI] [PubMed] [Google Scholar]
- 113. Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. 2002. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8:1288–1295 [DOI] [PubMed] [Google Scholar]
- 114. Miller RA, Chu Q, Le Lay J, Scherer PE, Ahima RS, Kaestner KH, Foretz M, Viollet B, Birnbaum MJ. 2011. Adiponectin suppresses gluconeogenic gene expression in mouse hepatocytes independent of LKB1-AMPK signaling. J Clin Invest 121:2518–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Berg AH, Combs TP, Scherer PE. 2002. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab 13:84–89 [DOI] [PubMed] [Google Scholar]
- 116. Zhou L, Deepa SS, Etzler JC, Ryu J, Mao X, Fang Q, Liu DD, Torres JM, Jia W, Lechleiter JD, Liu F, Dong LQ. 2009. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J Biol Chem 284:22426–22435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Inoki K, Zhu T, Guan KL. 2003. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115:577–590 [DOI] [PubMed] [Google Scholar]
- 118. Luo Z, Saha AK, Xiang X, Ruderman NB. 2005. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci 26:69–76 [DOI] [PubMed] [Google Scholar]
- 119. Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D, Matsumoto K, Toyonaga T, Asano T, Nishikawa T, Araki E. 2005. Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res 97:837–844 [DOI] [PubMed] [Google Scholar]
- 120. Chandrasekar B, Boylston WH, Venkatachalam K, Webster NJ, Prabhu SD, Valente AJ. 2008. Adiponectin blocks interleukin-18-mediated endothelial cell death via APPL1-dependent AMP-activated protein kinase (AMPK) activation and IKK/NF-κB/PTEN suppression. J Biol Chem 283:24889–24898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22:153–183 [DOI] [PubMed] [Google Scholar]
- 122. Wang C, Mao X, Wang L, Liu M, Wetzel MD, Guan KL, Dong LQ, Liu F. 2007. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J Biol Chem 282:7991–7996 [DOI] [PubMed] [Google Scholar]
- 123. Stefan N, Vozarova B, Funahashi T, Matsuzawa Y, Weyer C, Lindsay RS, Youngren JF, Havel PJ, Pratley RE, Bogardus C, Tataranni PA. 2002. Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes 51:1884–1888 [DOI] [PubMed] [Google Scholar]
- 124. Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. 1999. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100:2473–2476 [DOI] [PubMed] [Google Scholar]
- 125. Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y. 2001. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation 103:1057–1063 [DOI] [PubMed] [Google Scholar]
- 126. Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. 2003. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem 278:45021–45026 [DOI] [PubMed] [Google Scholar]
- 127. Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M, Nakamura T, Shimomura I, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. 2002. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation 105:2893–2898 [DOI] [PubMed] [Google Scholar]
- 128. Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. 2007. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 6:55–68 [DOI] [PubMed] [Google Scholar]
- 129. Corbetta S, Bulfamante G, Cortelazzi D, Barresi V, Cetin I, Mantovani G, Bondioni S, Beck-Peccoz P, Spada A. 2005. Adiponectin expression in human fetal tissues during mid- and late gestation. J Clin Endocrinol Metab 90:2397–2402 [DOI] [PubMed] [Google Scholar]
- 130. Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. 2003. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 46:459–469 [DOI] [PubMed] [Google Scholar]
- 131. Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, Mantzoros CS. 2003. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab 88:4823–4831 [DOI] [PubMed] [Google Scholar]
- 132. Qiao L, Shao J. 2006. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein α transcriptional complex. J Biol Chem 281:39915–39924 [DOI] [PubMed] [Google Scholar]
- 133. Bauche IB, Ait El Mkadem S, Rezsohazy R, Funahashi T, Maeda N, Miranda LM, Brichard SM. 2006. Adiponectin downregulates its own production and the expression of its AdipoR2 receptor in transgenic mice. Biochem Biophys Res Commun 345:1414–1424 [DOI] [PubMed] [Google Scholar]
- 134. Hivert MF, Sun Q, Shrader P, Mantzoros CS, Meigs JB, Hu FB. 2011. Higher adiponectin levels predict greater weight gain in healthy women in the Nurses' Health Study. Obesity (Silver Spring) 19:409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. 2006. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr 84:1033–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Christou NV, Lieberman M, Sampalis F, Sampalis JS. 2008. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg Obes Relat Dis 4:691–695 [DOI] [PubMed] [Google Scholar]
- 137. Sjöström L, Gummesson A, Sjöström CD, Narbro K, Peltonen M, Wedel H, Bengtsson C, Bouchard C, Carlsson B, Dahlgren S, Jacobson P, Karason K, Karlsson J, Larsson B, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Carlsson LM. 2009. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol 10:653–662 [DOI] [PubMed] [Google Scholar]
- 138. Rokling-Andersen MH, Reseland JE, Veierød MB, Anderssen SA, Jacobs DR, Jr, Urdal P, Jansson JO, Drevon CA. 2007. Effects of long-term exercise and diet intervention on plasma adipokine concentrations. Am J Clin Nutr 86:1293–1301 [DOI] [PubMed] [Google Scholar]
- 139. Friedenreich CM, Neilson HK, Woolcott CG, McTiernan A, Wang Q, Ballard-Barbash R, Jones CA, Stanczyk FZ, Brant RF, Yasui Y, Irwin ML, Campbell KL, McNeely ML, Karvinen KH, Courneya KS. 2011. Changes in insulin resistance indicators, IGFs, and adipokines in a year-long trial of aerobic exercise in postmenopausal women. Endocr Relat Cancer 18:357–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Moghadasi M, Mohebbi H, Rahmani-Nia F, Hassan-Nia S, Noroozi H, Pirooznia N. 2012. High-intensity endurance training improves adiponectin mRNA and plasma concentrations. Eur J Appl Physiol 112:1207–1214 [DOI] [PubMed] [Google Scholar]
- 141. Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. 2003. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab 88:2838–2843 [DOI] [PubMed] [Google Scholar]
- 142. Gale SM, Castracane VD, Mantzoros CS. 2004. Energy homeostasis, obesity and eating disorders: recent advances in endocrinology. J Nutr 134:295–298 [DOI] [PubMed] [Google Scholar]
- 143. Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, Matsuda M, Kondo H, Furuyama N, Kihara S, Nakamura T, Tochino Y, Funahashi T, Matsuzawa Y. 2002. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes 51:2734–2741 [DOI] [PubMed] [Google Scholar]
- 144. Page ST, Herbst KL, Amory JK, Coviello AD, Anawalt BD, Matsumoto AM, Bremner WJ. 2005. Testosterone administration suppresses adiponectin levels in men. J Androl 26:85–92 [PubMed] [Google Scholar]
- 145. Xu A, Chan KW, Hoo RL, Wang Y, Tan KC, Zhang J, Chen B, Lam MC, Tse C, Cooper GJ, Lam KS. 2005. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem 280:18073–18080 [DOI] [PubMed] [Google Scholar]
- 146. Siemińska L, Lenart J, Cichoń-Lenart A, Niedziołka D, Marek B, Kos-Kudła B, Kajdaniuk D, Nowak M. 2005. [Serum adiponectin levels in patients with acromegaly]. Pol Merkur Lekarski 19:514–516 [PubMed] [Google Scholar]
- 147. Sieminska L, Wojciechowska C, Niedziolka D, Marek B, Kos-Kudla B, Kajdaniuk D, Nowak M. 2005. Effect of postmenopause and hormone replacement therapy on serum adiponectin levels. Metabolism 54:1610–1614 [DOI] [PubMed] [Google Scholar]
- 148. Tworoger SS, Eliassen AH, Kelesidis T, Colditz GA, Willett WC, Mantzoros CS, Hankinson SE. 2007. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab 92:1510–1516 [DOI] [PubMed] [Google Scholar]
- 149. Hroussalas G, Kassi E, Dalamaga M, Delimaris I, Zachari A, Dionyssiou-Asteriou A. 2008. Leptin, soluble leptin receptor, adiponectin and resistin in relation to OGTT in overweight/obese postmenopausal women. Maturitas 59:339–349 [DOI] [PubMed] [Google Scholar]
- 150. Swarbrick MM, Havel PJ. 2008. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndr Relat Disord 6:87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Pischon T, Girman CJ, Rifai N, Hotamisligil GS, Rimm EB. 2005. Association between dietary factors and plasma adiponectin concentrations in men. Am J Clin Nutr 81:780–786 [DOI] [PubMed] [Google Scholar]
- 152. Qi L, Rimm E, Liu S, Rifai N, Hu FB. 2005. Dietary glycemic index, glycemic load, cereal fiber, and plasma adiponectin concentration in diabetic men. Diabetes Care 28:1022–1028 [DOI] [PubMed] [Google Scholar]
- 153. Mantzoros CS, Williams CJ, Manson JE, Meigs JB, Hu FB. 2006. Adherence to the Mediterranean dietary pattern is positively associated with plasma adiponectin concentrations in diabetic women. Am J Clin Nutr 84:328–335 [DOI] [PubMed] [Google Scholar]
- 154. Fargnoli JL, Fung TT, Olenczuk DM, Chamberland JP, Hu FB, Mantzoros CS. 2008. Adherence to healthy eating patterns is associated with higher circulating total and high-molecular-weight adiponectin and lower resistin concentrations in women from the Nurses' Health Study. Am J Clin Nutr 88:1213–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Williams CJ, Fargnoli JL, Hwang JJ, van Dam RM, Blackburn GL, Hu FB, Mantzoros CS. 2008. Coffee consumption is associated with higher plasma adiponectin concentrations in women with or without type 2 diabetes: a prospective cohort study. Diabetes Care 31:504–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Wedick NM, Brennan AM, Sun Q, Hu FB, Mantzoros CS, van Dam RM. 2011. Effects of caffeinated and decaffeinated coffee on biological risk factors for type 2 diabetes: a randomized controlled trial. Nutr J 10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Yannakoulia M, Yiannakouris N, Melistas L, Fappa E, Vidra N, Kontogianni MD, Mantzoros CS. 2008. Dietary factors associated with plasma high molecular weight and total adiponectin levels in apparently healthy women. Eur J Endocrinol 159:R5–R10 [DOI] [PubMed] [Google Scholar]
- 158. Aronis KN, Vamvini MT, Chamberland JP, Sweeney LL, Brennan AM, Magkos F, Mantzoros CS. 2012. Short-term walnut consumption increases circulating total adiponectin and apolipoprotein A concentrations, but does not affect markers of inflammation or vascular injury in obese humans with the metabolic syndrome: data from a double-blinded, randomized, placebo-controlled study. Metabolism 61:577–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Schulze MB, Rimm EB, Shai I, Rifai N, Hu FB. 2004. Relationship between adiponectin and glycemic control, blood lipids, and inflammatory markers in men with type 2 diabetes. Diabetes Care 27:1680–1687 [DOI] [PubMed] [Google Scholar]
- 160. Kassi E, Dalamaga M, Hroussalas G, Kazanis K, Merantzi G, Zachari A, Giamarellos-Bourboulis EJ, Dionyssiou-Asteriou A. 2010. Adipocyte factors, high-sensitive C-reactive protein levels and lipoxidative stress products in overweight postmenopausal women with normal and impaired OGTT. Maturitas 67:72–77 [DOI] [PubMed] [Google Scholar]
- 161. Okada T, Saito E, Kuromori Y, Miyashita M, Iwata F, Hara M, Harada K. 2006. Relationship between serum adiponectin level and lipid composition in each lipoprotein fraction in adolescent children. Atherosclerosis 188:179–183 [DOI] [PubMed] [Google Scholar]
- 162. Nakamura T, Kawachi K, Saito Y, Saito T, Morishita K, Hoshino J, Hosoi T, Iwasaki T, Ohyama Y, Kurabayashi M. 2009. Effects of ARB or ACE-inhibitor administration on plasma levels of aldosterone and adiponectin in hypertension. Int Heart J 50:501–512 [DOI] [PubMed] [Google Scholar]
- 163. Wanders D, Plaisance EP, Judd RL. 2010. Pharmacological effects of lipid-lowering drugs on circulating adipokines. World J Diabetes 1:116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Banga A, Unal R, Tripathi P, Pokrovskaya I, Owens RJ, Kern PA, Ranganathan G. 2009. Adiponectin translation is increased by the PPARγ agonists pioglitazone and omega-3 fatty acids. Am J Physiol Endocrinol Metab 296:E480–E489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Combs TP, Wagner JA, Berger J, Doebber T, Wang WJ, Zhang BB, Tanen M, Berg AH, O'Rahilly S, Savage DB, Chatterjee K, Weiss S, Larson PJ, Gottesdiener KM, Gertz BJ, Charron MJ, Scherer PE, Moller DE. 2002. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARγ agonists: a potential mechanism of insulin sensitization. Endocrinology 143:998–1007 [DOI] [PubMed] [Google Scholar]
- 166. Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. 2001. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50:2094–2099 [DOI] [PubMed] [Google Scholar]
- 167. Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM. 2002. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes 51:2968–2974 [DOI] [PubMed] [Google Scholar]
- 168. Hanefeld M, Pfützner A, Forst T, Kleine I, Fuchs W. 2011. Double-blind, randomized, multicentre, and active comparator controlled investigation of the effect of pioglitazone, metformin, and the combination of both on cardiovascular risk in patients with type 2 diabetes receiving stable basal insulin therapy: the PIOCOMB study. Cardiovasc Diabetol 10:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Pajvani UB, Scherer PE. 2003. Adiponectin: systemic contributor to insulin sensitivity. Curr Diab Rep 3:207–213 [DOI] [PubMed] [Google Scholar]
- 170. Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. 2003. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem 278:40352–40363 [DOI] [PubMed] [Google Scholar]
- 171. Kang ES, Magkos F, Kim BS, Zhai R, Su L, Kim YS, Christiani DC, Lee HC, Mantzoros CS. 2012. Variants of the adiponectin and adiponectin receptor-1 Genes and posttransplantation diabetes mellitus in renal allograft recipients. J Clin Endocrinol Metab 97:E129–E135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Richards JB, Waterworth D, O'Rahilly S, Hivert MF, Loos RJ, Perry JR, Tanaka T, Timpson NJ, Semple RK, Soranzo N, Song K, Rocha N, Grundberg E, Dupuis J, Florez JC, Langenberg C, Prokopenko I, Saxena R, Sladek R, Aulchenko Y, Evans D, Waeber G, Erdmann J, Burnett MS, Sattar N, Devaney J, Willenborg C, Hingorani A, Witteman JC, Vollenweider P, Glaser B, Hengstenberg C, Ferrucci L, Melzer D, Stark K, Deanfield J, Winogradow J, Grassl M, Hall AS, Egan JM, Thompson JR, Ricketts SL, König IR, Reinhard W, Grundy S, Wichmann HE, Barter P, Mahley R, Kesaniemi YA, Rader DJ, Reilly MP, Epstein SE, Stewart AF, Van Duijn CM, Schunkert H, Burling K, Deloukas P, Pastinen T, Samani NJ, McPherson R, Davey Smith G, Frayling TM, Wareham NJ, Meigs JB, Mooser V, Spector TD. 2009. A genome-wide association study reveals variants in ARL15 that influence adiponectin levels. PLoS Genet 5:e1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Mittal P, Chaiworapongsa T, Kim SK, Pacora P, Yeo L, Gotsch F, Dong Z, Yoon BH, Hassan SS, Kusanovic JP. 2009. Maternal serum adiponectin multimers in gestational diabetes. J Perinat Med 37:637–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Li S, Shin HJ, Ding EL, van Dam RM. 2009. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 302:179–188 [DOI] [PubMed] [Google Scholar]
- 175. Schulze MB, Shai I, Rimm EB, Li T, Rifai N, Hu FB. 2005. Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes 54:534–539 [DOI] [PubMed] [Google Scholar]
- 176. Mantzoros CS, Li T, Manson JE, Meigs JB, Hu FB. 2005. Circulating adiponectin levels are associated with better glycemic control, more favorable lipid profile, and reduced inflammation in women with type 2 diabetes. J Clin Endocrinol Metab 90:4542–4548 [DOI] [PubMed] [Google Scholar]
- 177. Durante-Mangoni E, Zampino R, Marrone A, Tripodi MF, Rinaldi L, Restivo L, Cioffi M, Ruggiero G, Adinolfi LE. 2006. Hepatic steatosis and insulin resistance are associated with serum imbalance of adiponectin/tumour necrosis factor-α in chronic hepatitis C patients. Aliment Pharmacol Ther 24:1349–1357 [DOI] [PubMed] [Google Scholar]
- 178. Delporte ML, Brichard SM, Hermans MP, Beguin C, Lambert M. 2003. Hyperadiponectinaemia in anorexia nervosa. Clin Endocrinol (Oxf) 58:22–29 [DOI] [PubMed] [Google Scholar]
- 179. Ebina K, Fukuhara A, Ando W, Hirao M, Koga T, Oshima K, Matsuda M, Maeda K, Nakamura T, Ochi T, Shimomura I, Yoshikawa H, Hashimoto J. 2009. Serum adiponectin concentrations correlate with severity of rheumatoid arthritis evaluated by extent of joint destruction. Clin Rheumatol 28:445–451 [DOI] [PubMed] [Google Scholar]
- 180. Senolt L, Pavelka K, Housa D, Haluzík M. 2006. Increased adiponectin is negatively linked to the local inflammatory process in patients with rheumatoid arthritis. Cytokine 35:247–252 [DOI] [PubMed] [Google Scholar]
- 181. Zoccali C, Mallamaci F. 2011. Adiponectin and leptin in chronic kidney disease: causal factors or mere risk markers? J Ren Nutr 21:87–91 [DOI] [PubMed] [Google Scholar]
- 182. Chen MP, Tsai JC, Chung FM, Yang SS, Hsing LL, Shin SJ, Lee YJ. 2005. Hypoadiponectinemia is associated with ischemic cerebrovascular disease. Arterioscler Thromb Vasc Biol 25:821–826 [DOI] [PubMed] [Google Scholar]
- 183. Söderberg S, Stegmayr B, Stenlund H, Sjöström LG, Agren A, Johansson L, Weinehall L, Olsson T. 2004. Leptin, but not adiponectin, predicts stroke in males. J Intern Med 256:128–136 [DOI] [PubMed] [Google Scholar]
- 184. Briana DD, Malamitsi-Puchner A. 2009. Reviews: adipocytokines in normal and complicated pregnancies. Reprod Sci 16:921–937 [DOI] [PubMed] [Google Scholar]
- 185. Dalamaga M, Srinivas SK, Elovitz MA, Chamberland J, Mantzoros CS. 2011. Serum adiponectin and leptin in relation to risk for preeclampsia: results from a large case-control study. Metabolism 60:1539–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Housa D, Housová J, Vernerová Z, Haluzík M. 2006. Adipocytokines and cancer. Physiol Res 55:233–244 [DOI] [PubMed] [Google Scholar]
- 187. Petridou E, Mantzoros C, Dessypris N, Koukoulomatis P, Addy C, Voulgaris Z, Chrousos G, Trichopoulos D. 2003. Plasma adiponectin concentrations in relation to endometrial cancer: a case-control study in Greece. J Clin Endocrinol Metab 88:993–997 [DOI] [PubMed] [Google Scholar]
- 188. Dal Maso L, Augustin LS, Karalis A, Talamini R, Franceschi S, Trichopoulos D, Mantzoros CS, La Vecchia C. 2004. Circulating adiponectin and endometrial cancer risk. J Clin Endocrinol Metab 89:1160–1163 [DOI] [PubMed] [Google Scholar]
- 189. Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, Papadiamantis Y, Markopoulos C, Spanos E, Chrousos G, Trichopoulos D. 2004. Adiponectin and breast cancer risk. J Clin Endocrinol Metab 89:1102–1107 [DOI] [PubMed] [Google Scholar]
- 190. Dalamaga M, Karmaniolas K, Papadavid E, Pelekanos N, Sotiropoulos G, Lekka A. 2011. Elevated serum visfatin/nicotinamide phosphoribosyl-transferase levels are associated with risk of postmenopausal breast cancer independently from adiponectin, leptin, and anthropometric and metabolic parameters. Menopause 18:1198–1204 [DOI] [PubMed] [Google Scholar]
- 191. Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. 2005. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst 97:1688–1694 [DOI] [PubMed] [Google Scholar]
- 192. Spyridopoulos TN, Petridou ET, Skalkidou A, Dessypris N, Chrousos GP, Mantzoros CS. 2007. Low adiponectin levels are associated with renal cell carcinoma: a case-control study. Int J Cancer 120:1573–1578 [DOI] [PubMed] [Google Scholar]
- 193. Petridou E, Mantzoros CS, Dessypris N, Dikalioti SK, Trichopoulos D. 2006. Adiponectin in relation to childhood myeloblastic leukaemia. Br J Cancer 94:156–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Petridou ET, Sergentanis TN, Dessypris N, Vlachantoni IT, Tseleni-Balafouta S, Pourtsidis A, Moschovi M, Polychronopoulou S, Athanasiadou-Piperopoulou F, Kalmanti M, Mantzoros CS. 2009. Serum adiponectin as a predictor of childhood non-Hodgkin's lymphoma: a nationwide case-control study. J Clin Oncol 27:5049–5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Dalamaga M, Karmaniolas K, Panagiotou A, Hsi A, Chamberland J, Dimas C, Lekka A, Mantzoros CS. 2009. Low circulating adiponectin and resistin, but not leptin, levels are associated with multiple myeloma risk: a case-control study. Cancer Causes Control 20:193–199 [DOI] [PubMed] [Google Scholar]
- 196. Dalamaga M, Karmaniolas K, Nikolaidou A, Chamberland J, Hsi A, Dionyssiou-Asteriou A, Mantzoros CS. 2008. Adiponectin and resistin are associated with risk for myelodysplastic syndrome, independently from the insulin-like growth factor-I (IGF-I) system. Eur J Cancer 44:1744–1753 [DOI] [PubMed] [Google Scholar]
- 197. Dalamaga M, Nikolaidou A, Karmaniolas K, Hsi A, Chamberland J, Dionyssiou-Asteriou A, Mantzoros CS. 2007. Circulating adiponectin and leptin in relation to myelodysplastic syndrome: a case-control study. Oncology 73:26–32 [DOI] [PubMed] [Google Scholar]
- 198. Dalamaga M, Crotty BH, Fargnoli J, Papadavid E, Lekka A, Triantafilli M, Karmaniolas K, Migdalis I, Dionyssiou-Asteriou A, Mantzoros CS. 2010. B-cell chronic lymphocytic leukemia risk in association with serum leptin and adiponectin: a case-control study in Greece. Cancer Causes Control 21:1451–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Ishikawa M, Kitayama J, Kazama S, Hiramatsu T, Hatano K, Nagawa H. 2005. Plasma adiponectin and gastric cancer. Clin Cancer Res 11:466–472 [PubMed] [Google Scholar]
- 200. Goktas S, Yilmaz MI, Caglar K, Sonmez A, Kilic S, Bedir S. 2005. Prostate cancer and adiponectin. Urology 65:1168–1172 [DOI] [PubMed] [Google Scholar]
- 201. Giovannucci E. 1995. Insulin and colon cancer. Cancer Causes Control 6:164–179 [DOI] [PubMed] [Google Scholar]
- 202. Bianchini F, Kaaks R, Vainio H. 2002. Overweight, obesity, and cancer risk. Lancet Oncol 3:565–574 [DOI] [PubMed] [Google Scholar]
- 203. Pais R, Silaghi H, Silaghi AC, Rusu ML, Dumitrascu DL. 2009. Metabolic syndrome and risk of subsequent colorectal cancer. World J Gastroenterol 15:5141–5148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. An W, Bai Y, Deng SX, Gao J, Ben QW, Cai QC, Zhang HG, Li ZS. 2012. Adiponectin levels in patients with colorectal cancer and adenoma: a meta-analysis. Eur J Cancer Prev 21:126–133 [DOI] [PubMed] [Google Scholar]
- 205. Xu XT, Xu Q, Tong JL, Zhu MM, Huang ML, Ran ZH, Xiao SD. 2011. Meta-analysis: circulating adiponectin levels and risk of colorectal cancer and adenoma. J Dig Dis 12:234–244 [DOI] [PubMed] [Google Scholar]
- 206. Byeon JS, Jeong JY, Kim MJ, Lee SM, Nam WH, Myung SJ, Kim JG, Yang SK, Kim JH, Suh DJ. 2010. Adiponectin and adiponectin receptor in relation to colorectal cancer progression. Int J Cancer 127:2758–2767 [DOI] [PubMed] [Google Scholar]
- 207. Sugiyama M, Takahashi H, Hosono K, Endo H, Kato S, Yoneda K, Nozaki Y, Fujita K, Yoneda M, Wada K, Nakagama H, Nakajima A. 2009. Adiponectin inhibits colorectal cancer cell growth through the AMPK/mTOR pathway. Int J Oncol 34:339–344 [PubMed] [Google Scholar]
- 208. Williams CJ, Mitsiades N, Sozopoulos E, Hsi A, Wolk A, Nifli AP, Tseleni-Balafouta S, Mantzoros CS. 2008. Adiponectin receptor expression is elevated in colorectal carcinomas but not in gastrointestinal stromal tumors. Endocr Relat Cancer 15:289–299 [DOI] [PubMed] [Google Scholar]
- 209. Kumor A, Daniel P, Pietruczuk M, Małecka-Panas E. 2009. Serum leptin, adiponectin, and resistin concentration in colorectal adenoma and carcinoma (CC) patients. Int J Colorectal Dis 24:275–281 [DOI] [PubMed] [Google Scholar]
- 210. Gonullu G, Kahraman H, Bedir A, Bektas A, Yücel I. 2010. Association between adiponectin, resistin, insulin resistance, and colorectal tumors. Int J Colorectal Dis 25:205–212 [DOI] [PubMed] [Google Scholar]
- 211. Guadagni F, Roselli M, Martini F, Spila A, Riondino S, D'Alessandro R, Del Monte G, Formica V, Laudisi A, Portarena I, Palmirotta R, Ferroni P. 2009. Prognostic significance of serum adipokine levels in colorectal cancer patients. Anticancer Res 29:3321–3327 [PubMed] [Google Scholar]
- 212. Ferroni P, Palmirotta R, Spila A, Martini F, Raparelli V, Fossile E, Mariotti S, Del Monte G, Buonomo O, Roselli M, Guadagni F. 2007. Prognostic significance of adiponectin levels in non-metastatic colorectal cancer. Anticancer Res 27:483–489 [PubMed] [Google Scholar]
- 213. Erarslan E, Turkay C, Koktener A, Koca C, Uz B, Bavbek N. 2009. Association of visceral fat accumulation and adiponectin levels with colorectal neoplasia. Dig Dis Sci 54:862–868 [DOI] [PubMed] [Google Scholar]
- 214. Gialamas SP, Petridou ET, Tseleni-Balafouta S, Spyridopoulos TN, Matsoukis IL, Kondi-Pafiti A, Zografos G, Mantzoros CS. 2011. Serum adiponectin levels and tissue expression of adiponectin receptors are associated with risk, stage, and grade of colorectal cancer. Metabolism 60:1530–1538 [DOI] [PubMed] [Google Scholar]
- 215. Kemik O, Sumer A, Kemik AS, Hasirci I, Purisa S, Dulger AC, Demiriz B, Tuzun S. 2010. The relationship among acute-phase response proteins, cytokines and hormones in cachectic patients with colon cancer. World J Surg Oncol 8:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Otake S, Takeda H, Fujishima S, Fukui T, Orii T, Sato T, Sasaki Y, Nishise S, Kawata S. 2010. Decreased levels of plasma adiponectin associated with increased risk of colorectal cancer. World J Gastroenterol 16:1252–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Catalán V, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Silva C, Rotellar F, Hernández-Lizoain JL, Baixauli J, Valentí V, Pardo F, Salvador J, Frühbeck G. 2011. Up-regulation of the novel proinflammatory adipokines lipocalin-2, chitinase-3 like-1 and osteopontin as well as angiogenic-related factors in visceral adipose tissue of patients with colon cancer. J Nutr Biochem 22:634–641 [DOI] [PubMed] [Google Scholar]
- 218. Lukanova A, Söderberg S, Kaaks R, Jellum E, Stattin P. 2006. Serum adiponectin is not associated with risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 15:401–402 [DOI] [PubMed] [Google Scholar]
- 219. Nakajima TE, Yamada Y, Hamano T, Furuta K, Matsuda T, Fujita S, Kato K, Hamaguchi T, Shimada Y. 2010. Adipocytokines as new promising markers of colorectal tumors: adiponectin for colorectal adenoma, and resistin and visfatin for colorectal cancer. Cancer Sci 101:1286–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Stocks T, Lukanova A, Johansson M, Rinaldi S, Palmqvist R, Hallmans G, Kaaks R, Stattin P. 2008. Components of the metabolic syndrome and colorectal cancer risk; a prospective study. Int J Obes (Lond) 32:304–314 [DOI] [PubMed] [Google Scholar]
- 221. Levy M, Visokai V, Lipska L, Topolcan O. 2008. Tumor markers in staging and prognosis of colorectal carcinoma. Neoplasma 55:138–142 [PubMed] [Google Scholar]
- 222. Yamamoto N, Oshima T, Yoshihara K, Sato T, Yamada R, Fujii S, Nagano Y, Shiozawa M, Akaike M, Wada N, Rino Y, Kunisaki C, Masuda M, Tanaka K, Imada T. 2009. Reduced expression of the AdipoR1 gene is correlated with venous invasion in colorectal cancer. Mol Med Report 2:555–559 [DOI] [PubMed] [Google Scholar]
- 223. Montecucco F, Mach F. 2009. Update on therapeutic strategies to increase adiponectin function and secretion in metabolic syndrome. Diabetes Obes Metab 11:445–454 [DOI] [PubMed] [Google Scholar]
- 224. Kaklamani VG, Wisinski KB, Sadim M, Gulden C, Do A, Offit K, Baron JA, Ahsan H, Mantzoros C, Pasche B. 2008. Variants of the adiponectin (ADIPOQ) and adiponectin receptor 1 (ADIPOR1) genes and colorectal cancer risk. JAMA 300:1523–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Pechlivanis S, Bermejo JL, Pardini B, Naccarati A, Vodickova L, Novotny J, Hemminki K, Vodicka P, Försti A. 2009. Genetic variation in adipokine genes and risk of colorectal cancer. Eur J Endocrinol 160:933–940 [DOI] [PubMed] [Google Scholar]
- 226. Carvajal-Carmona LG, Spain S, Kerr D, Houlston R, Cazier JB, Tomlinson I. 2009. Common variation at the adiponectin locus is not associated with colorectal cancer risk in the UK. Hum Mol Genet 18:1889–1892 [DOI] [PubMed] [Google Scholar]
- 227. Partida-Pérez M, de la Luz Ayala-Madrigal M, Peregrina-Sandoval J, Macías-Gómez N, Moreno-Ortiz J, Leal-Ugarte E, Cárdenas-Meza M, Centeno-Flores M, Maciel-Gutiérrez V, Cabrales E, Cervantes-Ortiz S, Gutiérrez-Angulo M. 2010. Association of LEP and ADIPOQ common variants with colorectal cancer in Mexican patients. Cancer Biomark 7:117–121 [DOI] [PubMed] [Google Scholar]
- 228. Gornick MC, Rennert G, Moreno V, Gruber SB. 2011. Adiponectin gene and risk of colorectal cancer. Br J Cancer 105:562–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. He B, Pan Y, Zhang Y, Bao Q, Chen L, Nie Z, Gu L, Xu Y, Wang S. 2011. Effects of genetic variations in the adiponectin pathway genes on the risk of colorectal cancer in the Chinese population. BMC Med Genet 12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Liu L, Zhong R, Wei S, Yin JY, Xiang H, Zou L, Chen W, Chen JG, Zheng XW, Huang LJ, Zhu BB, Chen Q, Duan SY, Rui R, Yang BF, Sun JW, Xie DS, Xu YH, Miao XP, Nie SF. 2011. Interactions between genetic variants in the adiponectin, adiponectin receptor 1 and environmental factors on the risk of colorectal cancer. PLoS One 6:e27301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Vrieling A, Buck K, Kaaks R, Chang-Claude J. 2010. Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status: a meta-analysis. Breast Cancer Res Treat 123:641–649 [DOI] [PubMed] [Google Scholar]
- 232. Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, Noguchi S. 2003. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res 9:5699–5704 [PubMed] [Google Scholar]
- 233. Hou WK, Xu YX, Yu T, Zhang L, Zhang WW, Fu CL, Sun Y, Wu Q, Chen L. 2007. Adipocytokines and breast cancer risk. Chin Med J (Engl) 120:1592–1596 [PubMed] [Google Scholar]
- 234. Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, Fu OY, Chen HY, Hou MF, Yuan SS. 2006. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett 237:109–114 [DOI] [PubMed] [Google Scholar]
- 235. Shahar S, Salleh RM, Ghazali AR, Koon PB, Mohamud WN. 2010. Roles of adiposity, lifetime physical activity and serum adiponectin in occurrence of breast cancer among Malaysian women in Klang Valley. Asian Pac J Cancer Prev 11:61–66 [PubMed] [Google Scholar]
- 236. Macis D, Gandini S, Guerrieri-Gonzaga A, Johansson H, Magni P, Ruscica M, Lazzeroni M, Serrano D, Cazzaniga M, Mora S, Feroce I, Pizzamiglio M, Sandri MT, Gulisano M, Bonanni B, Decensi A. 2012. Prognostic effect of circulating adiponectin in a randomized 2 x 2 trial of low-dose tamoxifen and fenretinide in premenopausal women at risk for breast cancer. J Clin Oncol 30:151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Fabian CJ. 2012. Adiponectin: a risk biomarker and attractive target for chemoprevention. J Clin Oncol 30:124–126 [DOI] [PubMed] [Google Scholar]
- 238. Jeong YJ, Bong JG, Park SH, Choi JH, Oh HK. 2011. Expression of leptin, leptin receptor, adiponectin, and adiponectin receptor in ductal carcinoma in situ and invasive breast cancer. J Breast Cancer 14:96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Kang JH, Yu BY, Youn DS. 2007. Relationship of serum adiponectin and resistin levels with breast cancer risk. J Korean Med Sci 22:117–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Gaudet MM, Falk RT, Gierach GL, Lacey JV, Jr, Graubard BI, Dorgan JF, Brinton LA. 2010. Do adipokines underlie the association between known risk factors and breast cancer among a cohort of United States women? Cancer Epidemiol 34:580–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Karaduman M, Bilici A, Ozet A, Sengul A, Musabak U, Alomeroglu M. 2007. Tissue levels of adiponectin in breast cancer patients. Med Oncol 24:361–366 [DOI] [PubMed] [Google Scholar]
- 242. Sonmez B, Seker M, Bilici A, Yavuz Erkal F, Oven Ustaalioglu BB, Gumus M, Ozturk Guler D, Karaduman M, Gezen C, Eser M, Bildik N, Salepci T. 2011. Is there any correlation among adiponectin levels in serum, tumor tissue and normal tissue of the same patients with breast cancer? J BUON 16:227–232 [PubMed] [Google Scholar]
- 243. Oh SW, Park CY, Lee ES, Yoon YS, Lee ES, Park SS, Kim Y, Sung NJ, Yun YH, Lee KS, Kang HS, Kwon Y, Ro J. 2011. Adipokines, insulin resistance, metabolic syndrome, and breast cancer recurrence: a cohort study. Breast Cancer Res 13:R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Tian YF, Chu CH, Wu MH, Chang CL, Yang T, Chou YC, Hsu GC, Yu CP, Yu JC, Sun CA. 2007. Anthropometric measures, plasma adiponectin, and breast cancer risk. Endocr Relat Cancer 14:669–677 [DOI] [PubMed] [Google Scholar]
- 245. Cust AE, Stocks T, Lukanova A, Lundin E, Hallmans G, Kaaks R, Jonsson H, Stattin P. 2009. The influence of overweight and insulin resistance on breast cancer risk and tumour stage at diagnosis: a prospective study. Breast Cancer Res Treat 113:567–576 [DOI] [PubMed] [Google Scholar]
- 246. Vona-Davis L, Rose DP. 2007. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer 14:189–206 [DOI] [PubMed] [Google Scholar]
- 247. Lorincz AM, Sukumar S. 2006. Molecular links between obesity and breast cancer. Endocr Relat Cancer 13:279–292 [DOI] [PubMed] [Google Scholar]
- 248. Duggan C, Irwin ML, Xiao L, Henderson KD, Smith AW, Baumgartner RN, Baumgartner KB, Bernstein L, Ballard-Barbash R, McTiernan A. 2011. Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J Clin Oncol 29:32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Teras LR, Goodman M, Patel AV, Bouzyk M, Tang W, Diver WR, Feigelson HS. 2009. No association between polymorphisms in LEP, LEPR, ADIPOQ, ADIPOR1, or ADIPOR2 and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev 18:2553–2557 [DOI] [PubMed] [Google Scholar]
- 250. Nyante SJ, Gammon MD, Kaufman JS, Bensen JT, Lin DY, Barnholtz-Sloan JS, Hu Y, He Q, Luo J, Millikan RC. 2011. Common genetic variation in adiponectin, leptin, and leptin receptor and association with breast cancer subtypes. Breast Cancer Res Treat 129:593–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Wolk A, Gridley G, Svensson M, Nyrén O, McLaughlin JK, Fraumeni JF, Adam HO. 2001. A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control 12:13–21 [DOI] [PubMed] [Google Scholar]
- 252. Moon HS, Chamberland JP, Aronis K, Tseleni-Balafouta S, Mantzoros CS. 2011. Direct role of adiponectin and adiponectin receptors in endometrial cancer: in vitro and ex vivo studies in humans. Mol Cancer Ther 10:2234–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Soliman PT, Wu D, Tortolero-Luna G, Schmeler KM, Slomovitz BM, Bray MS, Gershenson DM, Lu KH. 2006. Association between adiponectin, insulin resistance, and endometrial cancer. Cancer 106:2376–2381 [DOI] [PubMed] [Google Scholar]
- 254. Rzepka-Górska I, Bedner R, Cymbaluk-Płoska A, Chudecka-Głaz A. 2008. Serum adiponectin in relation to endometrial cancer and endometrial hyperplasia with atypia in obese women. Eur J Gynaecol Oncol 29:594–597 [PubMed] [Google Scholar]
- 255. Ashizawa N, Yahata T, Quan J, Adachi S, Yoshihara K, Tanaka K. 2010. Serum leptin-adiponectin ratio and endometrial cancer risk in postmenopausal female subjects. Gynecol Oncol 119:65–69 [DOI] [PubMed] [Google Scholar]
- 256. Cust AE, Kaaks R, Friedenreich C, Bonnet F, Laville M, Lukanova A, Rinaldi S, Dossus L, Slimani N, Lundin E, Tjønneland A, Olsen A, Overvad K, Clavel-Chapelon F, Mesrine S, Joulin V, Linseisen J, Rohrmann S, Pischon T, Boeing H, Trichopoulos D, Trichopoulou A, Benetou V, Palli D, Berrino F, Tumino R, Sacerdote C, Mattiello A, Quirós JR, Mendez MA, Sánchez MJ, Larrañaga N, Tormo MJ, Ardanaz E, Bueno-de-Mesquita HB, Peeters PH, van Gils CH, Khaw KT, Bingham S, Allen N, Key T, Jenab M, Riboli E. 2007. Plasma adiponectin levels and endometrial cancer risk in pre- and postmenopausal women. J Clin Endocrinol Metab 92:255–263 [DOI] [PubMed] [Google Scholar]
- 257. Soliman PT, Cui X, Zhang Q, Hankinson SE, Lu KH. 2011. Circulating adiponectin levels and risk of endometrial cancer: the prospective Nurses' Health Study. Am J Obstet Gynecol 204:167–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Chen X, Xiang YB, Long JR, Cai H, Cai Q, Cheng J, Wen W, Gao YT, Zheng W, Shu XO. 28 October 2011. Genetic polymorphisms in obesity-related genes and endometrial cancer risk. Cancer doi: 10.1002/cncr.26552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Ishikawa M, Kitayama J, Yamauchi T, Kadowaki T, Maki T, Miyato H, Yamashita H, Nagawa H. 2007. Adiponectin inhibits the growth and peritoneal metastasis of gastric cancer through its specific membrane receptors AdipoR1 and AdipoR2. Cancer Sci 98:1120–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Tsukada T, Fushida S, Harada S, Terai S, Yagi Y, Kinoshita J, Oyama K, Tajima H, Fujita H, Ninomiya I, Fujimura T, Ohta T. 2011. Adiponectin receptor-1 expression is associated with good prognosis in gastric cancer. J Exp Clin Cancer Res 30:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Nakajima TE, Yamada Y, Hamano T, Furuta K, Gotoda T, Katai H, Kato K, Hamaguchi T, Shimada Y. 2009. Adipocytokine levels in gastric cancer patients: resistin and visfatin as biomarkers of gastric cancer. J Gastroenterol 44:685–690 [DOI] [PubMed] [Google Scholar]
- 262. Seker M, Bilici A, Sonmez B, Ustaalioðlu BB, Gumus M, Gozu H, Sargin M, Orcun A, Gezen C, Eser M, Bildik N, Salepci T. 2010. The association of serum adiponectin levels with histopathological variables in gastric cancer patients. Med Oncol 27:1319–1323 [DOI] [PubMed] [Google Scholar]
- 263. Seker M, Bilici A, Guler DO, Ustaalioglu BB, Gozu H, Erkal FY, Sonmez B, Karaduman M, Kefeli U, Yildirim E, Salepci T, Gumus M. 2011. Tissue and serum adiponectin levels in patients with gastric cancer: are there any correlations between adiponectin levels and histopathological variables? Hepatogastroenterology 58:1841–1846 [DOI] [PubMed] [Google Scholar]
- 264. Thompson OM, Beresford SA, Kirk EA, Bronner MP, Vaughan TL. 2010. Serum leptin and adiponectin levels and risk of Barrett's esophagus and intestinal metaplasia of the gastroesophageal junction. Obesity (Silver Spring) 18:2204–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Akiyama T, Yoneda M, Maeda S, Nakajima A, Koyama S, Inamori M. 2011. Visceral obesity and the risk of Barrett's esophagus. Digestion 83:142–145 [DOI] [PubMed] [Google Scholar]
- 266. Yildirim A, Bilici M, Cayir K, Yanmaz V, Yildirim S, Tekin SB. 2009. Serum adiponectin levels in patients with esophageal cancer. Jpn J Clin Oncol 39:92–96 [DOI] [PubMed] [Google Scholar]
- 267. Konturek PC, Burnat G, Rau T, Hahn EG, Konturek S. 2008. Effect of adiponectin and ghrelin on apoptosis of Barrett adenocarcinoma cell line. Dig Dis Sci 53:597–605 [DOI] [PubMed] [Google Scholar]
- 268. Howard JM, Beddy P, Ennis D, Keogan M, Pidgeon GP, Reynolds JV. 2010. Associations between leptin and adiponectin receptor upregulation, visceral obesity and tumour stage in oesophageal and junctional adenocarcinoma. Br J Surg 97:1020–1027 [DOI] [PubMed] [Google Scholar]
- 269. Nakajima TE, Yamada Y, Hamano T, Furuta K, Oda I, Kato H, Kato K, Hamaguchi T, Shimada Y. 2010. Adipocytokines and squamous cell carcinoma of the esophagus. J Cancer Res Clin Oncol 136:261–266 [DOI] [PubMed] [Google Scholar]
- 270. Diao Y, Li H, Li H, Zhou Y, Ma Q, Wang Y, Li D. 2009. Association of serum levels of lipid and its novel constituents with the different stages of esophageal carcinoma. Lipids Health Dis 8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Chang MC, Chang YT, Su TC, Yang WS, Chen CL, Tien YW, Liang PC, Wei SC, Wong JM. 2007. Adiponectin as a potential differential marker to distinguish pancreatic cancer and chronic pancreatitis. Pancreas 35:16–21 [DOI] [PubMed] [Google Scholar]
- 272. Krechler T, Zeman M, Vecka M, Macasek J, Jachymova M, Zima T, Zak A. 2011. Leptin and adiponectin in pancreatic cancer: connection with diabetes mellitus. Neoplasma 58:58–64 [DOI] [PubMed] [Google Scholar]
- 273. Stolzenberg-Solomon RZ, Weinstein S, Pollak M, Tao Y, Taylor PR, Virtamo J, Albanes D. 2008. Prediagnostic adiponectin concentrations and pancreatic cancer risk in male smokers. Am J Epidemiol 168:1047–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Grote VA, Rohrmann S, Dossus L, Nieters A, Halkjaer J, Tjønneland A, Overvad K, Stegger J, Chabbert-Buffet N, Boutron-Ruault MC, Clavel-Chapelon F, Teucher B, Becker S, Montonen J, Boeing H, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Sieri S, Tumino R, Vineis P, Mattiello A, Argüelles M, Duell EJ, Molina-Montes E, Larrañaga N, Chirlaque MD, Gurrea AB, Jeurnink SM, Peeters PH, Ye W, Sund M, Lindkvist B, Johansen D, Khaw KT, Wareham N, Crowe FL, Romieu I, Rinaldi S, Jenab M, Romaguera D, Michaud DS, Riboli E, Bas Bueno-de-Mesquita H, Kaaks R. 2012. The association of circulating adiponectin levels with pancreatic cancer risk: a study within the prospective EPIC cohort. Int J Cancer 130:2428–2437 [DOI] [PubMed] [Google Scholar]
- 275. Pezzilli R, Barassi A, Corsi MM, Morselli-Labate AM, Campana D, Casadei R, Santini D, Corinaldesi R, D'Eril GM. 2010. Serum leptin, but not adiponectin and receptor for advanced glycation end products, is able to distinguish autoimmune pancreatitis from both chronic pancreatitis and pancreatic neoplasms. Scand J Gastroenterol 45:93–99 [DOI] [PubMed] [Google Scholar]
- 276. Kotani K, Wakai K, Shibata A, Fujita Y, Ogimoto I, Naito M, Kurozawa Y, Suzuki H, Yoshimura T, Tamakoshi A. 2009. Serum adiponectin multimer complexes and liver cancer risk in a large cohort study in Japan. Asian Pac J Cancer Prev 10 Suppl:87–90 [PubMed] [Google Scholar]
- 277. Sharma D, Wang J, Fu PP, Sharma S, Nagalingam A, Mells J, Handy J, Page AJ, Cohen C, Anania FA, Saxena NK. 2010. Adiponectin antagonizes the oncogenic actions of leptin in hepatocellular carcinogenesis. Hepatology 52:1713–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Monto A, Alonzo J, Watson JJ, Grunfeld C, Wright TL. 2002. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology 36:729–736 [DOI] [PubMed] [Google Scholar]
- 279. Jonsson JR, Barrie HD, O'Rourke P, Clouston AD, Powell EE. 2008. Obesity and steatosis influence serum and hepatic inflammatory markers in chronic hepatitis C. Hepatology 48:80–87 [DOI] [PubMed] [Google Scholar]
- 280. Ioannou GN, Splan MF, Weiss NS, McDonald GB, Beretta L, Lee SP. 2007. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 5:938–945, 945.e1–4 [DOI] [PubMed] [Google Scholar]
- 281. Ohki T, Tateishi R, Sato T, Masuzaki R, Imamura J, Goto T, Yamashiki N, Yoshida H, Kanai F, Kato N, Shiina S, Yoshida H, Kawabe T, Omata M. 2008. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin Gastroenterol Hepatol 6:459–464 [DOI] [PubMed] [Google Scholar]
- 282. Arano T, Nakagawa H, Tateishi R, Ikeda H, Uchino K, Enooku K, Goto E, Masuzaki R, Asaoka Y, Kondo Y, Goto T, Shiina S, Omata M, Yoshida H, Koike K. 29 March 2011. Serum level of adiponectin and the risk of liver cancer development in chronic hepatitis C patients. Int J Cancer doi: 10.1002/ijc.25861 [DOI] [PubMed] [Google Scholar]
- 283. Nkontchou G, Bastard JP, Ziol M, Aout M, Cosson E, Ganne-Carrie N, Grando-Lemaire V, Roulot D, Capeau J, Trinchet JC, Vicaut E, Beaugrand M. 2010. Insulin resistance, serum leptin, and adiponectin levels and outcomes of viral hepatitis C cirrhosis. J Hepatol 53:827–833 [DOI] [PubMed] [Google Scholar]
- 284. Sumie S, Kawaguchi T, Kuromatsu R, Takata A, Nakano M, Satani M, Yamada S, Niizeki T, Torimura T, Sata M. 2011. Total and high molecular weight adiponectin and hepatocellular carcinoma with HCV infection. PLoS One 6:e26840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Saxena NK, Fu PP, Nagalingam A, Wang J, Handy J, Cohen C, Tighiouart M, Sharma D, Anania FA. 2010. Adiponectin modulates C-jun N-terminal kinase and mammalian target of rapamycin and inhibits hepatocellular carcinoma. Gastroenterology 139:1762–1773, 1773.e1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Miuma S, Ichikawa T, Taura N, Shibata H, Takeshita S, Akiyama M, Motoyoshi Y, Ozawa E, Fujimoto M, Kawashimo H, Miyaaki H, Eguchi K, Nakao K. 2009. The level of fasting serum insulin, but not adiponectin, is associated with the prognosis of early stage hepatocellular carcinoma. Oncol Rep 22:1415–1424 [DOI] [PubMed] [Google Scholar]
- 287. Spyridopoulos TN, Petridou ET, Dessypris N, Terzidis A, Skalkidou A, Deliveliotis C, Chrousos GP. 2009. Inverse association of leptin levels with renal cell carcinoma: results from a case-control study. Hormones (Athens) 8:39–46 [DOI] [PubMed] [Google Scholar]
- 288. Pinthus JH, Kleinmann N, Tisdale B, Chatterjee S, Lu JP, Gillis A, Hamlet T, Singh G, Farrokhyar F, Kapoor A. 2008. Lower plasma adiponectin levels are associated with larger tumor size and metastasis in clear-cell carcinoma of the kidney. Eur Urol 54:866–873 [DOI] [PubMed] [Google Scholar]
- 289. Horiguchi A, Ito K, Sumitomo M, Kimura F, Asano T, Hayakawa M. 2008. Decreased serum adiponectin levels in patients with metastatic renal cell carcinoma. Jpn J Clin Oncol 38:106–111 [DOI] [PubMed] [Google Scholar]
- 290. Michalakis K, Williams CJ, Mitsiades N, Blakeman J, Balafouta-Tselenis S, Giannopoulos A, Mantzoros CS. 2007. Serum adiponectin concentrations and tissue expression of adiponectin receptors are reduced in patients with prostate cancer: a case control study. Cancer Epidemiol Biomarkers Prev 16:308–313 [DOI] [PubMed] [Google Scholar]
- 291. Arisan ED, Arisan S, Atis G, Palavan-Unsal N, Ergenekon E. 2009. Serum adipocytokine levels in prostate cancer patients. Urol Int 82:203–208 [DOI] [PubMed] [Google Scholar]
- 292. Li H, Stampfer MJ, Mucci L, Rifai N, Qiu W, Kurth T, Ma J. 2010. A 25-year prospective study of plasma adiponectin and leptin concentrations and prostate cancer risk and survival. Clin Chem 56:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Baillargeon J, Platz EA, Rose DP, Pollock BH, Ankerst DP, Haffner S, Higgins B, Lokshin A, Troyer D, Hernandez J, Lynch S, Leach RJ, Thompson IM. 2006. Obesity, adipokines, and prostate cancer in a prospective population-based study. Cancer Epidemiol Biomarkers Prev 15:1331–1335 [DOI] [PubMed] [Google Scholar]
- 294. Sher DJ, Oh WK, Jacobus S, Regan MM, Lee GS, Mantzoros C. 2008. Relationship between serum adiponectin and prostate cancer grade. Prostate 68:1592–1598 [DOI] [PubMed] [Google Scholar]
- 295. Housa D, Vernerová Z, Herácek J, Procházka B, Cechák P, Kuncová J, Haluzík M. 2008. Adiponectin as a potential marker of prostate cancer progression: studies in organ-confined and locally advanced prostate cancer. Physiol Res 57:451–458 [DOI] [PubMed] [Google Scholar]
- 296. Kaklamani V, Yi N, Zhang K, Sadim M, Offit K, Oddoux C, Ostrer H, Mantzoros C, Pasche B. 2011. Polymorphisms of ADIPOQ and ADIPOR1 and prostate cancer risk. Metabolism 60:1234–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Dhillon PK, Penney KL, Schumacher F, Rider JR, Sesso HD, Pollak M, Fiorentino M, Finn S, Loda M, Rifai N, Mucci LA, Giovannucci E, Stampfer MJ, Ma J. 2011. Common polymorphisms in the adiponectin and its receptor genes, adiponectin levels and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 20:2618–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Beebe-Dimmer JL, Zuhlke KA, Ray AM, Lange EM, Cooney KA. 2010. Genetic variation in adiponectin (ADIPOQ) and the type 1 receptor (ADIPOR1), obesity and prostate cancer in African Americans. Prostate Cancer Prostatic Dis 13:362–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Ross JA, Parker E, Blair CK, Cerhan JR, Folsom AR. 2004. Body mass index and risk of leukemia in older women. Cancer Epidemiol Biomarkers Prev 13:1810–1813 [PubMed] [Google Scholar]
- 300. Strom SS, Yamamura Y, Kantarijian HM, Cortes-Franco JE. 2009. Obesity, weight gain, and risk of chronic myeloid leukemia. Cancer Epidemiol Biomarkers Prev 18:1501–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Willett EV, Skibola CF, Adamson P, Skibola DR, Morgan GJ, Smith MT, Roman E. 2005. Non-Hodgkin's lymphoma, obesity and energy homeostasis polymorphisms. Br J Cancer 93:811–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Avcu F, Ural AU, Yilmaz MI, Bingol N, Nevruz O, Caglar K. 2006. Association of plasma adiponectin concentrations with chronic lymphocytic leukemia and myeloproliferative diseases. Int J Hematol 83:254–258 [DOI] [PubMed] [Google Scholar]
- 303. Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, Matsuzawa Y. 2000. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 96:1723–1732 [PubMed] [Google Scholar]
- 304. Ozturk K, Avcu F, Ural AU. 2012. Aberrant expressions of leptin and adiponectin receptor isoforms in chronic myeloid leukemia patients. Cytokine 57:61–67 [DOI] [PubMed] [Google Scholar]
- 305. Fitter S, Vandyke K, Schultz CG, White D, Hughes TP, Zannettino AC. 2010. Plasma adiponectin levels are markedly elevated in imatinib-treated chronic myeloid leukemia (CML) patients: a mechanism for improved insulin sensitivity in type 2 diabetic CML patients? J Clin Endocrinol Metab 95:3763–3767 [DOI] [PubMed] [Google Scholar]
- 306. Pamuk GE, Turgut B, Demir M, Vural O. 2006. Increased adiponectin level in non-Hodgkin lymphoma and its relationship with interleukin-10. Correlation with clinical features and outcome. J Exp Clin Cancer Res 25:537–541 [PubMed] [Google Scholar]
- 307. Reseland JE, Reppe S, Olstad OK, Hjorth-Hansen H, Brenne AT, Syversen U, Waage A, Iversen PO. 2009. Abnormal adipokine levels and leptin-induced changes in gene expression profiles in multiple myeloma. Eur J Haematol 83:460–470 [DOI] [PubMed] [Google Scholar]
- 308. Petridou ET, Dessypris N, Panagopoulou P, Sergentanis TN, Mentis AF, Pourtsidis A, Polychronopoulou S, Kalmanti M, Athanasiadou-Piperopoulou F, Moschovi M. 2010. Adipocytokines in relation to Hodgkin lymphoma in children. Pediatr Blood Cancer 54:311–315 [DOI] [PubMed] [Google Scholar]
- 309. Fowler JA, Lwin ST, Drake MT, Edwards JR, Kyle RA, Mundy GR, Edwards CM. 2011. Host-derived adiponectin is tumor-suppressive and a novel therapeutic target for multiple myeloma and the associated bone disease. Blood 118:5872–5882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Lauta VM. 2003. A review of the cytokine network in multiple myeloma: diagnostic, prognostic, and therapeutic implications. Cancer 97:2440–2452 [DOI] [PubMed] [Google Scholar]
- 311. Ajuwon KM, Spurlock ME. 2005. Adiponectin inhibits LPS-induced NF-κB activation and IL-6 production and increases PPARγ2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol 288:R1220–R1225 [DOI] [PubMed] [Google Scholar]
- 312. Molica S, Vitelli G, Cutrona G, Todoerti K, Mirabelli R, Digiesi G, Giannarelli D, Sperduti I, Molica M, Gentile M, Morabito F, Neri A, Ferrarini M. 2008. Prognostic relevance of serum levels and cellular expression of adiponectin in B-cell chronic lymphocytic leukemia. Int J Hematol 88:374–380 [DOI] [PubMed] [Google Scholar]
- 313. Molica S, Digiesi G, Vacca A, Mirabelli R, Todoerti K, Battaglia C, Morabito F, Neri A, Ribatti D. 2009. Does adiponectin act as an antiangiogenic factor in B-cell chronic lymphocytic leukemia? Adv Hematol 2009:287974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Moschovi M, Trimis G, Vounatsou M, Katsibardi K, Margeli A, Damianos A, Chrousos G, Papassotiriou I. 2010. Serial plasma concentrations of adiponectin, leptin, and resistin during therapy in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol 32:e8–e13 [DOI] [PubMed] [Google Scholar]
- 315. Petridou ET, Sergentanis TN, Antonopoulos CN, Dessypris N, Matsoukis IL, Aronis K, Efremidis A, Syrigos C, Mantzoros CS. 2011. Insulin resistance: an independent risk factor for lung cancer? Metabolism 60:1100–1106 [DOI] [PubMed] [Google Scholar]
- 316. Karapanagiotou EM, Tsochatzis EA, Dilana KD, Tourkantonis I, Gratsias I, Syrigos KN. 2008. The significance of leptin, adiponectin, and resistin serum levels in non-small cell lung cancer (NSCLC). Lung Cancer 61:391–397 [DOI] [PubMed] [Google Scholar]
- 317. Cui E, Deng A, Wang X, Wang B, Mao W, Feng X, Hua F. 2011. The role of adiponectin (ADIPOQ) gene polymorphisms in the susceptibility and prognosis of non-small cell lung cancer. Biochem Cell Biol 89:308–313 [DOI] [PubMed] [Google Scholar]
- 318. Wolin KY, Carson K, Colditz GA. 2010. Obesity and cancer. Oncologist 15:556–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Antoniadis AG, Petridou ET, Antonopoulos CN, Dessypris N, Panagopoulou P, Chamberland JP, Adami HO, Gogas H, Mantzoros CS. 2011. Insulin resistance in relation to melanoma risk. Melanoma Res 21:541–546 [DOI] [PubMed] [Google Scholar]
- 320. Mantzoros CS, Trakatelli M, Gogas H, Dessypris N, Stratigos A, Chrousos GP, Petridou ET. 2007. Circulating adiponectin levels in relation to melanoma: a case-control study. Eur J Cancer 43:1430–1436 [DOI] [PubMed] [Google Scholar]
- 321. Mitsiades N, Pazaitou-Panayiotou K, Aronis KN, Moon HS, Chamberland JP, Liu X, Diakopoulos KN, Kyttaris V, Panagiotou V, Mylvaganam G, Tseleni-Balafouta S, Mantzoros CS. 2011. Circulating adiponectin is inversely associated with risk of thyroid cancer: in vivo and in vitro studies. J Clin Endocrinol Metab 96:E2023–E2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Isobe K, Fu L, Tatsuno I, Takahashi H, Nissato S, Hara H, Yashiro T, Suzukawa K, Takekoshi K, Shimano H, Kawakami Y. 2009. Adiponectin and adiponectin receptors in human pheochromocytoma. J Atheroscler Thromb 16:442–447 [DOI] [PubMed] [Google Scholar]
- 323. Park JT, Yoo TH, Chang TI, Lee DH, Lee JH, Lee JE, Choi HY, Kang SW, Han DS, Ryu DR. 2011. Insulin resistance and lower plasma adiponectin increase malignancy risk in nondiabetic continuous ambulatory peritoneal dialysis patients. Metabolism 60:121–126 [DOI] [PubMed] [Google Scholar]
- 324. Comuzzie AG, Funahashi T, Sonnenberg G, Martin LJ, Jacob HJ, Black AE, Maas D, Takahashi M, Kihara S, Tanaka S, Matsuzawa Y, Blangero J, Cohen D, Kissebah A. 2001. The genetic basis of plasma variation in adiponectin, a global endophenotype for obesity and the metabolic syndrome. J Clin Endocrinol Metab 86:4321–4325 [DOI] [PubMed] [Google Scholar]
- 325. Menzaghi C, Trischitta V, Doria A. 2007. Genetic influences of adiponectin on insulin resistance, type 2 diabetes, and cardiovascular disease. Diabetes 56:1198–1209 [DOI] [PubMed] [Google Scholar]
- 326. Zakikhani M, Dowling RJ, Sonenberg N, Pollak MN. 2008. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res (Phila) 1:369–375 [DOI] [PubMed] [Google Scholar]
- 327. Ogunwobi OO, Beales IL. 2006. Adiponectin stimulates proliferation and cytokine secretion in colonic epithelial cells. Regul Pept 134:105–113 [DOI] [PubMed] [Google Scholar]
- 328. Habeeb BS, Kitayama J, Nagawa H. 2011. Adiponectin supports cell survival in glucose deprivation through enhancement of autophagic response in colorectal cancer cells. Cancer Sci 102:999–1006 [DOI] [PubMed] [Google Scholar]
- 329. Cong L, Gasser J, Zhao J, Yang B, Li F, Zhao AZ. 2007. Human adiponectin inhibits cell growth and induces apoptosis in human endometrial carcinoma cells, HEC-1-A and RL95 2. Endocr Relat Cancer 14:713–720 [DOI] [PubMed] [Google Scholar]
- 330. Grossmann ME, Mizuno NK, Bonorden MJ, Ray A, Sokolchik I, Narasimhan ML, Cleary MP. 2009. Role of the adiponectin leptin ratio in prostate cancer. Oncol Res 18:269–277 [DOI] [PubMed] [Google Scholar]
- 331. Bub JD, Miyazaki T, Iwamoto Y. 2006. Adiponectin as a growth inhibitor in prostate cancer cells. Biochem Biophys Res Commun 340:1158–1166 [DOI] [PubMed] [Google Scholar]
- 332. Sun Y, Lodish HF. 2010. Adiponectin deficiency promotes tumor growth in mice by reducing macrophage infiltration. PLoS One 5:e11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Drabkin HA, Gemmill RM. 2010. Obesity, cholesterol, and clear-cell renal cell carcinoma (RCC). Adv Cancer Res 107:39–56 [DOI] [PubMed] [Google Scholar]
- 334. Jardé T, Perrier S, Vasson MP, Caldefie-Chézet F. 2011. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer 47:33–43 [DOI] [PubMed] [Google Scholar]
- 335. Treeck O, Lattrich C, Juhasz-Boess I, Buchholz S, Pfeiler G, Ortmann O. 2008. Adiponectin differentially affects gene expression in human mammary epithelial and breast cancer cells. Br J Cancer 99:1246–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Li G, Cong L, Gasser J, Zhao J, Chen K, Li F. 2011. Mechanisms underlying the anti-proliferative actions of adiponectin in human breast cancer cells, MCF7-dependency on the cAMP/protein kinase-A pathway. Nutr Cancer 63:80–88 [DOI] [PubMed] [Google Scholar]
- 337. Jardé T, Caldefie-Chézet F, Goncalves-Mendes N, Mishellany F, Buechler C, Penault-Llorca F, Vasson MP. 2009. Involvement of adiponectin and leptin in breast cancer: clinical and in vitro studies. Endocr Relat Cancer 16:1197–1210 [DOI] [PubMed] [Google Scholar]
- 338. Grossmann ME, Nkhata KJ, Mizuno NK, Ray A, Cleary MP. 2008. Effects of adiponectin on breast cancer cell growth and signaling. Br J Cancer 98:370–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Dieudonne MN, Bussiere M, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. 2006. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun 345:271–279 [DOI] [PubMed] [Google Scholar]
- 340. Nakayama S, Miyoshi Y, Ishihara H, Noguchi S. 2008. Growth-inhibitory effect of adiponectin via adiponectin receptor 1 on human breast cancer cells through inhibition of S-phase entry without inducing apoptosis. Breast Cancer Res Treat 112:405–410 [DOI] [PubMed] [Google Scholar]
- 341. Grossmann ME, Ray A, Dogan S, Mizuno NK, Cleary MP. 2008. Balance of adiponectin and leptin modulates breast cancer cell growth. Cell Res 18:1154–1156 [DOI] [PubMed] [Google Scholar]
- 342. Liu J, Lam JB, Chow KH, Xu A, Lam KS, Moon RT, Wang Y. 2008. Adiponectin stimulates Wnt inhibitory factor-1 expression through epigenetic regulations involving the transcription factor specificity protein 1. Carcinogenesis 29:2195–2202 [DOI] [PubMed] [Google Scholar]
- 343. Dos Santos E, Benaitreau D, Dieudonne MN, Leneveu MC, Serazin V, Giudicelli Y, Pecquery R. 2008. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol Rep 20:971–977 [PubMed] [Google Scholar]
- 344. Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo RL, Wu D, Cooper GJ, Xu A. 2006. Adiponectin modulates the glycogen synthase kinase-3β/β-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res 66:11462–11470 [DOI] [PubMed] [Google Scholar]
- 345. Kang JH, Lee YY, Yu BY, Yang BS, Cho KH, Yoon DK, Roh YK. 2005. Adiponectin induces growth arrest and apoptosis of MDA-MB-231 breast cancer cell. Arch Pharm Res 28:1263–1269 [DOI] [PubMed] [Google Scholar]
- 346. Pfeiler GH, Buechler C, Neumeier M, Schäffler A, Schmitz G, Ortmann O, Treeck O. 2008. Adiponectin effects on human breast cancer cells are dependent on 17-β estradiol. Oncol Rep 19:787–793 [PubMed] [Google Scholar]
- 347. Lang K, Ratke J. 2009. Leptin and adiponectin: new players in the field of tumor cell and leukocyte migration. Cell Commun Signal 7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Nkhata KJ, Ray A, Schuster TF, Grossmann ME, Cleary MP. 2009. Effects of adiponectin and leptin co-treatment on human breast cancer cell growth. Oncol Rep 21:1611–1619 [DOI] [PubMed] [Google Scholar]
- 349. Arditi JD, Venihaki M, Karalis KP, Chrousos GP. 2007. Antiproliferative effect of adiponectin on MCF7 breast cancer cells: a potential hormonal link between obesity and cancer. Horm Metab Res 39:9–13 [DOI] [PubMed] [Google Scholar]
- 350. Taliaferro-Smith L, Nagalingam A, Zhong D, Zhou W, Saxena NK, Sharma D. 2009. LKB1 is required for adiponectin-mediated modulation of AMPK-S6K axis and inhibition of migration and invasion of breast cancer cells. Oncogene 28:2621–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Kim KY, Baek A, Hwang JE, Choi YA, Jeong J, Lee MS, Cho DH, Lim JS, Kim KI, Yang Y. 2009. Adiponectin-activated AMPK stimulates dephosphorylation of AKT through protein phosphatase 2A activation. Cancer Res 69:4018–4026 [DOI] [PubMed] [Google Scholar]
- 352. Man K, Ng KT, Xu A, Cheng Q, Lo CM, Xiao JW, Sun BS, Lim ZX, Cheung JS, Wu EX, Sun CK, Poon RT, Fan ST. 2010. Suppression of liver tumor growth and metastasis by adiponectin in nude mice through inhibition of tumor angiogenesis and downregulation of Rho kinase/IFN-inducible protein 10/matrix metalloproteinase 9 signaling. Clin Cancer Res 16:967–977 [DOI] [PubMed] [Google Scholar]
- 353. Tang CH, Lu ME. 2009. Adiponectin increases motility of human prostate cancer cells via adipoR, p38, AMPK, and NF-κB pathways. Prostate 69:1781–1789 [DOI] [PubMed] [Google Scholar]
- 354. Chiu YC, Shieh DC, Tong KM, Chen CP, Huang KC, Chen PC, Fong YC, Hsu HC, Tang CH. 2009. Involvement of AdipoR receptor in adiponectin-induced motility and α2β1 integrin upregulation in human chondrosarcoma cells. Carcinogenesis 30:1651–1659 [DOI] [PubMed] [Google Scholar]
- 355. Aaronson SA. 1991. Growth factors and cancer. Science 254:1146–1153 [DOI] [PubMed] [Google Scholar]
- 356. Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. 2005. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem 280:18341–18347 [DOI] [PubMed] [Google Scholar]
- 357. Fenton JI, Birmingham JM, Hursting SD, Hord NG. 2008. Adiponectin blocks multiple signaling cascades associated with leptin-induced cell proliferation in Apc Min/+ colon epithelial cells. Int J Cancer 122:2437–2445 [DOI] [PubMed] [Google Scholar]
- 358. Ogunwobi OO, Beales IL. 2008. Globular adiponectin, acting via adiponectin receptor-1, inhibits leptin-stimulated oesophageal adenocarcinoma cell proliferation. Mol Cell Endocrinol 285:43–50 [DOI] [PubMed] [Google Scholar]
- 359. Fenton JI, Birmingham JM. 2010. Adipokine regulation of colon cancer: adiponectin attenuates interleukin-6-induced colon carcinoma cell proliferation via STAT-3. Mol Carcinog 49:700–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Rondini EA, Harvey AE, Steibel JP, Hursting SD, Fenton JI. 2011. Energy balance modulates colon tumor growth: interactive roles of insulin and estrogen. Mol Carcinog 50:370–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Landskroner-Eiger S, Qian B, Muise ES, Nawrocki AR, Berger JP, Fine EJ, Koba W, Deng Y, Pollard JW, Scherer PE. 2009. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clin Cancer Res 15:3265–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Denzel MS, Hebbard LW, Shostak G, Shapiro L, Cardiff RD, Ranscht B. 2009. Adiponectin deficiency limits tumor vascularization in the MMTV-PyV-mT mouse model of mammary cancer. Clin Cancer Res 15:3256–3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Shackelford DB, Shaw RJ. 2009. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer 9:563–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Barb D, Neuwirth A, Mantzoros CS, Balk SP. 2007. Adiponectin signals in prostate cancer cells through Akt to activate the mammalian target of rapamycin pathway. Endocr Relat Cancer 14:995–1005 [DOI] [PubMed] [Google Scholar]
- 365. Sablina AA, Hahn WC. 2007. The role of PP2A A subunits in tumor suppression. Cell Adh Migr 1:140–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. 2004. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev 30:193–204 [DOI] [PubMed] [Google Scholar]
- 367. Lam JB, Chow KH, Xu A, Lam KS, Liu J, Wong NS, Moon RT, Shepherd PR, Cooper GJ, Wang Y. 2009. Adiponectin haploinsufficiency promotes mammary tumor development in MMTV-PyVT mice by modulation of phosphatase and tensin homolog activities. PLoS One 4:e4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Dhillon AS, Hagan S, Rath O, Kolch W. 2007. MAP kinase signalling pathways in cancer. Oncogene 26:3279–3290 [DOI] [PubMed] [Google Scholar]
- 369. Bowman T, Garcia R, Turkson J, Jove R. 2000. STATs in oncogenesis. Oncogene 19:2474–2488 [DOI] [PubMed] [Google Scholar]
- 370. Karim R, Tse G, Putti T, Scolyer R, Lee S. 2004. The significance of the Wnt pathway in the pathology of human cancers. Pathology 36:120–128 [DOI] [PubMed] [Google Scholar]
- 371. Turecková J, Kucerová D, Vojtechová M, Sloncová E, Tuhácková Z. 2006. Expression of β-catenin is regulated by PI-3 kinase and sodium butyrate in colorectal cancer cells. Int J Mol Med 17:69–75 [PubMed] [Google Scholar]
- 372. Karin M, Cao Y, Greten FR, Li ZW. 2002. NF-κB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2:301–310 [DOI] [PubMed] [Google Scholar]
- 373. Fruehauf JP, Meyskens FL., Jr 2007. Reactive oxygen species: a breath of life or death? Clin Cancer Res 13:789–794 [DOI] [PubMed] [Google Scholar]
- 374. Brown KA, Simpson ER. 2010. Obesity and breast cancer: progress to understanding the relationship. Cancer Res 70:4–7 [DOI] [PubMed] [Google Scholar]
- 375. Fox EM, Andrade J, Shupnik MA. 2009. Novel actions of estrogen to promote proliferation: integration of cytoplasmic and nuclear pathways. Steroids 74:622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Silva CM. 2004. Role of STATs as downstream signal transducers in Src family kinase-mediated tumorigenesis. Oncogene 23:8017–8023 [DOI] [PubMed] [Google Scholar]
- 377. Mutoh M, Teraoka N, Takasu S, Takahashi M, Onuma K, Yamamoto M, Kubota N, Iseki T, Kadowaki T, Sugimura T, Wakabayashi K. 2011. Loss of adiponectin promotes intestinal carcinogenesis in Min and wild-type mice. Gastroenterology 140:2000–2008, 2008.e1–2 [DOI] [PubMed] [Google Scholar]
- 378. Otani K, Kitayama J, Yasuda K, Nio Y, Iwabu M, Okudaira S, Aoki J, Yamauchi T, Kadowaki T, Nagawa H. 2010. Adiponectin suppresses tumorigenesis in Apc(Min)(/+) mice. Cancer Lett 288:177–182 [DOI] [PubMed] [Google Scholar]
- 379. Nishihara T, Baba M, Matsuda M, Inoue M, Nishizawa Y, Fukuhara A, Araki H, Kihara S, Funahashi T, Tamura S, Hayashi N, Iishi H, Shimomura I. 2008. Adiponectin deficiency enhances colorectal carcinogenesis and liver tumor formation induced by azoxymethane in mice. World J Gastroenterol 14:6473–6480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, Oshima M, Taketo MM. 2001. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Delta 716) knockout mice. Nat Med 7:1048–1051 [DOI] [PubMed] [Google Scholar]
- 381. Kamada Y, Matsumoto H, Tamura S, Fukushima J, Kiso S, Fukui K, Igura T, Maeda N, Kihara S, Funahashi T, Matsuzawa Y, Shimomura I, Hayashi N. 2007. Hypoadiponectinemia accelerates hepatic tumor formation in a nonalcoholic steatohepatitis mouse model. J Hepatol 47:556–564 [DOI] [PubMed] [Google Scholar]
- 382. White PB, True EM, Ziegler KM, Wang SS, Swartz-Basile DA, Pitt HA, Zyromski NJ. 2010. Insulin, leptin, and tumoral adipocytes promote murine pancreatic cancer growth. J Gastrointest Surg 14:1888–1893; discussion 1893–1894 [DOI] [PubMed] [Google Scholar]
- 383. Endo H, Hosono K, Fujisawa T, Takahashi H, Sugiyama M, Yoneda K, Nozaki Y, Fujita K, Yoneda M, Inamori M, Wada K, Nakagama H, Nakajima A. 2009. Involvement of JNK pathway in the promotion of the early stage of colorectal carcinogenesis under high-fat dietary conditions. Gut 58:1637–1643 [DOI] [PubMed] [Google Scholar]
- 384. Yan L, DeMars LC. 2010. Effects of dietary fat on spontaneous metastasis of Lewis lung carcinoma in mice. Clin Exp Metastasis 27:581–590 [DOI] [PubMed] [Google Scholar]
- 385. Yun JP, Behan JW, Heisterkamp N, Butturini A, Klemm L, Ji L, Groffen J, Müschen M, Mittelman SD. 2010. Diet-induced obesity accelerates acute lymphoblastic leukemia progression in two murine models. Cancer Prev Res (Phila) 3:1259–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Zyromski NJ, Mathur A, Pitt HA, Wade TE, Wang S, Nakshatri P, Swartz-Basile DA, Nakshatri H. 2009. Obesity potentiates the growth and dissemination of pancreatic cancer. Surgery 146:258–263 [DOI] [PubMed] [Google Scholar]
- 387. Rogozina OP, Bonorden MJ, Seppanen CN, Grande JP, Cleary MP. 2011. Effect of chronic and intermittent calorie restriction on serum adiponectin and leptin and mammary tumorigenesis. Cancer Prev Res (Phila) 4:568–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Fujisawa T, Endo H, Tomimoto A, Sugiyama M, Takahashi H, Saito S, Inamori M, Nakajima N, Watanabe M, Kubota N, Yamauchi T, Kadowaki T, Wada K, Nakagama H, Nakajima A. 2008. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut 57:1531–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Ealey KN, Archer MC. 2009. Elevated circulating adiponectin and elevated insulin sensitivity in adiponectin transgenic mice are not associated with reduced susceptibility to colon carcinogenesis. Int J Cancer 124:2226–2230 [DOI] [PubMed] [Google Scholar]
- 390. Lin S, Thomas TC, Storlien LH, Huang XF. 2000. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord 24:639–646 [DOI] [PubMed] [Google Scholar]
- 391. Pollak MN, Schernhammer ES, Hankinson SE. 2004. Insulin-like growth factors and neoplasia. Nat Rev Cancer 4:505–518 [DOI] [PubMed] [Google Scholar]
- 392. Manousos O, Souglakos J, Bosetti C, Tzonou A, Chatzidakis V, Trichopoulos D, Adami HO, Mantzoros C. 1999. IGF-I and IGF-II in relation to colorectal cancer. Int J Cancer 83:15–17 [DOI] [PubMed] [Google Scholar]
- 393. Moschos SJ, Mantzoros CS. 2002. The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology 63:317–332 [DOI] [PubMed] [Google Scholar]
- 394. Petridou E, Dessypris N, Spanos E, Mantzoros C, Skalkidou A, Kalmanti M, Koliouskas D, Kosmidis H, Panagiotou JP, Piperopoulou F, Tzortzatou F, Trichopoulos D. 1999. Insulin-like growth factor-I and binding protein-3 in relation to childhood leukaemia. Int J Cancer 80:494–496 [DOI] [PubMed] [Google Scholar]
- 395. Bohlke K, Cramer DW, Trichopoulos D, Mantzoros CS. 1998. Insulin-like growth factor-I in relation to premenopausal ductal carcinoma in situ of the breast. Epidemiology 9:570–573 [PubMed] [Google Scholar]
- 396. Wolk A, Mantzoros CS, Andersson SO, Bergström R, Signorello LB, Lagiou P, Adami HO, Trichopoulos D. 1998. Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. J Natl Cancer Inst 90:911–915 [DOI] [PubMed] [Google Scholar]
- 397. Spentzos D, Cannistra SA, Grall F, Levine DA, Pillay K, Libermann TA, Mantzoros CS. 2007. IGF axis gene expression patterns are prognostic of survival in epithelial ovarian cancer. Endocr Relat Cancer 14:781–790 [DOI] [PubMed] [Google Scholar]
- 398. Petridou E, Skalkidou A, Dessypris N, Moustaki M, Mantzoros C, Spanos E, Trichopoulos D. 2001. Insulin-like growth factor binding protein-3 predicts survival from acute childhood leukemia. Oncology 60:252–257 [DOI] [PubMed] [Google Scholar]
- 399. Petridou E, Skalkidou A, Dessypris N, Moustaki M, Mantzoros C, Spanos E, Trichopoulos D. 2000. Endogenous risk factors for childhood leukemia in relation to the IGF system (Greece). The Childhood Haematologists-Oncologists Group. Cancer Causes Control 11:765–771 [DOI] [PubMed] [Google Scholar]
- 400. Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. 2002. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8:731–737 [DOI] [PubMed] [Google Scholar]
- 401. Cowherd RB, Cowerd RB, Asmar MM, Alderman JM, Alderman EA, Garland AL, Busby WH, Bodnar WM, Rusyn I, Medoff BD, Tisch R, Mayer-Davis E, Swenberg JA, Zeisel SH, Combs TP. 2010. Adiponectin lowers glucose production by increasing SOGA. Am J Pathol 177:1936–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Furukawa K, Hori M, Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Miyazaki A, Nakayama H, Horiuchi S. 2004. Adiponectin down-regulates acyl-coenzyme A:cholesterol acyltransferase-1 in cultured human monocyte-derived macrophages. Biochem Biophys Res Commun 317:831–836 [DOI] [PubMed] [Google Scholar]
- 403. Paillasse MR, de Medina P, Amouroux G, Mhamdi L, Poirot M, Silvente-Poirot S. 2009. Signaling through cholesterol esterification: a new pathway for the cholecystokinin 2 receptor involved in cell growth and invasion. J Lipid Res 50:2203–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Mantovani A, Allavena P, Sica A, Balkwill F. 2008. Cancer-related inflammation. Nature 454:436–444 [DOI] [PubMed] [Google Scholar]
- 405. Kim S, Keku TO, Martin C, Galanko J, Woosley JT, Schroeder JC, Satia JA, Halabi S, Sandler RS. 2008. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res 68:323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. 2004. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun 316:924–929 [DOI] [PubMed] [Google Scholar]
- 407. Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, Nagasawa A, Funahashi T, Matsuzawa Y. 2004. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation 109:2046–2049 [DOI] [PubMed] [Google Scholar]
- 408. Lovren F, Pan Y, Quan A, Szmitko PE, Singh KK, Shukla PC, Gupta M, Chan L, Al-Omran M, Teoh H, Verma S. 2010. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am J Physiol Heart Circ Physiol 299:H656–H663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Ouedraogo R, Gong Y, Berzins B, Wu X, Mahadev K, Hough K, Chan L, Goldstein BJ, Scalia R. 2007. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J Clin Invest 117:1718–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. 2007. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest 117:375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Kim KY, Kim JK, Han SH, Lim JS, Kim KI, Cho DH, Lee MS, Lee JH, Yoon DY, Yoon SR, Chung JW, Choi I, Kim E, Yang Y. 2006. Adiponectin is a negative regulator of NK cell cytotoxicity. J Immunol 176:5958–5964 [DOI] [PubMed] [Google Scholar]
- 412. Tsang JY, Li D, Ho D, Peng J, Xu A, Lamb J, Chen Y, Tam PK. 2011. Novel immunomodulatory effects of adiponectin on dendritic cell functions. Int Immunopharmacol 11:604–609 [DOI] [PubMed] [Google Scholar]
- 413. Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK, Colvin RA, Kihara S, Funahashi T, Luster AD, Libby P. 2008. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res 102:218–225 [DOI] [PubMed] [Google Scholar]
- 414. Yokota T, Meka CS, Kouro T, Medina KL, Igarashi H, Takahashi M, Oritani K, Funahashi T, Tomiyama Y, Matsuzawa Y, Kincade PW. 2003. Adiponectin, a fat cell product, influences the earliest lymphocyte precursors in bone marrow cultures by activation of the cyclooxygenase-prostaglandin pathway in stromal cells. J Immunol 171:5091–5099 [DOI] [PubMed] [Google Scholar]
- 415. Folco EJ, Rocha VZ, López-Ilasaca M, Libby P. 2009. Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10. J Biol Chem 284:25569–25575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Kerbel RS. 2000. Tumor angiogenesis: past, present and the near future. Carcinogenesis 21:505–515 [DOI] [PubMed] [Google Scholar]
- 417. Bergers G, Hanahan D. 2008. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 8:592–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y. 2004. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res 94:e27–e31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. 2004. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem 279:28670–28674 [DOI] [PubMed] [Google Scholar]
- 420. Shibata R, Skurk C, Ouchi N, Galasso G, Kondo K, Ohashi T, Shimano M, Kihara S, Murohara T, Walsh K. 2008. Adiponectin promotes endothelial progenitor cell number and function. FEBS Lett 582:1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Yang H, Zhang R, Mu H, Li M, Yao Q, Chen C. 2006. Adiponectin promotes endothelial cell differentiation from human peripheral CD14+ monocytes in vitro. J Cell Mol Med 10:459–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Nakamura N, Naruse K, Matsuki T, Hamada Y, Nakashima E, Kamiya H, Matsubara T, Enomoto A, Takahashi M, Oiso Y, Nakamura J. 2009. Adiponectin promotes migration activities of endothelial progenitor cells via Cdc42/Rac1. FEBS Lett 583:2457–2463 [DOI] [PubMed] [Google Scholar]
- 423. Leicht SF, Schwarz TM, Hermann PC, Seissler J, Aicher A, Heeschen C. 2011. Adiponectin pretreatment counteracts the detrimental effect of a diabetic environment on endothelial progenitors. Diabetes 60:652–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Adachi Y, Takeuchi T, Sonobe H, Ohtsuki Y. 2006. An adiponectin receptor, T-cadherin, was selectively expressed in intratumoral capillary endothelial cells in hepatocellular carcinoma: possible cross talk between T-cadherin and FGF-2 pathways. Virchows Arch 448:311–318 [DOI] [PubMed] [Google Scholar]
- 425. Rose DP, Komninou D, Stephenson GD. 2004. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev 5:153–165 [DOI] [PubMed] [Google Scholar]
- 426. Talmadge JE, Singh RK, Fidler IJ, Raz A. 2007. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol 170:793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427. Sikder H, Huso DL, Zhang H, Wang B, Ryu B, Hwang ST, Powell JD, Alani RM. 2003. Disruption of Id1 reveals major differences in angiogenesis between transplanted and autochthonous tumors. Cancer Cell 4:291–299 [DOI] [PubMed] [Google Scholar]
- 428. Sata M, Nishimatsu H, Osuga J, Tanaka K, Ishizaka N, Ishibashi S, Hirata Y, Nagai R. 2004. Statins augment collateral growth in response to ischemia but they do not promote cancer and atherosclerosis. Hypertension 43:1214–1220 [DOI] [PubMed] [Google Scholar]
- 429. Otvos L, Jr, Haspinger E, La Russa F, Maspero F, Graziano P, Kovalszky I, Lovas S, Nama K, Hoffmann R, Knappe D, Cassone M, Wade J, Surmacz E. 2011. Design and development of a peptide-based adiponectin receptor agonist for cancer treatment. BMC Biotechnol 11:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Higgins LS, Mantzoros CS. 2008. The development of INT131 as a selective PPARγ modulator: approach to a safer insulin sensitizer. PPAR Res 2008:936906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Mueller E, Smith M, Sarraf P, Kroll T, Aiyer A, Kaufman DS, Oh W, Demetri G, Figg WD, Zhou XP, Eng C, Spiegelman BM, Kantoff PW. 2000. Effects of ligand activation of peroxisome proliferator-activated receptor γ in human prostate cancer. Proc Natl Acad Sci USA 97:10990–10995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokán M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. 2005. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366:1279–1289 [DOI] [PubMed] [Google Scholar]
- 433. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G. 2006. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 434. Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R. 2004. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care 27:256–263 [DOI] [PubMed] [Google Scholar]
- 435. Higgins LS, Depaoli AM. 2010. Selective peroxisome proliferator-activated receptor γ (PPARγ) modulation as a strategy for safer therapeutic PPARγ activation. Am J Clin Nutr 91:267S–272S [DOI] [PubMed] [Google Scholar]