Abstract
Proopiomelanocortin (POMC)-derived peptides such as melanocortins and β-endorphin (β-ED) exert their pleiotropic effects via binding to melanocortin receptors (MCR) and opioid receptors (OR). There is now compelling evidence for the existence of a functional POMC system within the osteoarticular system. Accordingly, distinct cell types of the synovial tissue and bone have been identified to generate POMC-derived peptides like β-ED, ACTH, or α-MSH. MCR subtypes, especially MC1R, MC2R (the ACTH receptor), MC3R, and MC4R, but also the μ-OR and δ-OR, have been detected in various cells of the synovium, cartilage, and bone. The respective ligands of these POMC-derived peptide receptors mediate an increasing number of newly recognized biological effects in the osteoarticular system. These include bone mineralization and longitudinal growth, cell proliferation and differentiation, extracellular matrix synthesis, osteoprotection, and immunomodulation. Importantly, bone formation is also regulated by the central melanocortin system via a complex hormonal interplay with other organs and tissues involved in energy metabolism. Among the POMC-derived peptides examined in cell culture systems from osteoarticular tissue and in animal models of experimentally induced arthritis, α-MSH, ACTH, and MC3R-specific agonists appear to have the most promising antiinflammatory actions. The effects of these melanocortin peptides may be exploited in future for the treatment of patients with inflammatory and degenerative joint diseases.
Introduction
-
Basic Biology of POMC, POMC-Derived Peptides, and Their Receptors
Regulation of POMC gene expression
POMC processing into biologically active peptides
Receptors for POMC-derived peptides
-
Basic Biology of the Osteoarticular System
Anatomy of the subchondral bone in diarthroidal joints
Cartilage types of the osteoarticular system
The process of endochondral ossification
-
Osteoarticular Pathologies: OA and RA
Inflammatory factors
Aging factors
Genetic association and reinduction of developmental processes
Mechanical loading and biomechanical factors
Contribution of underlying subchondral bone
Biomarkers in OA and RA
-
Clinical Observations Pointing Toward a Role of POMC-Derived Peptides in Bone and Cartilage in Man
Familial endocrinopathies
Acquired endocrinopathies
In vivo administration of therapeutics activating POMC peptide receptors
-
The Osteoarticular System: Direct Target Organ and Source of POMC-Derived Peptides
Synovium and synovial cell types
Cartilage and chondrocytes
Bone, osteoblasts, and osteoclastic cells
-
In Vitro and in Vivo Effects of Melanocortin Peptides and β-ED in the Osteoarticular System
Growth and differentiation
Inflammatory and immune responses
Tissue protection
Lessons from ablation of specific genes of the POMC system
-
POMC-Derived Peptides and Derivatives: Future Tools for the Treatment of Inflammatory and Degenerative Joint Diseases?
Preclinical studies with melanocortin peptides
Preclinical studies exploiting β-ED and related peptides
Open questions and perspectives for POMC-derived peptides in the future treatment of inflammatory and degenerative joint diseases
Conclusions
I. Introduction
Proopiomelanocortin (POMC) is a multifunctional precursor protein for several biologically active hormones that include ACTH, the MSH (α-, β-, and γ-MSH), and the endogenous opioid β-endorphin (β-ED). These peptides play an important role in a diversity of physiological processes including energy homeostasis, adrenal function, sexual activity, thermoregulation, nociception, exocrine gland activity, immune function, and pigmentation. Although originally characterized as neurohormones induced by stressful signals in context of the classical hypothalamic-pituitary-adrenal (HPA) stress axis, it is now established that POMC and its derived peptides can also be autonomously generated in a number of peripheral tissues, e.g. the skin (1). Here, receptors for POMC peptides have been identified in various resident cell types where they elicit biological effects far beyond the initially identified action of their ligands. A very fascinating aspect of POMC-derived peptides is their immunomodulatory potential, especially that of melanocortin-derived peptides (2).
This review aims at bringing the POMC system in bone and cartilage into the spotlight of physicians and scientists working in related fields. First, the basic biology of POMC gene regulation and POMC processing is presented followed by a brief description of the various POMC peptides receptors and their principal signal transduction pathways. Subsequently, the basic biology of the osteoarticular system and pathologies of the two most common osteoarticular diseases affecting large segments of the Western population, osteoarthritis (OA) and rheumatoid arthritis (RA), are described. This is followed by a chapter dedicated to clinical observations pointing toward a functional role of POMC-derived peptides in bone and cartilage of man. After that, the many lines of evidence are presented that collectively demonstrate that the osteoarticular system is a direct target organ and source of POMC peptides. Finally, results from preclinical studies with melanocortin peptides as well as β-ED in animal models of arthritis and OA will be reviewed. Special focus will be on open questions and perspectives of POMC-derived peptides as a future treatment for degenerative and inflammatory joint diseases.
II. Basic Biology of POMC, POMC-Derived Peptides, and Their Receptors
A.Regulation of POMC gene expression
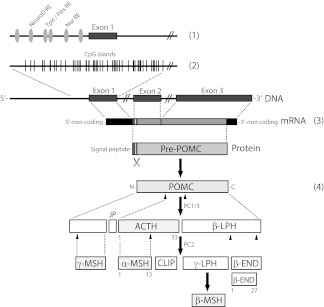
The human POMC gene is composed of three exons (86, 152, and 833 bp long) and two large introns (Fig. 1). Exon 1 is untranslated, whereas parts of exon 2 code for the signal sequence. Exon 3 codes for the bioactive POMC-derived peptides that are generated after proteolytic processing of POMC (3). Only the full-length POMC transcript (1200 bp) is functional because smaller transcripts, e.g. the 800-bp transcript lacking exons 1 and 2, are neither processed nor secreted (4). POMC gene expression is highly complex and involves several cis-acting elements in the 5′ region upstream of exon 1 but also within the intron 2 of the POMC gene. The trans-acting elements that regulate POMC transcription in the pituitary include on one hand transcription factors with limited neuroendocrine tissue expression, i.e. pituitary homeobox 1, T box factor, and neurogenic differentiation factor, and on the other hand ubiquitously expressed factors including members of the orphan nuclear receptor family (Nur77, Nurr1, and NOR1), nuclear factor-κB (NF-κB), and signal transducers and activators of transcription (5) (Fig. 1). Glucocorticoids acting via glucocorticoid-responsive elements repress POMC transcription. Interestingly, the POMC promoter contains neither a cAMP-responsive element consensus site nor a Ca2+-responsive element. CRH, the most upstream player of the HPA axis and the factor responsible for the circadian expression of the POMC gene in the pituitary, thus uses some of the above trans-acting elements in a synergistic and not fully understood manner to drive POMC transcription. In peripheral cell types, e.g. those of the skin, proinflammatory stressors including UV light irradiation, proinflammatory cytokines such as IL-1 and TNF are typical inducers of POMC gene expression (1). POMC gene induction by proinflammatory signals may be associated with up-regulation of POMC peptide receptors to confer a concerted action of POMC-derived peptides during the stress response as demonstrated for melanin pigmentation of mammalian skin (6).
Figure 1.
Regulation of POMC gene expression and POMC peptide synthesis. POMC expression is tightly orchestrated involving 1) transcriptional regulation at various cis-acting elements within the central pituitary promoter located upstream of exon 1; 2) epigenetic regulation, i.e. methylation of CpG islands of the pituitary promoter region as well as of exon 1; 3) transcription of all three exons to generate full-length POMC transcripts including the signal sequence of POMC protein encoded by exon 2; and 4) posttranslational processing of POMC protein by the action of specific PC. PC cleavage sites are indicated as arrowheads pointing upward. Dotted lines depict enlarged regions within the gene, mRNA, or protein. Note that additional cis-acting elements outside the central pituitary promoter region, e.g. within exon 1 or those within intron 2 (so-called weak peripheral promoter) are not depicted for the sake of clarity. Numbers indicate the length of selected POMC-derived peptides. CLIP, Corticotropin-like intermediate lobe peptide; JP, junctional peptide; LPH, lipotropic hormone; NT, N-terminal peptide.
Expression of the POMC gene is further controlled by an epigenetic mechanism involving hypermethylation of a CpG-rich island upstream of the exon 1 in the 5′ POMC promoter region (7) (Fig. 1). In contrast to the pars anterior of the human pituitary gland in which POMC is physiologically expressed, the POMC promoter of many peripheral tissues is constantly methylated.
B. POMC processing into biologically active peptides
After translation, the approximately 30-kDa POMC precursor protein is further processed by prohormone convertases (PC). These are Ca2+-dependent serine endoproteases of the subtilisin/kexin type that cleave behind the consensus sequence (K/R)-(X)n-(K/R) ↓ (cleavage site), where n = 0, 2, 4, or 6 and X is any amino acid except Cys (8). PC1/PC3 and PC2 are considered the classical POMC processing enzymes, although paired basic amino acid-cleaving enzyme 4 (PACE4) and furin convertase have been shown to process POMC as well. PC1 cleaves into ACTH and β-lipotropic hormone, whereas PC2 further processes ACTH and β-lipotropic hormone to the various MSH as well as β-ED (Fig. 1).
Of note, the N-terminal amino acid sequence of ACTH is contained within the tridecapeptide α-MSH. Moreover, ACTH and the various MSH share the central pharmacophore His6-Phe7-Arg8-Trp9. Based on this structural homology and the originally discovered stimulatory effect on frog melanophores and cells of the adrenal gland, these peptides are the bona fide melanocortin peptides. In contrast, β-ED is grouped within the family of endogenous opioids. α-MSH but not ACTH requires N-acetylation and C-amidation by C-terminal carboxypeptidases, α-amidating monooxygenase, and N-acetyltransferase to obtain full biological activity. Although POMC is considered a precursor protein for the above biologically active peptide hormones it should be noted that recently POMC has been demonstrated to possess weak melanotropic activity in vitro (9). The mechanism of this effect of POMC is unknown.
C. Receptors for POMC-derived peptides
1. Melanocortin receptors (MCR)
The melanocortin peptides ACTH as well as α-, β-, and γ-MSH bind with high affinity to MCR. They belong to the superfamily of G protein-coupled receptors with seven transmembrane domains (10). Five MCR subtypes, MC1R–MC5R, have been cloned that have a sequence homology of 39–61% to one another at the amino acid level. The MCR subtypes bind melanocortin with different affinities. MC1R binds both ACTH and α-MSH with almost equal affinity, whereas MC2R is selective for ACTH. However, ACTH also binds to MC3R, MC4R, and MC5R, although γ-MSH is the preferred ligand for MC3R and α-MSH the preferred ligand for the MC5R (Table 1). Ligand stimulation of all MCR leads to activation of Gs followed by adenylate cyclase activation and intracellular increase of cAMP (11). The cAMP response of cells after melanocortin peptide stimulation depends on the expressed MCR subtype but is also largely influenced by the experimental conditions as well as by cell type-specific determinants of cAMP signaling. Examples have been described in which MCR-expressing cells respond significantly to nanomolar doses of α-MSH with various biological responses, whereas changes in intracellular cAMP are minimal or even nondetectable (12, 13). Calcium mobilization from intracellular stores after stimulation of cells with melanocortin peptides has also been demonstrated for MC1R and MC3–5R-expressing cells (14). Controversial results on calcium flux, however, were found in MC2R-expressing cells including those of the adrenal gland upon treatment with ACTH (15–17). There is increasing evidence that at least MC1R and MC4R stimulation with respective ligands can activate MAPK including MAPK1/2 and p38 (18–20). Of note, when combined with proinflammatory costimuli such as IL-1 or TNF, melanocortin peptides have been widely shown to suppress signal transduction pathways linked to inflammation, and immune responses. This has been most extensively demonstrated for various MC1R-expressing cell types in which α-MSH can significantly attenuate activation of the redox-sensitive nuclear factor-κB NF-κB (2).
Table 1.
Binding affinities of POMC-derived peptides to their respective receptors
| POMC peptide receptor | Natural ligand and affinity |
|---|---|
| MCR | |
| MC1R | α-MSH > ACTH ≫ γ-MSH |
| MC2R | ACTH |
| MC3R | γ-MSH = ACTH ≥ α-MSH |
| MC4R | α-MSH = ACTH ≫ γ-MSH |
| MC5R | α-MSH ≥ ACTH > γ-MSH |
| OR | |
| MOR | EM-1/2 > β-ED |
| DOR | Enkephalinsa ≫ β-ED |
| KOR | Dynorphinsb ≫ β-ED |
Derived from proenkephalin.
Derived from prodynorphin.
Gene expression analyses, in vivo studies using targeted disruption of MCR and human genetics, have provided combined evidence that pigmentation, adrenocortical steroidogenesis, energy homeostasis, natriuresis, erectile responses, energy homeostasis, and exocrine gland secretion are physiologically regulated by the melanocortin system (21). However, advances in the detection methods of MCR and application of novel biological readouts suggest a role of the POMC system beyond the originally discovered functions of melanocortin peptides. MC1R was originally detected mainly in melanocytes and neural cells but has been detected in a myriad of other resident cell types of many nonneural organs. These include cell types of the immune system and of skin and other organs (2). MC2R is primarily expressed by the adrenal gland but has also been detected outside this classical endocrine organ as will be described below. Although MC3R and MC4R are considered receptors typically expressed by neural tissues, there is further recent evidence that functional MC4R are present in some nonneuronal cell types, e.g. in specialized cells of the hair follicle and epidermal melanocytes (22, 23). MC5R expression, in contrast, has early been considered to be broadly expressed in a diversity of tissues including muscle, heart, and exocrine glands (2).
2. Opioid receptors (OR)
The POMC peptide β-ED binds to OR which are also G protein-coupled receptors (Table 1). β-ED has high affinity for both the μ-OR (MOR) and δ-OR (DOR) (24). In contrast, β-ED has little affinity for the κ-OR (KOR), the bona fide receptor for dynorphins. The signaling events elicited by β-ED are complex and different compared with MCR-mediated responses. Typically, stimulation of cells with β-ED reduces forskolin-induced cAMP synthesis. However, both, inhibitory and stimulatory effects on adenylate cyclase have been reported (25). Moreover, activation of phospholipase C, MAPK, or inwardly rectifying potassium channels as well as induction of calcium influx can occur upon β-ED stimulation. Interestingly, some of the opioid-mediated effects of β-ED appear to be MOR independent (26). In analogy to MCR, MOR and DOR have been detected in an increasing number of extraneural tissues and nonneuronal cells indicating functions of β-ED far beyond antinociception (24).
III. Basic Biology of the Osteoarticular System
A. Anatomy of the subchondral bone in diarthroidal joints
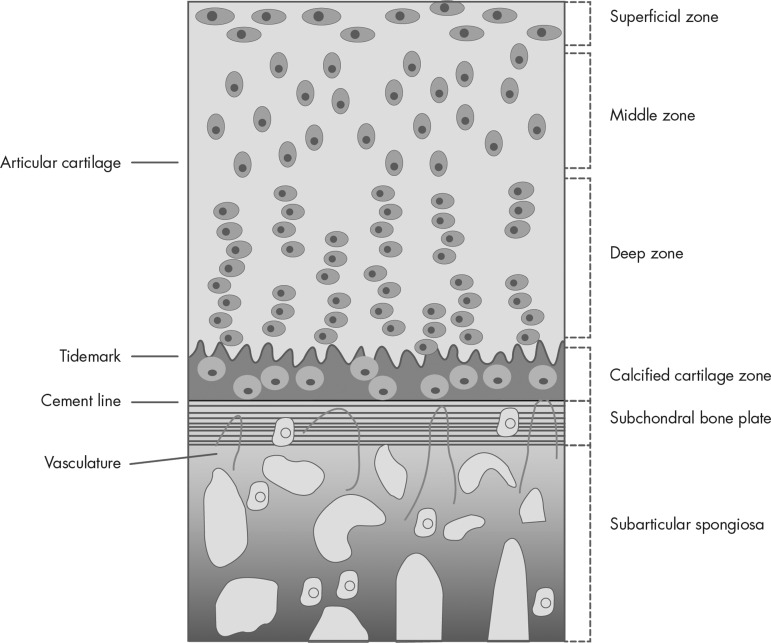
In the human knee joint, the hyaline articular cartilage is separated from the bone marrow cavity by the subchondral bone plate (Fig. 2). This plate is normally composed of two layers: the mineralized cartilage, which is contiguous with the deepest part of the articular cartilage, and a layer of lamellar bone, which is in contact with the marrow and gives rise to the supporting subarticular trabeculae. At the interface between the layer of calcified cartilage and the adjacent articular cartilage is the so-called tidemark, a region that is also referred to as the mineralization front or calcified line. At the junction of the tidemark and the articular cartilage, a higher level of calcification is present compared with surrounding areas; this demarcation is recognized radiographically as the limit of the joint space. The collagen fibers, which are arranged as a meshwork within the articular cartilage, pass through the region of the tidemark and are embedded within the layer of calcified cartilage. In some instances, these collagen fibers can be traced through the layer of calcified cartilage as far as the so-called cement line, which is the interface between the calcified cartilage and the underlying, closely attached lamellar bone (27). Depending upon the joint, the subchondral bone plate varies in thickness. The trabeculae arising from there are referred to as supporting trabeculae and, together with the additional bony components, are included under the term subarticular. Thus, the subarticular spongiosa and the subchondral bone plate form the subchondral bone (28).
Figure 2.
Schematic representation of the general structure of human articular cartilage. This drawing is representative for a large adult diathroidal joint (knee or hip) and shows the different horizontal zones of articular cartilage in connection with the subsequent layers of subchondral bone and the subarticular spongiosa. The chondrocytes residing in the calcified cartilage zone are hypertrophic. All terms are explained in the text.
The anatomy of the subchondral region is highly variable. These variations include the contour of the tidemark and cement line, the number and type of perforations in the subchondral bone plate, and its thickness, density, and composition. Differences in the trabecular structure and mechanical properties of weight-bearing and non-weight-bearing areas have also been recognized (29, 30).
B. Cartilage types of the osteoarticular system
Hyaline cartilage, the predominant cartilage tissue of diathroidal joints, is a highly specialized connective tissue of mesenchymal origin (Fig. 2). The only cell type within this tissue is the chondrocyte embedded in its self-contrived extracellular matrix (ECM). This ECM is composed of a fibrillar compartment of collagen fibrils filled with an extrafibrillar compartment, rich in proteoglycans. Unlike other connective tissues, cartilage does not contain blood vessels and is not deeply innervated by nerve fibers. Therefore, oxygen and nutrient supply of chondrocytes is low and depends on diffusion from surrounding tissues, e.g. from the perichondrium. This leads to low growth rates and, after injury, to limited repair (31, 32).
Hyaline cartilage occurs in two types in various locations within the body. As permanent cartilage, the tissue persists throughout life in ears, in various structures of the airways, and in diarthroidal joints. Permanent articular joint cartilage covers the surface of bones and is able to bear very large compressive loads without distortion and allows smooth, frictionless movement of the joints. The resident cells, the articular chondrocytes, produce and maintain their surrounding ECM comprising collagens (predominantly collagen II, with smaller amounts of collagens VI, IX, and XI) (33) and proteoglycans (predominantly aggrecan with smaller amounts of decorin, biglycan, and fibromodulin) and further noncollagenous matrix proteins (34).
Articular cartilage is composed of four different horizontal regions (Fig. 2). 1) The superficial tangential zone is composed of thin collagen fibrils in tangential array that are associated with a high concentration of decorin and a low concentration of aggrecan. Here the chondrocytes are flattened and disc shaped. 2) The middle, transitional zone is filled with radial bundles of thicker collagen fibrils and with chondrocytes that adopt a rounded or spherical shape. 3) In the deep radial zone, the proteoglycan content is high and collagen bundles are thickest and are arranged in a radial fashion. Here, the chondrocytes are rounded and arranged in columns. 4) The calcified cartilage zone islocated immediately below the tidemark and above the subchondral bone. It is characterized by rounded chondrocytes in uncalcified lagunae and the absence of proteoglycans. The calcified zone persists after growth plate closure as the tidemark and serves as an important mechanical buffer between the uncalcified articular cartilage and the subchondral bone. From the superficial to the deep zone, cell density progressively decreases, whereas cell volume and the proportion of proteoglycan relative to collagen increase (35–37).
In addition to the horizontal matrix subdivisions, a circumferential differentiation of matrix components into pericellular, territorial, and interterritorial matrices around each chondrocyte is described. The pericellular matrix is composed mainly of thin filaments of collagen VI associated with noncollagenous proteins and small proteoglycans as decorin and biglycan (38, 39). The territorial and interterritorial zones consist of collagen networks with differing diameter and with the thickest fibrils being located in the interterritorial area. This collagen network consists primarily of type II collagen fibrils with type XI collagen located within the fibril core and type IX collagen integrated into the fibril surface with the noncollagen domain projecting outward, permitting association with other matrix components and retention of proteoglycans (32, 40).
Resilience resistance of the cartilage matrix to load is provided by the large proteoglycan aggrecan, which is attached to hyaluronic acid polymers via link protein. The half-life of aggrecan core protein ranges from 3–24 yr and is essential for protecting the collagen network, which has a half-life of more than 100 yr if not subjected to inappropriate degradation. The collagen network provides the tensile strength to resist compressive forces.
A large number of other noncollagenous molecules, including biglycan, decorin, fibromodulin, the matrilins, and cartilage oligomeric matrix protein (COMP), are also present in the matrix. Interactions between type IX collagen and COMP or matrilin-3 are essential for proper formation and maintenance of the integrity of the articular cartilage matrix (41). Perlecan enhances fibril formation (42), and collagen VI microfibrils connect to collagen II and aggrecan via complexes of matrilin-1 and biglycan or decorin (38).
In contrast, transient cartilage or growth cartilage is destined to be replaced by bone, e.g. during skeletal development in the process of endochondral ossification. Skeletal development is initiated by the formation of the cartilage templates, located in the growth plates, for the future bones. The so-called bone anlagen derive from mesenchymal progenitor cells (MPC) recruited into areas where bones are to be built. In these locations, the MPC condensate, differentiate into chondrocytes, and prefigure the future bone. In transient growth cartilages, the chondrocytes do not have a unique pheno- and genotype. They change their morphology and the expression of their gene and protein repertoire according to the progression of the chondrogenic differentiation process. This process of bone formation including chondrogenic differentiation of MPC, chondrocyte proliferation, chondrocyte differentiation, and mineralization is termed endochondral ossification. Temporary cartilage occurs also in long bones located between the epiphyseal and metaphyseal regions, where it enables long bone growth until adulthood and in bone fracture calli, where it is essential for bone reunion and fracture repair after injuries (43).
C. The process of endochondral ossification
To form growth cartilage as template for long bone growth chondroprogenitor cells, the MPC have to be recruited. MPC are not necessarily located in the region where they are needed but reside at specialized sites close to the diarthroidal joint as in the bone marrow or in differentiated tissues such as synovium and periosteum. Although a well-defined site for MPC in synovial tissue is not described, periosteal tissue is known to contain a specialized region that is the home for MPC. The best and most described source for MPC is the bone marrow. Mammalian bone consists of bone cells at different developmental stages (including osteocytes, preosteoblasts, and osteoblasts), collagen fibrils, and mineral deposits such as calcium and phosphate. The bone cavity is filled with soft bone marrow and blood vessels. MPC also reside in this bone cavity and are proposed to give rise to the majority of marrow stromal cell lineages, including chondrocytes and osteocytes (44). MPC exist in different commitment and differentiation states, most likely the so-called naive MPC with true stem cell attributes reside as part of the stroma, but the MPC with committed osteo-chondroblastic progenitor status reside in the osteoblastic niche (45).
After commitment of MPC to the chondrogenic lineage, they usually enter a process called endochondral ossification (Fig. 3). Endochondral ossification is particularly important for bone development during embryogenesis and repair as well as for longitudinal growth of long bones. Within the highly organized structures of growth plates, located between the epiphyseal and the metaphyseal part of long bones, differentiated cartilage cells transit through a temporospatial cascade of late differentiation events that sequentially include proliferation and several steps of maturation, culminating in chondrocyte hypertrophy. Longitudinal growth is collectively achieved by proliferation and hypertrophic differentiation of chondrocytes, along with appropriate matrix synthesis. After invasion of blood vessels from the subchondral bone, the majority of hypertrophic cells undergo apoptosis, and the cartilage template is remodeled into trabecular bone. The overall process is regulated by hormones as well as by locally acting autocrine signals derived from chondrocytes themselves or by paracrine signals originating from cells of surrounding tissues, e.g. the perichondrium or subchondral blood vessels. The interaction of chondrocytes with their surrounding matrix via cell surface receptors is thought to play a key role in the regulation of survival, proliferation, and maturation of cartilage cells (46–50).
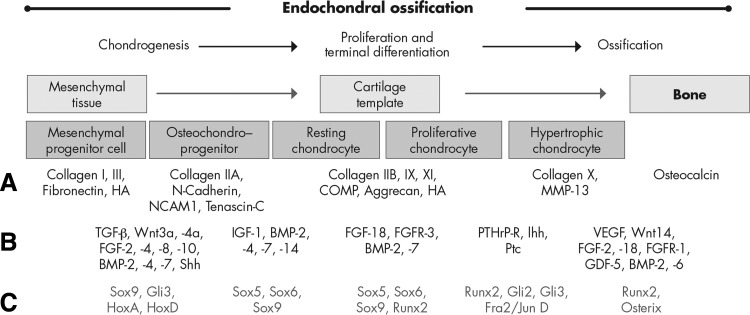
Figure 3.
Sequential steps during development of long bones. A, Different stages of endochondral ossification are shown schematically along with stage-specific ECM marker proteins; B and C, underlying signaling/growth factors (B) and transcription factors (C) that are responsible for the transition of the cells into the next differentiation stage and translation into required stage-specific matrix production are indicated below. [Reproduced from S. Grässel and R. Dreier 2011 Effect of cellular microenvironment and paracrine signals on chondrogenic differentiation of mesenchymal progenitor cells In: Singh SR, ed. Stem cells, regenerative medicine and cancer. Nova Science Publishers, New York, p 441–467 (236), with permission.]
Endochondral ossification can be divided into five main stages (Fig. 3): 1) commitment and condensation of mesenchymal progenitor cells to differentiate into cartilage, which is caused by paracrine factors, such as fibroblast growth factors (FGF), bone morphogenetic proteins (BMP), and Hedgehog signaling; 2) differentiation of the condensed MPC into chondrocytes in a process where the master transcription factor Sox9 plays an essential role in controlling the expression of many downstream genes specific for cartilage tissue development as aggrecan, and collagens IIA, IX, and XI; 3) rapid division and proliferation of chondrocytes and massive production of the cartilage-specific ECM mainly consisting of collagen IIB, aggrecan, and hyaluronic acid; 4) chondrocytes in the center of cartilage templates stop to proliferate and increase in size severalfold, thereby entering the hypertrophic state, the composition of the ECM (mainly collagen type X and fibronectin) is remodeled, and chondrocytes start to mineralize their environment with calcium salts; and 5) invasion of blood vessels is induced, and the hypertrophic chondrocytes die by apoptosis. At this stage, osteoblast precursors invade the remodeled intermediate tissue and start forming bone using the cartilaginous matrix as template and replacing it by a mineralized, bony matrix.
The regulation of this cascade is highly complex and involves numerous transcription factors besides Sox9 (Sox family, Hox family), growth factors such as TGF-β, BMP, wingless/int (Wnt), and FGF as well as and members of the hedgehog/PTH family (see reviews in Refs. 43, 46, 51, and 52). The tightly regulated transition of MPC to chondrocytes involves not only the time- and space-dependent expression of transcription and growth factors but also cell-cell and cell-matrix interactions with the immediate microenvironment. Cells interact with the ECM or with other cells using membrane receptors or different types of adhesion molecules, i.e. N-cadherin, which plays a highly decisive role in mesenchymal condensation, and these recognitions translate into intracellular signals affecting gene expression, cell proliferation, and migration (53–55). Cell-matrix interactions play a profound role in establishing the mechanical strength necessary to pull the MPC together to form a condensed cell mass. Cells condense and subsequently migrate out from the condensation areas, a process that requires substantial ECM remodeling. Thus, it is not surprising that the ECM composition is tightly regulated throughout chondrogenesis to meet the specific mechanical needs in each step of this morphogenetic process (51).
In summary, ECM greatly affects mesenchymal condensation in two different ways: it establishes the adequate mechanical environment and moreover modulates the concentration or activity of important growth factors like BMP or FGF. These complex and important roles of the ECM during mesenchymal condensation still are elusive in part and are in need of further investigation (51).
A complex interplay between numerous factors derived from neighboring cells and the immediate tissue environment is responsible for proper joint formation through endochondral ossification during skeletogenesis and proper joint homeostasis throughout adult life. However, disturbance of the delicate balance in tissue turnover toward increased catabolic and anabolic reactions and recapitulation of embryonic developmental processes lead to osteoarticular pathologies such as OA, the most common form of chronic degenerative joint diseases. A second major osteoarticular pathology, RA, arises mainly from inflammatory and proliferative processes in the synovium; however, both disease entities promote extensive cartilage destruction resulting in loss of joint function. The next section will highlight known basic mechanisms that underlie these osteoarticular pathologies.
IV. Osteoarticular Pathologies: OA and RA
A. Inflammatory factors
OA and RA are conditions that affect many tissues of the joint. However, the basic cellular mechanisms regulating chondrocyte responses are very different in OA and RA. Although characterized by different etiologies, both diseases have in common that the activated, inflamed synovial tissue contributes to the disease pathology, although more prevalent in RA, where a proliferating synovial pannus is a highlight of the disease. The most extensive studied tissue with respect to OA pathophysiology is the articular cartilage and its degradative changes.
In both pathologies, however, the equilibrium in cartilage matrix turnover and formation is disrupted, and the rate of loss of collagens and proteoglycans from the matrix exceeds the rate of deposition of newly synthesized molecules. This is largely the result of elevated expression and increased activity of proteolytic enzymes. In OA, these degradative enzymes are mainly produced by the chondrocytes, whereas in RA, the inflamed synovium is the major source of cytokine-induced proteinases. However, synovial inflammation is also occurring episodically in OA, then being a source of cytokines and cartilage-degrading enzymes (56). In addition, chondrocytes are capable of secreting several proinflammatory cytokines such as IL-1β and TNF, which are found in both RA and OA joint tissues and fluids. Chondrocytes respond to these inflammatory mediators by increasing the synthesis of prostaglandins, proteinases, and nitric oxide (NO) (57). In both entities, matrix metalloproteases (MMP) and aggrecanases are the best known and described proteinases in joint tissues and synovial fluid because they degrade collagens (native and gelatinized) and proteoglycans (58, 59). In OA, proteoglycan and collagen II loss occurs initially at the cartilage surface in the superficial layer (60), whereas in RA, chondrocyte-derived proteolytic activity is highest at the cartilage-pannus interface and in deeper zones of the cartilage tissue (61). Additionally, the synovium is responsible for elevated MMP levels in the synovial fluid. With respect to matrix synthesis activity, in early RA, collagen II synthesis is impaired, whereas in early OA, overall gene expression is up-regulated and collagen II and aggrecan biosynthesis is enhanced, which is considered as a compensatory response to matrix degradation (62, 63).
Interestingly, adipokines have recently received increased attention in joint diseases because they can have pro- or antiinflammatory effects in joint tissues and may serve as a link between the neuroendocrine and immune systems (64). Leptin expression is enhanced during acute inflammation, correlating negatively with inflammatory markers in RA sera (65). Expression of leptin is elevated in OA cartilage and in osteophytes, and it stimulates IGF-I and TGF-β1 synthesis in chondrocytes (66, 67). Possibly, a dysregulated balance between leptin and other adipokines, such as adiponectin, promotes destructive inflammatory processes (68). Another adipokine, resistin, plays a role in early stages of trauma-induced OA and in RA at local sites of inflammation where the serum level of resistin is an indicator of disease activity (69).
B. Aging factors
OA is the most common cause for chronic disability in older adults and rises directly with age. It is of note that OA is not an inevitable consequence of aging because not all older adults develop OA and not all joints are equally affected. It is becoming apparent that aging changes in the musculoskeletal system contribute to the development of OA in conjunction with other factors as obesity, joint injuries, sex, and genetic disposition. Joint failure in OA is a consequence of progressive changes in several compartments of the muskuloskeletal system that include subchondral bone, muscle, synovium, ligaments, tendons, and menisci (70). Chondrocyte senescence promoted by reactive oxygen species, DNA damage, and mitochondrial dysfunction likely contributes to the development of OA in older adults (71). In addition, reduced repair response most likely due to the reduced ability of cells to respond properly to growth factor stimulation is a key event in aging cartilage. Chondrocytes in aged and OA cartilage are less responsive to TGF-β and IGF-I. The alteration in signaling in the case of TGF-β seems to occur at the level of the receptor (altered ratio of the activin receptor-like kinases) (72). Loss of IGF-I responsiveness might be connected to oxidative stress and increasing reactive oxygen species levels. Excessive reactive oxygen species level might lead to aberrant MAPK activation which can contribute to cell senescence (73, 74).
A gradual deterioration of the immune system known as immunosenescence occurs with aging. The resulting failure to mount effective immune responses renders elderly individuals more susceptible to infections and to development of certain cancers (75). Aging is also associated with an increase in autoimmunity and in the incidence of certain autoimmune diseases, such as RA (76–78). Individuals with RA are immunocompromised (79), and accumulating evidence suggests that premature aging of the immune system contributes to the pathogenesis of RA (80, 81). The progressive deterioration of the immune system with advancing age may therefore predispose to the development of RA late in life. In addition, the chronic immune stimulation characteristic of RA may itself age the immune system and lead to progression of the disease (82, 83).
C. Genetic association and reinduction of developmental processes
Evidence exists that developmental disruption of chondrogenesis and/or joint formation might predispose to OA pathogenesis. Mutations in a variety of genes coding for ECM components or transcription or growth factors cause dyschondroplastic effects that are often accompanied by early-onset OA (84, 85). Genes involved in cartilage development and chondrodysplasia might have equally vital roles in articular cartilage homeostasis. Possibly, subtle polymorphisms in these genes might not cause developmental defects but may predispose to OA and increased susceptibility to RA. Additional, ectopic activation of these pathways later in life might also contribute to OA development (50). Similarly, microarray analysis of cocultures of synovial fibroblasts with chondrocytes in alginate has identified markers of inflammation and cartilage destruction associated with RA pathogenesis (86).
OA pathogenesis commonly includes a switch from articular to the growth-plate chondrocyte phenotype and reinitiation of proliferation and hypertrophic differentiation (87). Many processes associated with chondrocyte hypertrophy, such as inhibition of Sox9, collagen II, and aggrecan, induction of MMP-13 expression, chondrocyte apoptosis, ECM mineralization, and recruitment of blood vessels and osteoclasts, contribute to OA pathophysiology (50). A study by Fuerst et al. (88) including 100 patients with end-stage OA supports the relation between chondrocyte hypertrophy and OA. They demonstrated that cartilage calcification correlates with clinical OA scores and collagen X expression. The authors state that induction of hypertrophy in articular chondrocytes leads to matrix calcification and thus is a general feature of OA. This observation underscores the clinical relevance of chondrocyte maturation associated processes for OA pathogenesis. There are functional studies that suggest that inhibition of chondrocyte hypertrophy is a promising approach for preventing or treating OA. However, those studies are preclinical attempts in mouse models where OA was surgically induced (89, 90). Whether there are clinical and therapeutic implications for the human situation, where nonsurgical and nontraumatic forms of OA predominate, remains unclear at this time.
It appears well established that disruption of normal development of cartilage and joint formation and ectopic reinduction of developmental processes in adult articular cartilage both contribute to pathophysiology of OA. However, early diagnosis of the disease and treatment strategies that stop, halt, or reverse degenerative processes in OA are still to be implemented in the clinic.
D. Mechanical loading and biomechanical factors
Biomechanical factors due to trauma and injurious mechanical stress are strongly implicated in initiating OA lesions. Mechanical disruption of cell-matrix interactions may lead to altered chondrocyte behavior, contributing to fibrillations, cell clusters, and changes in quantity, distribution, or composition of matrix proteins (91, 92).
Traumatic injury induces general gene expression patterns that lead to increased expression of inflammatory mediators, cartilage-degrading proteinases, and stress-response factors (93, 94). Many receptors for ECM components on chondrocytes respond well to mechanical stimulation (95). Among these are several integrin receptors that bind to fibronectin and type II collagen fragments, which then upon activation stimulate the production of proteinases, cytokines, and chemokines (96).
The main determinants of mechanical properties of bone are the amount of mineral, the collagen content, the orientation of the collagen fibers and minerals, and the accumulation of microcracks in the bone matrix. In a mouse model of arthritis, mechanical testing has shown that arthritic femurs have a significantly lower Young's modulus, yield stress, and work until ultimate stress. This evidence suggests that one of the major explanations for the increased fracture risk in RA is related to the changes on bone components induced by inflammation that result in compromised biomechanical properties (97).
Discoidin domain receptor 2, which binds native type II collagen fibrils, is activated on chondrocytes after loss of proteoglycans in OA and preferentially induces MMP-13 via p38 MAPK (98). On the other hand, in RA, the cell-cell adhesion molecule, cadherin-11, is expressed at the interface between the RA synovial pannus and cartilage and facilitates cartilage invasion and erosion in human RA tissues in vitro and ex vivo dependent on the presence of TNF (99, 100). Recent studies indicate that lubricin is an important secreted product of chondrocytes, synovial cells, and other joint tissues that is down-regulated in OA and RA modulated by cytokines and growth factors (101). It is possible that normal accumulation of lubricin is impaired in OA, leading to a net loss of protein. The content of this protein produced by the superficial layer of cartilage is damaged with aging and OA, and the lubricin gene is differently expressed in the synovium of RA and OA, implying a possible role in the pathogenesis of these diseases (102).
E. Contribution of underlying subchondral bone
OA and RA are not exclusively disorders of articular cartilage. The subchondral bone tightly connected to the articular cartilage is an important shock absorber, and due to its intimate connection to the articular cartilage, it plays an important role in cartilage metabolism. Changes in the periarticular and subchondral bone may contribute to cartilage and joint pathology in both RA and OA. These alterations include the presence of microcracks, microedema, microbleeding within the subchondral region, and the development of subchondral bone cysts (103).
Receptor activator of NF-κB (RANK), a member of the TNF receptor family, RANK ligand (RANKL), and the soluble receptor osteoprotegerin regulate osteoclast differentiation and activity and are important mediators of bone destruction in RA. Although RANK and RANKL are expressed in adult articular chondrocytes, a direct action in cartilage has not been identified (104). In inflammatory models, cartilage destruction is not blocked directly by the inhibition of RANKL, indirect effects may occur through protection of the bone (105, 106). Runx2-dependent expression of RANKL is observed in hypertrophic chondrocytes at the boundary to the calcifying cartilage during embryogenesis in the growth plate (107). A link between RANKL and Wnt has been suggested by findings in human TNF transgenic mice and RA tissues, in which decreased β-catenin and high Dickkopf-1, a Wnt inhibitor, were demonstrated in synovium and in cartilage adjacent to inflammatory tissue (106). In contrast, increased β-catenin was observed in OA cartilage and conditional overexpression in mouse cartilage leads to premature chondrocyte differentiation and development of an OA-like phenotype (108).
F. Biomarkers in OA and RA
To provide means for monitoring the efficacy of disease-modifying therapies and to possibly identify patients at risk for developing joint pathologies, assays to quantify specific biological markers that indicate altered bone and cartilage metabolism would be of high clinical interest.
Molecules released from the articular cartilage, mainly aggrecan fragments, which contain chondroitin sulfate and keratan sulfate, type II collagen fragments, and collagen pyridinoline cross-links, are usually secreted as degradation products as a result of catabolic processes. Specific antibodies that detect either synthetic or cleavage epitopes have been developed to study biological markers of cartilage metabolism in synovial fluids, sera, and urine of patients with OA or RA (109–111). Additional markers in synovial fluid or sera are complete molecules such as COMP (112), cartilage-derived retinoic-acid-sensitive protein [also known as melanoma inhibitory activity (113)], C-reactive protein, IL-6, and MMP-3, which might be useful as potential biomarkers in RA and OA patients (35). Assays for biomarkers are frequently applied in research, and their usefulness in monitoring cartilage degradation or repair in patient populations needs to be evaluated carefully. Currently, there is no individual biomarker known for the diagnosis and/or monitoring of the progression of the disease that can be used without being correlated with structural changes in cartilage identified by magnetic resonance imaging techniques (112) or radiographic imaging.
In summary, novel mechanisms and molecules have been identified in addition to a better understanding of known mediators that contribute to the development and etiology of OA and RA. There is increasing knowledge about catabolic and anabolic factors that modify the responses of joint cells, i.e. synoviocytes, chondrocytes, osteocytes, matrix-degrading cells, and immune cells to their environment. Inflammatory processes during OA and RA increase bone and cartilage turnover and promote degradation of these tissues. The osteoarticular system responds to this with an evolutionarily conserved stress response in analogy to the classical HPA axis, that is increased synthesis of POMC-derived peptides. The next sections will provide clinical and experimental evidence that the POMC system and its respective receptors mediate an increasing number of newly recognized biological effects in the osteoarticular system, which includes bone mineralization and longitudinal growth, cell proliferation and differentiation, ECM synthesis, osteoprotection, and immunomodulation. There is increasing evidence for a role of the POMC system in joint tissue homeostasis that opens perspectives for POMC-derived peptides as a future treatment for degenerative and inflammatory joint diseases.
V. Clinical Observations Pointing Toward a Role of POMC-Derived Peptides in Bone and Cartilage in Man
A. Familial endocrinopathies
In at least two hereditary endocrinopathies with mutations of distinct genes of the POMC system, familial glucocorticoid deficiency (FGD) and obesity associated with MC4R deficiency, abnormalities of the osteoarticular system have been detected.
FGD, also known as familial Addison's disease or hereditary unresponsiveness to ACTH, is an autosomal recessive ACTH insensitivity syndrome, characterized by glucocorticoid deficiency in absence of mineralocorticoid deficiency (114). Due to the feedback loop to the pituitary gland, glucocorticoid deficiency results in abnormally high peripheral blood levels of ACTH. Recent advances in the genetics of this disorder have shown that approximately 25% of FGD cases are due to mutations in the MC2R, whereas an additional 15–20% of FGD cases have mutations in MC2R accessory protein (115). This small single transmembrane domain protein is required for proper trafficking of the MC2R to the cell surface (116). Of note, a tall stature has been reported in several cases with FGD (117–123), including the originally described two sisters by Shepard et al. (124). In a case series of five patients with FGD and mutations of the MC2R, all affected individuals had excessive linear growth over that predicted from parental indices as well as increased head circumference (125). However, tall stature and advanced bone age has also been noted in patients with FGD with no mutations in the coding and promoter region of the MC2R gene (126). Upon treatment of these patients with glucocorticosteroids, peripheral ACTH levels drop, and the growth patterns of the bone appear to normalize. Because no endocrinological explanation for these disturbances of bone and/or cartilage growth have been found in patients with FGD, one can speculate on a direct impact of increased ACTH levels and/or MC2R deficiency in cells of the osteoarticular system (see Sections VI.B, VI.C, and VII.A). Moreover, it is puzzling that in another ACTH insensitivity syndrome, triple A syndrome, different abnormalities in bone and cartilage have been reported in a subset of patients (114). Triple A syndrome is characterized by the clinical triad ACTH-resistant adrenal insufficiency, achalasia of the cardia, and alacrimia. Most cases are diagnosed in the first decade of life by severe hypoglycemic episodes (due to isolated glucocorticoid deficiency) and cutaneous hyperpigmentation (due to increased ACTH peripheral blood) levels. A minority of patients with triple A syndrome display short stature, microcephaly, and other skeletal abnormalities such as kyphosis and scoliosis (114). The completely different molecular base of triple A syndrome, i.e. mutations in the ALADIN protein (for alacrima/achalasia/adrenal insufficiency/neurological disorder) (127) may explain these phenotypic differences in the osteoarticular system between patients with the former syndrome and FGD.
Morbid obesity associated with MC4R deficiency is the second familial endocrinopathy of the POMC system that is linked with changes in the osteoarticular system. This disorder represents the most common monogenic cause of human obesity with a mostly recessive mode of inheritance (21).
Using whole-body dual-energy x-ray absorptiometry, a marked increase in bone mineral density was detected in affected subjects (128). Moreover, total bone mineral content was enhanced in affected individuals compared with age- and gender-specific data. Cortical thickness and cancellous bone pattern appeared to be normal. Subsequent studies on the potential mechanism related to this phenomenon revealed that individuals with loss of one functional MC4R allele display a decrease in bone resorption parameters as demonstrated by reduced serum levels of Crosslaps carboxyl-terminal collagen cross-links, a bone resorption marker (129). On the other hand, serum levels of cocaine- and amphetamine-regulated transcript (CART), a leptin-regulated inhibitor of bone formation (130), were markedly increased in subjects with MC4R mutations. As will be discussed below (Section VII.D), these findings in humans with MC4R deficiency could be recapitulated and extended in MC4R knockout mice.
B. Acquired endocrinopathies
For several decades, calcification of the auricles has been reported as a rare clinical sign of Addison's disease. The condition mostly occurs in males and often involves both ears. About 30 cases have been described in the literature until now (131–145). The true incidence of this condition in Addison's disease may be higher because palpation of the auricles is not routinely performed during medical examination, and patients do not necessarily report complaints. Based on an older roentgenological observational study, calcification of the auricular cartilage was detected in six of 120 of patients (5%) with Addison's disease (131). Clinically, the auricles are stone-hard and move only as a rigid unit. The pinna is spared in marked contrast to gouty arthritis. Anatomically, the auricle is composed of elastic cartilage, which represents the only cartilage in the human body that contains elastin. Histological and radiological examination revealed true ossification of the auricular cartilage with development of trabecular structures. Interestingly, other sites of metaplastic calcification including costal cartilage, spinal ligaments, and the lungs have also been noted in patients with Addison's disease (131, 145). The pathogenesis of ectopic ossification in Addison's disease remains unclear, and several hypotheses have been suggested. Importantly, there are no signs of proceeding inflammation (such as frostbite), and the calcium phosphorous product in peripheral blood of the affected patients is often normal excluding dystrophic and metastatic calcification. However, hypercalcemia due to increased calcium mobilization from the bone, and reduced calcium removal by the kidney is a well-described complication in Addison's disease (146–150). It was therefore suggested that enhanced input of calcium into the extracellular space and the lack of endogenous corticosteroids via reduced expression of the calcification inhibitor α2-Heremans-Schmid glycoprotein/fetuitin-A promote calcium deposits (139, 144). Cohen et al. (139) also suggested that the reduced availability of corticosteroids could lead to increased proliferation of mesenchymal stem cells, and decreased synthesis of proteoglycans may promote auricular ossification. The presence of functional MCR in both human mesenchymal stem cells (151, 152) and chondrocytes points to additional mechanisms how increased peripheral blood levels of ACTH and/or MSH in patients with Addison's disease could affect chondrogenic differentiation and ossification (Sections VI.B and VII.A)
C. In vivo administration of therapeutics activating POMC peptide receptors
Evidence that melanocortin peptides can control inflammatory conditions in the osteoarticular system comes from clinical studies on patients with OA and gouty arthritis. As early as 1949, it was noticed that parenteral administration of full-length ACTH has beneficial effects on OA and gouty arthritis (153–160). Although ACTH administered to patients with interval gout did not have ameliorating effects and even appeared to provoke acute gouty arthritis in some patients (161), it has rapid effects in acute gout. In one of the initial reports, ACTH was given in decreasing doses of 100–20 mg/d for 4 consecutive days, which had ameliorating effects in seven of 11 patients. In some of the responding individuals, the effect of ACTH was more pronounced than treatment with colchicine (156). Since then, a number of trials have been performed on ACTH in acute gouty arthritis that collectively confirmed the role of this POMC peptide as a valuable therapeutic tool, especially in patients with multiple medical problems or those with contraindications against colchicine or nonsteroidal antiinflammatory drugs (162). Although these early observations were initially explained by the stimulatory effect of ACTH on the adrenal gland resulting in subsequent induction of endogenous corticosteroid synthesis, others already speculated on an alternative mode of action (160).
The second clinical observation that further supported the idea that the osteoarticular system could be a direct target organ for POMC-derived peptides stems from patients receiving intraarticular injection of morphine after arthroscopic knee surgery (163). In a double-blind, randomized trial, it could be demonstrated that intraarticular injection of morphine has a stronger antinociceptive effect than the same dose given iv. These findings together with results from animal studies (164, 165) strongly supported the existence of functional OR in the knee. It is now apparent that various cell types (neuronal as well as nonneuronal cell types) of the osteoarticular system express functional receptors for POMC-derived peptides that can mediate antiinflammatory, immunomodulatory, and antinociceptive effects of the above therapeutic agents (Section VI).
VI. The Osteoarticular System: Direct Target Organ and Source of POMC-Derived Peptides
A. Synovium and synovial cell types
As early as 1986, POMC-derived peptides have been reported to be present in the synovial fluid. Denko et al. (166) examined the synovial fluid of 120 patients with various rheumatic disorders, especially RA and OA for the presence of β-ED using RIA. One third of the patients with RA had synovial fluid levels of β-ED severalfold higher than serum levels, whereas in two thirds, the opposite was seen. In accordance with the former authors, significantly elevated levels of β-ED were found in the synovial fluid of patients with RA compared with those from OA patients as reported by a recent study (167). Others confirmed the presence of β-ED in the synovial fluid of patients with RA and OA but detected amounts of β-ED higher than in the plasma only in RA (168). In addition to β-ED, α-MSH was identified in the synovial fluid of patients with RA, OA, and juvenile chronic arthritis (169). The levels of α-MSH were higher in patients with RA than those with OA. Moreover, in patients with polyarticular/systemic-onset juvenile chronic arthritis, the concentrations of α-MSH in the synovial fluid were greater than in those with pauciarticular disease. There is accumulating evidence that the synovium itself is capable of generating POMC-derived peptides with subsequent release into the synovial fluid, especially in response to stress and inflammatory signals. Synovial tissue explants from patients with RA were shown to release β-ED (170). Using an immunohistochemical approach, it was further shown that macrophage-like (CD68+) and fibroblast-like (CD68−) cells within the synovial lining layers from patients with OR, RA, and joint trauma express β-ED immunoreactivity (171). In the synovial sublining, layers of β-ED immunoreactivity were mostly detected in granulocytes, macrophages/monocytes, lymphocytes, and plasma cells. In contrast to the above studies that revealed higher levels of synovial β-ED in RA than in OA, cellular β-ED immunostaining was more pronounced in patients with OA than in those with RA. Of note, β-ED immunoreactivity in synovial cells was rarely seen in noninflamed synovial tissue with minor trauma (171). In support of a stress or inflammatory-signal-mediated regulation of POMC-derived peptides within the joint is the observation that β-ED immunoreactivity increases in synovial membrane of the dog after a low-impact exercise program (172). These data demonstrate that the synovium as an important interface within the joint is capable of producing their own POMC peptides. Future in vitro studies, e.g. on synovial fibroblasts, are needed to clarify to which extent and under what specific stimuli different cell types contribute to the autonomous POMC system in this tissue. It will be of interest to investigate the whole POMC processing machinery, e.g. the various PC responsible for POMC processing, in such cell types.
The synovium, however, does not only possess the capacity to produce POMC-derived peptides. Various cell types within this tissue express receptors for POMC-derived peptides. High-affinity binding sites for β-ED were detected by radioreceptor assays in synovial cells from patients with RA. Here, RT-PCR revealed transcripts for both the MOR and DOR in cultured synovial cells as well as MOR immunoreactivity in synovial lining and sublining cells in situ (173). Reduced phosphorylation of cAMP-responsive element-binding protein and biological responses of RA synoviocytes to nanomolar amounts of β-ED but not of the KOR ligand dynorphin strongly suggested the presence of functionally active MOR and DOR in human synovium, although the precise cell type that expresses these OR remained unclear. It was subsequently shown that fibroblast-like synoviocytes from healthy individuals express functional MOR and DOR in vitro, whereas this expression is reduced in patients with in OA and RA. Interestingly, IL-1β and TNF, both key proinflammatory cytokines in RA and OA, suppressed the mRNA level of both OR in RA- and OA-derived fibroblast-like synoviocytes (174). Others, however, detected MOR and DOR immunoreactivity only in nerve fibers within the sublining tissue of the synovium from patients with OA and RA (171). Interestingly, neuronal MOR expression became undetectable in rat synovium in adjuvant monoarthritic knee joints (175). These findings are in accordance with the reduction of OR expression in fibroblast-like synoviocytes from patients with RA and OA (174) and suggest that OR in the synovium are regulated in a stress- and/or disease-dependent manner.
B. Cartilage and chondrocytes
Compared with synovium, less is known about production of POMC peptides by chondrocytes. We and others (176, 177) detected truncated forms of POMC mRNA (transcripts related to exon 2) in human articular chondrocytes obtained from patients with end-stage OA. However, POMC transcripts related to exon 2–3 were not detectable (176), suggesting that articular chondrocytes cannot make POMC protein. In support of these findings, immunoreactivity for β-lipotropin or β-ED was not detectable in human articular chondrocytes in culture. These findings suggest that expression of functional POMC transcripts in human chondrocytes is silenced by methylation of the POMC promoter. However, no such studies have been performed on chondrocytes to the best of our knowledge. In the dog, β-ED immunoreactivity has been detected in articular cartilage after low-impact exercise (172), indicating species-specific differences in POMC gene regulation in chondrocytes, differences in POMC peptide detection between cell culture systems and cartilage in situ, or the possibility that POMC expression is turned on under distinct proinflammatory conditions.
In contrast to the yet to be established role of chondrocytes as potential producers of POMC peptides, it is now recognized that cartilage is a direct target for POMC-derived peptides. Before cloning of POMC peptide receptors, the analgesic effect of intraarticular morphine after arthroscopic knee surgery as outlined above (Section V.C) as well as radioligand studies already suggested the presence of opioid binding sites within the joint. Accordingly, specific binding of [125I]β-ED was detected in articular cartilage and in growth plate chondrocytes of the rat (178, 179). The binding of the radioligand could be reduced by naloxone and morphine. Intra- and periarticular opioid binding was also demonstrated in the dog where experimentally induced arthritis increased [3H]morphine binding (180). Definite expression of the MOR was later confirmed by RT-PCR analysis, Western immunoblotting, and immunohistochemistry in human osteoarthritic cartilage and chondrocytes. In vitro stimulation of human articular chondrocytes with β-ED decreased phosphorylation of the cAMP-responsive element-binding protein indicating a functional MOR (181). In addition to the MOR, there is increasing evidence for expression of MCR in chondrocytes. Initially, the MC3R was detected by Western blotting in rat bone marrow stromal-like cells, in the clonal multipotential cell line RCJ3.1 as well as in chondrocytes isolated from the ribcages of young rats (182). However, in the human system other MCR than MC3R appear to be more relevant. We could recently demonstrate the presence of MC1R, MC2R, and MC5R transcripts in human articular chondrocytes derived from patients with OA (177). Protein expression of the MC1R in these cells was confirmed by Western blotting and by immunohistochemical staining of OA cartilage. Here, chondrocytes located in the middle and deep cartilage layers were prevalently immunoreactive for MC1R, whereas chondrocytes in the superficial zone were negative. Treatment of these chondrocytes with α-MSH was associated with functional coupling as shown by cAMP assays but not with a Ca2+ response (177). The detection of MC1R in human articular chondrocytes is in accordance with the observation that also HTB-94, a human chondrosarcoma cell line, likewise expresses functional MC1R (183).
C. Bone, osteoblasts, and osteoclastic cells
Most studies on POMC peptides and their receptors in bone have focused on effects of melanocortin peptides rather than on expression of POMC, POMC-derived peptides, and effects of endogenous opioids. ACTH immunoreactivity and ACTH secretion was described in rat osteoclastic cells (12), but the significance of this finding remains to be shown. In addition to cells of the cartilage, bone-derived cells express multiple MCR subtypes as demonstrated by independent studies. Using various molecular biology approaches (RT-PCR, Northern blotting, ribonuclease protection assay, and in situ hybridization), Dumont et al. (184) reported the presence of MC4R in UMR106.06 rat osteosarcoma cells and in primary rat osteoblasts as well as in the periosteum of newborn Swiss mouse tibial bones. In primary rat osteoblasts, MC2R and MC5R transcripts were also detected but not MC1R and MC3R mRNA. In another study focusing more on the human system, transcripts of all five MCR were found in normal human osteoblasts as well as in MG63 and SaOs2 osteosarcoma cells, albeit not all receptors were present in each cell type (12). All of the above cell types exhibited MC2R immunoreactivity. Using the MG63 osteosarcoma cell line as a model, it was further demonstrated that these cells bind radiolabeled POMC-derived peptides, with the highest affinity for ACTH and α-MSH. However, stimulation of MG63 osteosarcoma cells with only α-MSH but not ACTH resulted in a weak (∼20%) increase in cellular cAMP content, although ACTH affected cell proliferation dose dependently and modulated a battery of target genes as will be discussed in Section VII.A (12). In situ hybridization of normal mouse bone tissue further revealed MC2R labeling in osteocytes and osteoblasts, whereas labeling for all MCR was found in chondrocytes in the proliferative zone of the epiphyseal growth plate. Interestingly, the same authors also detected transcripts for all MCR subtypes in the murine osteoclastic cell line RAW 264.7 as well as in primary rat osteoclasts (12). With regard to bone cells, it was further reported that MG63 cells, a human osteoblast-like osteosarcoma cell line, expresses DOR and MOR transcripts as well as the protein (185). Whether OR are expressed in normal osteoblasts or osteoclasts remains to be determined.
In summary, these findings show that multiple nonneuronal and resident cell types of the osteoarticular system express OR and MCR (Table 2). Via these receptors, POMC peptides originating from the central nervous system (pituitary-osteoarticular axis), from the osteoarticular system itself (via autonomous POMC peptide production), or from other peripheral POMC sources (e.g. from the immune system) can affect the function of bone, cartilage, and synovium.
Table 2.
POMC peptide receptors expressed in tissues and cell types of the osteoarticular system
| Receptors and tissue (cell type) | In vitro cell culture system |
|---|---|
| MOR | |
| Articular OA cartilage (h) | Primary articular chondrocytes (h) |
| Fetal long bone (r) | Primary fibroblast-like synoviocytes (h) |
| Growth plate (r) | MG63 (h) |
| Endosteum (r) | |
| Periosteum (r) | |
| Synovium (nerve fibers) (h) | |
| DOR | |
| Synovium (nerve fibers) (h) | Primary fibroblast-like synoviocytes (h) |
| MC1R | |
| Articular OA cartilage (h) | MG63 (h) |
| Growth plate (chondrocytes) (m) | Primary articular chondrocytes (h) |
| HTB-94 (h) | |
| Primary osteoblasts (h) | |
| MG63 (h), SaOs2 (h) | |
| RAW264.7 (m) | |
| MC2R | |
| Bone (osteoblasts, osteocytes) (m) | Primary articular chondrocytes (h) |
| Growth plate (chondrocytes) (m) | Primary osteoblasts (h), (r) |
| MG63 (h), SaOs2 (h) | |
| Primary osteoclasts (r) | |
| RAW264.7 (m) | |
| MC3R | |
| Growth plate (chondrocytes) (m) | RCJ3.1 (r) |
| Bone marrow stromal cells (r) | |
| Resting chondrocytes (r) | |
| MG63 (h) | |
| Primary osteoclasts (r) | |
| RAW264.7 (m) | |
| MC4R | |
| Periosteum (m) | UMR106.06 (r) |
| Growth plate (chondrocytes) (m) | Primary osteoblasts (h), (r) |
| Primary articular chondrocytes (h) | |
| SaOs2 (h) | |
| Primary osteoclasts (r) | |
| RAW264.7 (m) | |
| MC5R | |
| Growth plate (chondrocytes) (m) | Primary articular chondrocytes (h) |
| MG63 (h) | |
| RAW264.7 (m) |
h, Human; m, mouse; r, rat.
VII. In Vitro and in Vivo Effects of Melanocortin Peptides and β-ED in the Osteoarticular System
A. Growth and differentiation
As pointed out above (Section V), a number of clinical observations already suggest direct or indirect effects of the POMC system in cartilage and bone physiology and pathophysiology.
In support of a role of melanocortin peptides in chondrocyte differentiation during endochondral ossification, adrenalectomized (ADX) rats with elevated ACTH blood levels have an increase of the proliferative index and the hypertrophic zone height of the femoral growth plate (186). It was subsequently shown that ACTH in vitro increases proliferation of rat chondroprogenitor cells, which raises the possibility that full-length ACTH (amino acids 1–39) at 10−7 m acts on the stem-cell-like layer of the growth plate and thus increases the number of cells transitioning from the stem cell phenotype to the proliferative growth plate layer, thereby increasing the height (182). Also, melanocortins stimulated longitudinal growth because sc administration of 50 μg/d γ-MSH increased growth plate proliferative indices in ADX mice above ADX alone (186). The detection of immunoreactivity for all five MCR in the femoral growth plate of adult mice, in particular in the proliferative zone (12), further indicated susceptibility of chondrocytes for all melanocortin peptides. Because γ-MSH is a MC3R agonist, possibly this is the major receptor involved in regulation of endochondral ossification, which is supported by its high expression in rat chondrocytes and chondroprogenitor cells (182). Additionally, ACTH was shown to promote rat chondrocyte differentiation from chondroprogenitor cells and, together with 10−6 m α-MSH positively to influence chondrogenic matrix production (collagen II and aggrecan expression) in committed chondrocytes. Because both melanocortins also induce collagen X expression, ACTH and α-MSH presumably enhance matrix maturation toward the hypertrophic terminal differentiation stage (177, 182). The actions of the melanocortin peptides on chondrocytes appear to be mediated via at least two signaling pathways. In articular chondrocytes from patients with OA, administration of α-MSH weakly increased intracellular cAMP levels but had no detectable impact on intracellular calcium mobilization (177). On the contrary, in costal chondrocytes derived from the rat, which are considered equivalent to resting chondrocytes of the growth plate, 10−7 m ACTH (amino acids 1–39) evoked transient elevation of free intracellular Ca2+ levels (17). It is likely that in costal chondrocytes, the ACTH signal is mediated via the MC3R (17), which is not expressed in articular OA chondrocytes where expression of only MC1R, MC2R, and MC5R was detectable (177).
Melanocortins may also target the bone either directly via MCR expressed in certain bone cells or indirectly via distinct central circuits within the brain (Section VII.D). It was originally observed some time ago that α-MSH (and to a lesser extent also ACTH) administered sc to hypophysectomized rats modulates periosteal bone resorption. Accordingly, α-MSH reduced periosteal resorption but had no effect on the endostal apposition, resulting in an increased total cortical width (187). Systemic sc administration of 4.5 μg α-MSH per day to adult mice resulted in increased bone turnover and a net loss of trabecular bone (188), whereas α-MSH has no effect on cortical bone turnover (188, 189). The observation that humans who are deficient in MC4R have increased bone mass (Section V.A) suggests that melanocortin peptides may modulate bone turnover possibly via this POMC peptide receptor. Of note, in vitro administration of α-MSH at 10−8 m increased proliferation of fetal rat osteoblasts (and chondrocytes) without affecting their differentiation. In cultures of mouse bone marrow, α-MSH also stimulated the development of osteoclasts from their precursors but has no effect on mature osteoclasts. Contrary to α-MSH, ACTH appears to modulate osteogenic differentiation. In the human osteoblast-like SaOs2 cells derived from an osteosarcoma, ACTH had a biphasic effect on transcripts of collagen type I, the major collagen in bone for which expression is strongly up-regulated during osteoblast differentiation. At 10 nm, ACTH increases collagen I mRNA and thus differentiation, whereas at lower concentrations, it also can oppose osteoblast differentiation (190).
B. Inflammatory and immune responses
Proteolytic degradation of the articular cartilage matrix is a hallmark of inflammatory and degenerative joint diseases such as OA and RA. A key mediator of cartilage degradation is MMP-13 (known as collagenase-3), with clearly elevated levels in joint disorders. Yoon et al. (183) demonstrated that 200 nm α-MSH inhibits TNF-induced MMP-13 expression by decreasing p38 kinase phosphorylation and thus preventing subsequent activation of the NF-κB in HTB-94 chondrosarcoma cells. This is in line with the observation that α-MSH at 10−6 m reduced secretion of pro-MMP-13 and pro-MMP-2 in addition to suppressing mRNA levels of IL-1β and TNF in articular chondrocytes (177).
In contrast to ACTH and MSH peptides that uniformly acted in an antiinflammatory manner (see Section VIII.A), the role of β-ED appears to be more complex within the osteoarticular system. Depending on the examined cell type and the experimental conditions, both proinflammatory and antiinflammatory effects have been observed with β-ED. Administered in physiological concentrations to articular OA chondrocytes, β-ED increased production of TNF and IL-1β (191) and dose-dependently (60–6000 ng/ml) affected the phosphorylation of several MAPK. Pharmacological inhibition of p38 and c-Jun N-terminal kinase furthermore reduced the inductive effect of 60 and 600 ng/ml β-ED on IL-1β production by OA chondrocytes, suggesting that these MAPK are intracellular target molecules of this opioid (192). On the other hand, β-ED reduced spontaneous release of both IL-6 and IL-8 in synovial fibroblasts from RA patients at concentrations between 10−7 and 10−9 m. In synovial fibroblasts from OA patients the modulatory effect of β-ED had a different dose response. At very low concentrations (10−11 to 10−12 m), β-ED significantly inhibited whereas at higher doses it stimulated basal secretion of both cytokines (193). It was suggested that this shift in the dose-response curve of β-ED is due to the more proinflammatory situation in RA compared with OA, a concept supported by the absence of sympathetic nerve fibers in synovial tissue of patients with RA (194). Interestingly, β-ED at subnanomolar doses had multiple modulatory effects in synovial cells cultured ex vivo from rats with collagen-induced arthritis (CIA). It reduced TNF-induced proliferation and induced apoptosis. β-ED also reduced NF-κB activity and attenuated lipopolysaccharide-induced mRNA expression of TNF, IL-1β, IL-6, regulated activation, normal T cell expressed and secreted (RANTES), MMP-2, and MMP-9 (195).
C. Tissue protection
Recently, another modulatory effect of melanocortin peptides was unraveled in the context of cytoprotection. Daily sc administration of 0.2 μg/kg ACTH (amino acids 1–24) protected against glucocorticoid-induced osteonecrosis of the femoral head in a mouse model (196). Here, ACTH acted by stimulating vascular endothelial growth factor (VEGF) production, which supported the maturation and survival of osteoblasts. Induction of VEGF expression and secretion from osteoblasts appeared to be mediated by MC2R. Glucocorticoids inhibited VEGF synthesis and thus subjected osteoblasts and the adjacent bony vascular network to severe damage. This microvascular necrosis was associated with low ACTH levels, whereas administration of ACTH reduced such osteonecrotic symptoms (190).
Endogenous opioids are involved, along with other humoral mediators, in stress-response generation (197). It is known that stressful situations associated with tissue injury, such as traumatic fractures, induce a decrease in serum osteocalcin concentration (198). Because reduced osteocalcin production is considered to be a marker of low osteoblastic activity, this could be related to the pathogenesis of secondary forms of osteoporosis. With regard to bone cells, it was shown that high doses (10−4 m) of the MOR agonist d-Ala2,N-MePhe4,Gly5-ol-enkephalin inhibit osteocalcin secretion by MG63 osteosarcoma cells, whereas the alkaline phosphatase level was unaffected. Thus, endogenous opioids may be involved in the reduction of osteocalcin in stressful situations associated with tissue injury (185). Most recently, an orally active MOR/DOR antagonist, H-Dmt-Tic-Lys-NH-CH2-Ph (MZ-2), was administered at 10 mg/kg · d to female ob/ob (B6.V-Lep<ob>/J homozygous) and lean wild-type mice with or without voluntary exercise. Interestingly, this agent decreased fat content and serum insulin and glucose levels and enhanced bone mineral density in obese mice. MZ-2, furthermore, increased mineral nodule formation in MG63 osteosarcoma cells at 30 μm. Thus, MZ-2 was suggested to be of potential usefulness in the clinical management of obesity and insulin and glucose levels and the amelioration of osteoporosis (199). It will be therefore interesting to investigate whether normal osteoblasts likewise respond to MOR/DOR agonistic and antagonistic peptides with modulation of mineralization and thus osteoporosis. In summary, these in vitro and in vivo data indicate that both melanocortin peptides and β-ED affect various functions of bone and cells that are listed in Tables 3 and 4.
Table 3.
In vitro effects of melanocortin peptides and β-ED in the osteoarticular system
| Process | Effect | Molecule | Cell type | Ref. |
|---|---|---|---|---|
| Cell survival and differentiation | VEGF induction, increased survival and maturation of osteoblasts | ACTH | BMSC (h), MG63 (h), osteoblasts (h), BHSC (h), MC3T3-E1 (m) | 196 |
| Proliferation and differentiation | Induction of proliferation and differentiation of osteoblasts | ACTH | SaOs2 (h), osteoblasts (h) | 190 |
| Gene expression | Modulation of mRNA and protein expression of MMP, IL-6, TNF, Sox9, and collagens | α-MSH | Chondrocytes (h) | 177 |
| Modulation of inflammatory cell responses | Reduction of TNF-mediated MMP-13 expression, reduced p38 phosphorylation and NF-κB activation | α-MSH | HTB-94 (h) | 183 |
| Cytokine induction | Increased IL-1β secretion, increase of MAPK 1/2 and JNK phosphorylation, decrease of p38 phosphorylation | β-ED | Chondrocytes (h) | 192 |
| Terminal differentiation | Increase of intracellular free Ca2 + | ACTH | Chondrocytes (r) | 17 |
| Proliferation and cell survival | Decrease in metabolic activity and induction of apoptosis | β-ED | Synovial cells from CIA (r) | 195 |
| Cytokine production | Reduction of LPS-induced mRNA expression of TNF, IL-1β RANTES, iNOS, MMP-2 and MMP9 | β-ED | Synovial cells from CIA (r) | 195 |
| Proliferation | Increase of [3H]thymidine incorporation | ACTH, α-, β-, γ-MSH | MG63 (h), osteoblasts (h) | 184 |
| Cytokine induction | Increased IL-1β and TNF secretion | β-ED | Chondrocytes (h) | 191 |
| Proliferation and differentiation | Increased proliferation and osteoclastogenesis | α-MSH | Osteoblasts (r), chondrocytes (d),, BMSC (m), calvaria organ culture (m) | 12 |
| Proliferation and chondrogenesis | Increased proliferation, proteoglycan matrix deposition, total collagen production, and col2a1, col8a1, and col10a1 gene expression | ACTH | BMSC (r), RCJ3.1 (r), chondrocytes (r), RCJ3.1C5.18 (r) | 182 |
| Transcription factor modulation | Decreased CREB phosphorylation | β-ED | Chondrocytes (h) | 181 |
| Cytokine production | Modulation (mostly reduction) in IL-6 and IL-8 secretion | β-ED | Synovial fibroblasts (h) | 193 |
Effects are arranged in chronological order. BMSC, Bone marrow-derived mesenchymal stem cells; BHSC, bone marrow-derived hematopoietic stem cells; d, dog; h, human; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; m, murine; MC3T3, murine chondrocytic cell line; r, rat; RCJ3.1C5.18, rat chondrocytic cell line.
Table 4.
In vivo effects of melanocortin peptides and β-ED in the osteoarticular system
| Process | Effect | Molecule | Ref. |
|---|---|---|---|
| Antiinflammation | Reduction of CIA (m) | α-MSH fused to LAP | 233 |
| Osteoprotection | Reduction of steroid-induced femoral head necrosis (r) | ACTH | 196 |
| Antiinflammation | Reduction of arthritis scores of serum-induced arthritis (m) | γ-MSH | 205 |
| Antiinflammation | Reduced arthritis indices of collagen-induced arthritis, reduced mRNA of TNF-α, IL-2, IL-6, iNOS, RANTES, MMP-2/9 (r) | β-ED | 208 |
| Longitudinal growth | Increased tibial length, increase in hypertrophic differentiation and growth plate turnover (m) | γ2-MSH | 186 |
| Bone turnover and bone volume | Reduction of tibial perimeter and length, decrease of fat mass (m) | α-MSH | 188 |
| Antiinflammation | Reduction of neutrophil accumulation, reduced IL-1β and IL-6 amounts, reduction of joint swelling in gouty arthritis (r) | ACTH | 203 |
| Antiinflammation | Reduction of albumin extravasation from knee joint tissue and reduced leukocyte infiltration in HCl-induced cartilage damage (d) | β-ED | 207 |
| Antiinflammation | Increase in body weight, reduction of arthritis scores and articular erosions in adjuvant arthritis (r) | α-MSH | 202 |
Effects are arranged in chronological order. d, Dog; m, mouse; r, rat.
D. Lessons from ablation of specific genes of the POMC system
In light of the accumulating evidence that the osteoarticular system (via expression of both MCR and OR) is a direct target and even a source of POMC-derived peptides, it is of special interest if experimentally induced ablation of genes of the POMC system will result in any abnormality in bone or cartilage. Although such an approach may not be sufficient to clarify the functional role of the POMC system in specific human diseases such as RA or OA, it will certainly define whether such genes of the POMC system have a physiological role in the given animal model.
In accordance with the clinical observations on patients with morbid obesity due to MC4R deficiency (Section V.A), targeted disruption of the MC4R not only increases the mean body length (naso-anal) of homozygous and heterozygous mutant females (200) but also leads to increased bone mass. In an elegant study, it was demonstrated that the observed abnormal bone parameters in MC4R-knockout mice are due to increased expression of CART in the hypothalamus. Accordingly, removal of one allele of Cart from mice that are either homozygous or heterozygous for MC4R either normalized or significantly improved bone resorption without changing energy metabolism (129). These findings suggest that CART signaling (within the hypothalamus) may be the main molecular pathway accounting for the increased bone mass in mice and man with MC4R deficiency.
Another recent study further extended our current view on the role of the central melanocortin system in regulation of bone formation. Based on the above studies, it would be expected that ablation of the primary ligand of MC4R in the hypothalamus, α-MSH, recapitalizes the bone phenotype of MC4R-knockout mice. However, mice with knockout of carboxypeptidase E (CPE), an enzyme cleaving carboxyl-terminally extended lysine and arginine from POMC and other prohormones, are obese but have an abnormally low bone mineral density. These animals exhibited low bone mineral density and elevated plasma carboxyl-terminal collagen cross-links 1 and osteocalcin, both indicators for enhanced bone turnover. CPE-knockout mice had increased bone expression of RANKL, an indicator of osteoclastic activity and osteodestruction (201). Furthermore, expression of CART was undetectable in the hypothalamus of these mutant mice. As expected, POMC processing into α-MSH and ACTH in the brain, i.e. in the anterior and neurointermediate lobe of the pituitary gland, was impaired in CPE-knockout mice, whereas interestingly, peripheral blood levels of ACTH were normal between control mice and mutant mice.
VIII. POMC-Derived Peptides and Derivatives: Future Tools for the Treatment of Inflammatory and Degenerative Joint Diseases?
A. Preclinical studies with melanocortin peptides
The first evidence that melanocortins can modulate inflammation in the osteoarticular system was provided by Lipton and co-workers (202). α-MSH (50 μg) attenuated adjuvant-induced arthritis of the rat when injected ip twice daily for 20 d. In contrast to prednisolone (100 mg/kg), which also prevented development of arthritis, α-MSH ameliorated the weight loss observed in control animals (202). Inspired by the beneficial effect of ACTH in patients with acute gout (Section V.C), ACTH was later evaluated in an animal model of gouty arthritis. Local micromolar amounts of ACTH reduced joint swelling and neutrophil accumulation as well as the IL-1β and IL-6 levels in a rat model of gouty arthritis in which monosodium urate monohydrate crystals are injected into knee joints (203). Notably, intraarticularly injected ACTH did not alter plasma cortisol and was also operative in ADX animals, confirming a local action of this melanocortin peptide within the joint. The same authors could further show that rat knee macrophages, presumably derived from synovium, express MC3R immunoreactivity. The rat knee macrophages responded to ACTH with an increased cAMP level, suggesting a functional MC3R on these cells. The antiinflammatory effect of ACTH on experimentally induced gouty arthritis was blocked by the pharmacological MC3R antagonist SHU9119, whereas the selective MC3R agonist γ2-MSH mimicked the action of ACTH (203). The modulatory effects of γ2-MSH in this model of gouty arthritis are in accordance with the inhibitory action of this peptide on IL-1β production by mouse macrophages, which likewise express MC3R (204).
An endogenous inhibitory and/or protective action of the MC3R on joint inflammation has been confirmed in mice with targeted disruption of MC3R. Mice deficient for MC3R displayed increased joint inflammatory indices (arthritis score, paw volume, cumulative disease, and expression of various proinflammatory cytokines) compared with wild-type mice in a model of K/BxN serum-induced arthritis (205). Interestingly, MC3R−/− mice also had prominent bone erosion and higher articular expression of RANKL, a factor crucial for differentiation of macrophages into mature osteoclasts. Joint expression of MC3R but not of MC1R increased during the course of serum-induced arthritis. Daily sc treatment of wild-type mice with the MC3R agonist d[Trp8]γ-MSH (twice with 5 μg or twice with 200 μg) for 10–12 d attenuated serum-induced arthritis from d 2 onward, whereas this antiinflammatory action was lost in mice deficient for MC3R. Interestingly, osteoclastogenesis in MC3R−/− bone marrow cells revealed enhanced responsiveness to RANKL along with sustained DNA binding of NF-κB and increased secretion of NF-κB-dependent chemokines.
In accordance with an antiinflammatory effect of POMC peptides in the joint is a recent report on POMC gene delivery in experimentally induced OA (206). Here, OA was elicited by anterior cruciate ligament-transection (ACLT) in the knees of Wistar rats. Two weeks after surgery, adenoviral vector encoding human POMC or control vector was injected intraarticularly. Expression of POMC was monitored by immunoblotting and immunohistochemistry in the knee joints 72 h after POMC gene delivery. POMC protein amounts appeared to be increased in joint extracts of animals treated with POMC gene delivery. Immunoreactivity of POMC was markedly enhanced in chondrocytes and synoviocytes of the knee joints of rats treated with the POMC adenoviral vector. Histological evaluation 90 d after ACLT further revealed lesser cartilage damage, fewer synovial hyperplasia, and hypertrophia as well as reduced expression of MMP-13 in the medial femoral condyles from rats treated with POMC delivery. In addition, the number of microvessels in the synovium was significantly lower in rats treated with the POMC adenoviral vector. Although it is unclear from the above study how ectopically expressed human POMC is processed within the rat joint and which POMC-derived peptide actually exerts the observed beneficial effects on experimentally induced OA, these findings support the concept of chondroprotection by the POMC system.
B. Preclinical studies exploiting β-ED and related peptides
With regard to β-ED there is also evidence for an attenuating property of this POMC-derived peptide in a limited number of animal models of experimentally induced arthritis when administered locally or systemically. β-ED had a dose-dependent antiinflammatory effect on HCl-induced cartilage damage in the dog. Here, there was no significant difference between pre- and postinjury injection of β-ED (207); however, the used doses of β-ED were supraphysiological, ranging from 250-1000 μg. More recently, Yin et al. (208) reported that systemically injected β-ED at nearly physiological doses ameliorates arthritis indices (pad thickness, synoviocyte proliferation, pannus formation, and bone erosion) in CIA of the rat. β-ED treatment was associated with reduced mRNA levels of proinflammatory cytokines (TNF, IL-2, IL-6, and RANTES), inducible NO synthase, MMP-2, and MMP-9 and also modulated the T helper type 1/T helper type 2 response (208). In light of the proinflammatory actions of β-ED when administered to human articular chondrocytes in vitro (see above), these findings are puzzling and indicate a complex role of endogenous opioids in the osteoarticular system with possibly species-specific differences with respect to the effects of these peptides on inflammatory responses. Another reason for the partially contradicting effects of β-ED in vitro in human chondrocytes and in models of experimentally induced arthritis may be the fact that this POMC-derived peptide is not specific for MOR. Recently, endomorphin (EM)-1 and -2, two highly MOR-specific agonists (209), were investigated in their antiinflammatory potential in a superfusion model of synovial tissue from patients with RA and OA as well as in experimentally induced polyarthritis (210). EM-1 was more potent than EM-2 with regard to suppression of IL-6 and IL-8 secretion by synovial tissue. EM-1 had also beneficial effects on the paw volume of rats after adjuvant (Mycobacterium butyricum)-induced polyarthritis. These findings underscore the role of the MOR as an endogenous modulator of inflammation within the osteoarticular system. Moreover, it was suggested by the same authors that local targeting of the MOR in the osteoarticular system could be an interesting new strategy for the future therapy of inflammatory and/or degenerative joint diseases (211).
C. Open questions and perspectives for POMC-derived peptides in the future treatment of inflammatory and degenerative joint diseases
Based on the multiple in vitro and the so far reported in vivo effects of melanocortin and opioid peptides on cells of the osteoarticular system, the question arises of whether POMC-derived peptides and derivatives are promising future candidates for the treatment of RA or OA. As described above, melanocortin peptides and β-ED, via specific POMC peptide receptors, were shown to target osteoarticular key cells in these diseases and to modulate pathogenetically relevant key players including proinflammatory cytokines, MMP, NF-κB, or RANKL. However, in contrast to the reported salutary effects of melanocortin and opioid peptides on experimentally induced arthritis (adjuvant-induced arthritis, CIA, or monosodium urate-induced or serum-induced arthritis), comparatively little is known on the specific impact of POMC-derived peptides in models of OA (Table 4). It was reported that POMC gene delivery into knee joints of rats with ACLT ameliorates the course of OA in rats (206). However, which POMC-derived peptides mediate this antiarthrotic effect remains to be shown. Future studies have to confirm this interesting observation and should define the relative potency of exogenously administered melanocortin peptides and opioids in large, medium, and small animal models of OA, e.g. in surgically induced models with meniscectomy and/or ACLT (212). In this context, it will be highly interesting to clarify which specific MCR or OR confer a chondroprotective function in such models. In mice with serum-induced arthritis, at least the MC3R has been shown to exert a tonic antiinflammatory and/or protective role in the joint (205), although little is known of the role of other MCR subtypes or OR in these models. Using MCR- and OR-knockout mice, it should be informative to dissect the role of the other POMC peptide receptors in both inducible arthritis models as well as in transgenic models, e.g. in TNF-transgenic mice, IL-1α-transgenic, and IL-1 receptor antagonist-deficient mice (213–215). Because NF-κB is a well-described intracellular target of melanocortin peptides (2), direct comparative studies of MSH peptides vs. established antirheumatic biologicals targeting IL-1α and TNF, e.g. anakinra and etanercept (216, 217), will be of great interest.
Clinically, administration of melanocortin peptides to patients with inflammatory (or degenerative) joint disease may be a priori more advantageous than application of opioids due to the lack of addiction of the former agents. The fact that melanocortins target not only virtually all cells of the osteoarticular system but also those of the immune system (2, 218) makes these peptides even more attractive as future therapeutic candidates for RA and related diseases where abnormalities in cell-mediated immunity have been found. There is compelling evidence that abnormal cell-mediated immune responses, especially those of T cells residing in the synovia, play a crucial role in autoimmune pathogenesis of RA (219). α-MSH, on the other hand, has been demonstrated to be capable of diminishing severity and tempo of experimental autoimmune encephalomyelitis presumably by induction of specific regulatory T cells (220, 221). Therefore, the immunodulatory effects of melanocortin peptides may be of special value for the therapeutic success of these agents in patients with immune-mediated inflammatory diseases such as RA. Importantly, melanocortin peptides, albeit targeting immune cells, are immunododulators rather than immunosuppressants. This is in marked contrast to the majority of established disease-modifying antirheumatic drugs for RA, e.g. methotrexate or anti-TNF biologicals, which always bear the inheritable risk of uncontrolled infection and sepsis. Interestingly, α-MSH and related peptides have been shown to have antimicrobial effects against gram-positive bacteria as well as against Candida species (222).
One major challenge encountered with the clinical use of melanocortin peptides is their rapid degradation in vivo. Oral administration of natural melanocortin peptides may be therefore considered impossible. Even iv administered full-length α-MSH has a half-life of only a few minutes (223) due to the action of serum proteases such as trypsin, α-chymotrypsin, aminopeptidase, angiotensin-converting enzyme, prolyl endopeptidase, and neutral endopeptidase 24.11 (224–228). Recently, another α-MSH-degrading enzyme, prolylcarboxypeptidase (PRCP), also received special attention because it decreases the amounts of α-MSH in the hypothalamus and thus via MC4R-expressing neurons regulates food intake (229). PRCP is also expressed in immune cells and fibroblasts (230), and interestingly, PRCP activity had been detected in synovial fluid of patients with RA some time ago (231). The latter finding is in accordance with the detection of PRCP mRNA and protein expression in both synovial fibroblasts and chondrocytes from patients with OA (Böhm, M., and S. Grässel, unpublished). Therefore, development of protease-stable MSH analogs with MCR selectivity, increased potency, but maintained antiinflammatory efficacy is a prerequisite to successfully bring melanocortins into the clinic. With regard to cutaneous pigmentation and photoprotection, both physiological effects of α-MSH, phase III trials with the protease-resistant melanocortin peptide [Nle4, d-Phe7]-α-MSH are already underway in patients with erythropoietic porphyria based on promising pilot studies (232). Here, [Nle4, d-Phe7]-α-MSH is slowly released from an implant that is injected sc in intervals of 2 months. Another interesting approach for increasing the protease stability of α-MSH in the context of the osteoarticular system was recently introduced by Perretti and co-workers (233). The authors engineered plasmids encoding α-MSH fused to the latency-associated peptide of TGF-β and a MMP cleavage site. Gene therapy with this latent form of α-MSH ameliorated established CIA in mice as demonstrated by significantly reduced cellular infiltration and bone and cartilage erosion. Latency-associated peptide-MMP α-MSH treatment furthermore reduced total anti-collagen type II IgG, IgG2a, and IgG1 levels and decreased circulating amounts of both TNF and γ-interferon. Finally, another promising and yet to be validated strategy in the treatment of inflammatory (or degenerative) joint diseases may be the exploitation of protease-resistant small peptide derivatives from the C-terminal domain of α-MSH. MSH tripeptide derivatives such as Lys-d-Pro-Thr do not bind to the MC1R but have potent antiinflammatory effects against IL-1β in vitro (234). In accordance with the detected antiinflammatory effects of Lys-d-Pro-Thr in vitro, this small peptide turned out to possess antiinflammatory power in vivo as shown in several models of experimentally induced colitis (235).
IX. Conclusions
The data presented in this review highlight a significant number of biological effects of POMC-derived peptides via both MCR and OR expressed by the majority of nonneuronal resident cell types within the osteoarticular system. Thus, we propose several possible modes of actions of POMC peptides in bone and cartilage (Fig. 4). Inflammatory processes originated from diseases such as OA and RA increase bone and cartilage turnover and promote degradation of these tissues. The osteoarticular system responds to this with an evolutionarily conserved stress response in analogy to the classical HPA axis, that is increased synthesis of POMC-derived peptides. The release of such POMC peptides will subsequently modulate the activity not only of immune cells of the osteoarticular system but also of resident cells such as osteoclasts, osteoblasts, chondrocytes, synoviocytes, and their progenitor cells. In line with such a local endocrine stress response of the osteoarticular system is also the newly discovered osteoprotective effect of ACTH on osteonecrosis of the femoral head. ACTH produced either locally within the hip joint or released into the circulation by induction of the classical endocrine HPA axis appears to promote osteogenic-chondrogenic differentiation of multipotent precursor cells. Supported by studies on mice with ablated genes of the POMC system (129, 201), a complex interplay appears to exist between the central melanocortin system, bone, and additional organs involved in energy metabolism such as adipose tissue or pancreas. Understanding precisely the functional role of POMC-derived peptides in cells of the osteoarticular system should finally help to better tailor more efficient future therapies against inflammatory or degenerative joint diseases.
Figure 4.
Molecular targets and antiinflammatory mechanisms of melanocortin peptides in the joint. POMC-derived peptides affect several cellular and molecular targets in synovium, bone, and articular and growth cartilage in the joint. ACTH induces cartilage matrix production by increasing collagen II and aggrecan expression [1] and collagen X production [2]; it induces proliferation of chondroprogenitor cells and growth plate chondrocytes [3]; it promotes chondrocyte differentiation from progenitor cells [4]; it modulates osteogenic differentiation [8]; it protects against osteonecrosis by stimulating VEGF production [9]; and it inhibits TNF and IL-6 expression. α-MSH induces cartilage matrix production (collagen II and aggrecan) [1]; it induces collagen X production and thus hypertrophy [2]; it increases proliferation of osteoblasts [5] and stimulates osteoclastic differentiation from precursors [6]; it induces bone turnover of trabecular bone [7]; and it inhibits cytokine-induced MMP-13 expression. β-ED reduces proliferation of synoviocytes [10] and can modulate TNF, IL-1β, IL-2, IL-6, MMP-2, MMP-9, inducible NO synthase (iNOS), and RANTES production in a bidirectional manner. Black arrows indicate origin of POMC peptides within the joint. ACTH, α-MSH, and β-ED are secreted by the synovium (solid arrows) and presumably from osteoclasts (dotted arrows with question marks). Green arrows indicate stimulatory effects culminating in cell differentiation, proliferation, and modulation of gene expression; red bars indicate inhibition, and the brown curved arrow indicates bone turnover.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) to S.G. and M.B. (GR 1301/9-1). The subject of this review is also part of the Research Unit Forschergruppe 696 “Molecular analysis and interaction at articular interfaces. Role of neuroendocrine immune mechanisms.”
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACLT
- Anterior cruciate ligament transection
- ADX
- adrenalectomized
- BMP
- bone morphogenetic protein
- CART
- cocaine- and amphetamine-regulated transcript
- CIA
- collagen-induced arthritis
- COMP
- cartilage oligomeric matrix protein
- CPE
- carboxypeptidase E
- DOR
- δ-OR
- ECM
- extracellular matrix
- β-ED
- β-endorphin
- EM
- endomorphin
- FGD
- familial glucocorticoid deficiency
- FGF
- fibroblast growth factor
- HPA
- hypothalamic-pituitary-adrenal
- KOR
- κ-OR
- MCR
- melanocortin receptor
- MMP
- matrix metalloproteinase
- MOR
- μ-OR
- MPC
- mesenchymal progenitor cell
- MZ-2
- H-Dmt-Tic-Lys-NH-CH2-Ph
- NF-κB
- nuclear factor-κB
- NO
- nitric oxide
- OA
- osteoarthritis
- OR
- opioid receptor
- PC
- prohormone convertase
- POMC
- proopiomelanocortin
- PRCP
- prolylcarboxypeptidase
- RA
- rheumatoid arthritis
- RANK
- receptor activator of NF-κB
- RANKL
- RANK ligand
- RANTES
- regulated activation, normal T cell expressed and secreted
- VEGF
- vascular endothelial growth factor
- Wnt
- wingless/int.
References
- 1. Slominski A, Wortsman J, Luger T, Paus R, Solomon S. 2000. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev 80:979–1020 [DOI] [PubMed] [Google Scholar]
- 2. Brzoska T, Luger TA, Maaser C, Abels C, Böhm M. 2008. α-Melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev 29:581–602 [DOI] [PubMed] [Google Scholar]
- 3. Autelitano DJ, Lundblad JR, Blum M, Roberts JL. 1989. Hormonal regulation of POMC gene expression. Annu Rev Physiol 51:715–726 [DOI] [PubMed] [Google Scholar]
- 4. Clark AJ, Lavender PM, Coates P, Johnson MR, Rees LH. 2009. In vitro and in vivo analysis of the processing and fate of the peptide products of the short proopiomelanocortin mRNA. Mol Endocrinol 4:1737–1743 [DOI] [PubMed] [Google Scholar]
- 5. Jenks BG. 2009. Regulation of proopiomelanocortin gene expression: an overview of the signaling cascades, transcription factors, and responsive elements involved. Ann NY Acad Sci 1163:17–30 [DOI] [PubMed] [Google Scholar]
- 6. Slominski A, Tobin DJ, Shibahara S, Wortsman J. 2004. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 84:1155–1228 [DOI] [PubMed] [Google Scholar]
- 7. Newell-Price J. 2003. Proopiomelanocortin gene expression and DNA methylation: implications for Cushing's syndrome and beyond. J Endocrinol 177:365–372 [DOI] [PubMed] [Google Scholar]
- 8. Seidah NG, Benjannet S, Hamelin J, Mamarbachi AM, Basak A, Marcinkiewicz J, Mbikay M, Chrétien M, Marcinkiewicz M. 1999. The subtilisin/kexin family of precursor convertases. Emphasis on PC1, PC2/7B2, POMC and the novel enzyme SKI-1. Ann NY Acad Sci 885:57–74 [DOI] [PubMed] [Google Scholar]
- 9. Rousseau K, Kauser S, Pritchard LE, Warhurst A, Oliver RL, Slominski A, Wei ET, Thody AJ, Tobin DJ, White A. 2007. Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB J 21:1844–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schiöth HB. 2001. The physiological role of melanocortin receptors. Vitam Horm 63:195–232 [DOI] [PubMed] [Google Scholar]
- 11. Cone RD, Lu D, Koppula S, Vage DI, Klungland H, Boston B, Chen W, Orth DN, Pouton C, Kesterson RA. 1996. The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent Prog Horm Res 51:287–317; discussion 318 [PubMed] [Google Scholar]
- 12. Zhong Q, Sridhar S, Ruan L, Ding KH, Xie D, Insogna K, Kang B, Xu J, Bollag RJ, Isales CM. 2005. Multiple melanocortin receptors are expressed in bone cells. Bone 36:820–831 [DOI] [PubMed] [Google Scholar]
- 13. Böhm M, Apel M, Sugawara K, Brehler R, Jurk K, Luger TA, Haas H, Paus R, Eiz-Vesper B, Walls AF, Ponimaskin E, Gehring M, Kapp A, Raap U. 2012. Modulation of basophil activity: a novel function of the neuropeptide α-melanocyte-stimulating hormone. J Allergy Clin Immunol 129:1085–1093 [DOI] [PubMed] [Google Scholar]
- 14. Mountjoy KG, Kong PL, Taylor JA, Willard DH, Wilkison WO. 2001. Melanocortin receptor-mediated mobilization of intracellular free calcium in HEK293 cells. Physiol Genomics 5:11–19 [DOI] [PubMed] [Google Scholar]
- 15. Coyne MD, Rodriguez O, Wilson Y, Wang G, Lemos JR. 1997. Voltage dependent calcium and potassium currents in Y-1 adrenocortical cells are unresponsive to ACTH. Endocr Res 23:245–275 [DOI] [PubMed] [Google Scholar]
- 16. Omura M, Suematsu S, Nishikawa T. 2007. Role of calcium messenger systems in ACTH-induced cortisol production in bovine adrenal fasciculo-reticularis cells. Endocr J 54:585–592 [DOI] [PubMed] [Google Scholar]
- 17. Evans JF, Shen CL, Pollack S, Aloia JF, Yeh JK. 2005. Adrenocorticotropin evokes transient elevations in intracellular free calcium ([Ca2+]i) and increases basal [Ca2+]i in resting chondrocytes through a phospholipase C-dependent mechanism. Endocrinology 146:3123–3132 [DOI] [PubMed] [Google Scholar]
- 18. Sutton GM, Duos B, Patterson LM, Berthoud HR. 2005. Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology 146:3739–3747 [DOI] [PubMed] [Google Scholar]
- 19. Bellei B, Maresca V, Flori E, Pitisci A, Larue L, Picardo M. 2010. p38 regulates pigmentation via proteasomal degradation of tyrosinase. J Biol Chem 285:7288–7299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herraiz C, Journé F, Abdel-Malek Z, Ghanem G, Jiménez-Cervantes C, García-Borrón JC. 2011. Signaling from the human melanocortin 1 receptor to ERK1 and ERK2 mitogen-activated protein kinases involves transactivation of cKIT. Mol Endocrinol 25:138–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cone RD. 2006. Studies on the physiological functions of the melanocortin system. Endocr Rev 27:736–749 [DOI] [PubMed] [Google Scholar]
- 22. Böhm M, Eickelmann M, Li Z, Schneider SW, Oji V, Diederichs S, Barsh GS, Vogt A, Stieler K, Blume-Peytavi U, Luger TA. 2005. Detection of functionally active melanocortin receptors and evidence for an immunoregulatory activity of α-melanocyte-stimulating hormone in human dermal papilla cells. Endocrinology 146:4635–4646 [DOI] [PubMed] [Google Scholar]
- 23. Spencer JD, Schallreuter KU. 2009. Regulation of pigmentation in human epidermal melanocytes by functional high-affinity β-melanocyte-stimulating hormone/melanocortin-4 receptor signaling. Endocrinology 150:1250–1258 [DOI] [PubMed] [Google Scholar]
- 24. Ballet S, Pietsch M, Abell AD. 2008. Multiple ligands in opioid research. Protein Pept Lett 15:668–682 [DOI] [PubMed] [Google Scholar]
- 25. Sarne Y, Fields A, Keren O, Gafni M. 1996. Stimulatory effects of opioids on transmitter release and possible cellular mechanisms: overview and original results. Neurochem Res 11:1353–1361 [DOI] [PubMed] [Google Scholar]
- 26. Hashimoto K, Amano T, Kasakura A, Uhl GR, Sora I, Sakai N, Kuzumaki N, Suzuki T, Narita M. 2009. μ-Opioid receptor-independent fashion of the suppression of sodium currents by μ-opioid analgesics in thalamic neurons. Neurosci Lett 453:62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duncan H, Jundt J, Riddle JM, Pitchford W, Christopherson T. 1987. The tibial subchondral plate. A scanning electron microscopic study. J Bone Joint Surg Am 69:1212–1220 [PubMed] [Google Scholar]
- 28. Madry H, van Dijk CN, Mueller-Gerbl M. 2010. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc 18:419–433 [DOI] [PubMed] [Google Scholar]
- 29. Armstrong SJ, Read RA, Price R. 1995. Topographical variation within the articular cartilage and subchondral bone of the normal ovine knee joint: a histological approach. Osteoarthritis Cartilage 3:25–33 [DOI] [PubMed] [Google Scholar]
- 30. Bullough PG, Yawitz PS, Tafra L, Boskey AL. 1985. Topographical variations in the morphology and biochemistry of adult canine tibial plateau articular cartilage. J Orthop Res 3:1–16 [DOI] [PubMed] [Google Scholar]
- 31. Poole AR, Kojima T, Yasuda T, Mwale F, Kobayashi M, Laverty S. 2001. Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop Relat Res S26–S33 [DOI] [PubMed] [Google Scholar]
- 32. Eikenberry EF, Bruckner P. 1999. Supramolecular structure of cartilage matrix. In: Seibel MJ, Robins SP, Bilezikian JP, eds. Bone and cartilage metabolism. San Diego, CA: Academic Press; 289–300 [Google Scholar]
- 33. Wu JJ, Woods PE, Eyre DR. 1992. Identification of cross-linking sites in bovine cartilage type IX collagen reveals an antiparallel type II-type IX molecular relationship and type IX to type IX bonding. J Biol Chem 267:23007–23014 [PubMed] [Google Scholar]
- 34. Buckwalter JA, Mankin HJ, Grodzinsky AJ. 2005. Articular cartilage and osteoarthritis. Instr Course Lect 54:465–480 [PubMed] [Google Scholar]
- 35. Goldring MB, Marcu KB. 2009. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther 11:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Poole CA. 1997. Articular cartilage chondrons: form, function and failure. J Anat 191(Pt 1):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Poole AR, Kojima T, Yasuda T, Mwale F, Kobayashi M, Laverty S. 2001. Composition and structure of articular cartilage. Clin Orthop Relat Res. 391S:S26–S33 [DOI] [PubMed] [Google Scholar]
- 38. Wiberg C, Klatt AR, Wagener R, Paulsson M, Bateman JF, Heinegård D, Mörgelin M. 2003. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J Biol Chem 278:37698–37704 [DOI] [PubMed] [Google Scholar]
- 39. Klatt AR, Nitsche DP, Kobbe B, Mörgelin M, Paulsson M, Wagener R. 2000. Molecular structure and tissue distribution of matrilin-3, a filament-forming extracellular matrix protein expressed during skeletal development. J Biol Chem 275:3999–4006 [DOI] [PubMed] [Google Scholar]
- 40. Eyre DR, Weis MA, Wu JJ. 2006. Articular cartilage collagen: an irreplaceable framework? Eur Cell Mater 12:57–63 [DOI] [PubMed] [Google Scholar]
- 41. Budde B, Blumbach K, Ylöstalo J, Zaucke F, Ehlen HW, Wagener R, Ala-Kokko L, Paulsson M, Bruckner P, Grässel S. 2005. Altered integration of matrilin-3 into cartilage extracellular matrix in the absence of collagen IX. Mol Cell Biol 25:10465–10478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kvist AJ, Johnson AE, Mörgelin M, Gustafsson E, Bengtsson E, Lindblom K, Aszódi A, Fässler R, Sasaki T, Timpl R, Aspberg A. 2006. Chondroitin sulfate perlecan enhances collagen fibril formation. Implications for perlecan chondrodysplasias. J Biol Chem 281:33127–33139 [DOI] [PubMed] [Google Scholar]
- 43. Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. 2008. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol 40:46–62 [DOI] [PubMed] [Google Scholar]
- 44. Yin T, Li L. 2006. The stem cell niches in bone. J Clin Invest 116:1195–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moore KA, Lemischka IR. 2006. Stem cells and their niches. Science 311:1880–1885 [DOI] [PubMed] [Google Scholar]
- 46. Goldring MB, Tsuchimochi K, Ijiri K. 2006. The control of chondrogenesis. J Cell Biochem 97:33–44 [DOI] [PubMed] [Google Scholar]
- 47. Kronenberg HM. 2003. Developmental regulation of the growth plate. Nature 423:332–336 [DOI] [PubMed] [Google Scholar]
- 48. Lefebvre V, Smits P. 2005. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res C Embryo Today 75:200–212 [DOI] [PubMed] [Google Scholar]
- 49. Olsen BR. 1996. Role of cartilage collagens in formation of the skeleton. Ann NY Acad Sci 785:124–130 [DOI] [PubMed] [Google Scholar]
- 50. Pitsillides AA, Beier F. 2011. Cartilage biology in osteoarthritis-lessons from developmental biology. Nat Rev Rheumatol 7:654–663 [DOI] [PubMed] [Google Scholar]
- 51. Quintana L, zur Nieden NI, Semino CE. 2009. Morphogenetic and regulatory mechanisms during developmental chondrogenesis: new paradigms for cartilage tissue engineering. Tissue Eng Part B Rev 15:29–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Olsen BR, Reginato AM, Wang W. 2000. Bone development. Annu Rev Cell Dev Biol 16:191–220 [DOI] [PubMed] [Google Scholar]
- 53. Woods A, Wang G, Dupuis H, Shao Z, Beier F. 2007. Rac1 signaling stimulates N-cadherin expression, mesenchymal condensation, and chondrogenesis. J Biol Chem 282:23500–23508 [DOI] [PubMed] [Google Scholar]
- 54. DeLise AM, Tuan RS. 2002. Alterations in the spatiotemporal expression pattern and function of N-cadherin inhibit cellular condensation and chondrogenesis of limb mesenchymal cells in vitro. J Cell Biochem 87:342–359 [DOI] [PubMed] [Google Scholar]
- 55. Delise AM, Tuan RS. 2002. Analysis of N-cadherin function in limb mesenchymal chondrogenesis in vitro. Dev Dyn 225:195–204 [DOI] [PubMed] [Google Scholar]
- 56. Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. 2005. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis 64:1263–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Loeser RF. 2006. Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide. Arthritis Rheum 54:1357–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cawston TE, Wilson AJ. 2006. Understanding the role of tissue degrading enzymes and their inhibitors in development and disease. Best Pract Res Clin Rheumatol 20:983–1002 [DOI] [PubMed] [Google Scholar]
- 59. Murphy G, Nagase H. 2008. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat Clin Pract Rheumatol 4:128–135 [DOI] [PubMed] [Google Scholar]
- 60. Wu CW, Tchetina EV, Mwale F, Hasty K, Pidoux I, Reiner A, Chen J, Van Wart HE, Poole AR. 2002. Proteolysis involving matrix metalloproteinase 13 (collagenase-3) is required for chondrocyte differentiation that is associated with matrix mineralization. J Bone Miner Res 17:639–651 [DOI] [PubMed] [Google Scholar]
- 61. Kane D, Jensen LE, Grehan S, Whitehead AS, Bresnihan B, Fitzgerald O. 2004. Quantitation of metalloproteinase gene expression in rheumatoid and psoriatic arthritis synovial tissue distal and proximal to the cartilage-pannus junction. J Rheumatol 31:1274–1280 [PubMed] [Google Scholar]
- 62. Aigner T, Fundel K, Saas J, Gebhard PM, Haag J, Weiss T, Zien A, Obermayr F, Zimmer R, Bartnik E. 2006. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum 54:3533–3544 [DOI] [PubMed] [Google Scholar]
- 63. Hermansson M, Sawaji Y, Bolton M, Alexander S, Wallace A, Begum S, Wait R, Saklatvala J. 2004. Proteomic analysis of articular cartilage shows increased type II collagen synthesis in osteoarthritis and expression of inhibin βA (activin A), a regulatory molecule for chondrocytes. J Biol Chem 279:43514–43521 [DOI] [PubMed] [Google Scholar]
- 64. Hunter DJ, Li J, LaValley M, Bauer DC, Nevitt M, DeGroot J, Poole R, Eyre D, Guermazi A, Gale D, Felson DT. 2007. Cartilage markers and their association with cartilage loss on magnetic resonance imaging in knee osteoarthritis: the Boston Osteoarthritis Knee Study. Arthritis Res Ther 9:R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Otero M, Lago R, Gomez R, Dieguez C, Lago F, Gomez-Reino J, Gualillo O. 2006. Towards a pro-inflammatory and immunomodulatory emerging role of leptin. Rheumatology (Oxford) 45:944–950 [DOI] [PubMed] [Google Scholar]
- 66. Dumond H, Presle N, Terlain B, Mainard D, Loeuille D, Netter P, Pottie P. 2003. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum 48:3118–3129 [DOI] [PubMed] [Google Scholar]
- 67. Popa C, Netea MG, Radstake TR, van Riel PL, Barrera P, van der Meer JW. 2005. Markers of inflammation are negatively correlated with serum leptin in rheumatoid arthritis. Ann Rheum Dis 64:1195–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lago F, Dieguez C, Gómez-Reino J, Gualillo O. 2007. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev 18:313–325 [DOI] [PubMed] [Google Scholar]
- 69. Senolt L, Housa D, Vernerová Z, Jirásek T, Svobodová R, Veigl D, Anderlová K, Müller-Ladner U, Pavelka K, Haluzík M. 2007. Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Ann Rheum Dis 66:458–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Loeser RF. 2009. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage 17:971–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Loeser RF. 2011. Aging and osteoarthritis. Curr Opin Rheumatol 23:492–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. van der Kraan PM, Blaney Davidson EN, van den Berg WB. 2010. A role for age-related changes in TGFβ signaling in aberrant chondrocyte differentiation and osteoarthritis. Arthritis Res Ther 12:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yin W, Park JI, Loeser RF. 2009. Oxidative stress inhibits insulin-like growth factor-I induction of chondrocyte proteoglycan synthesis through differential regulation of phosphatidylinositol 3-Kinase-Akt and MEK-ERK MAPK signaling pathways. J Biol Chem 284:31972–31981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Krejci P, Prochazkova J, Smutny J, Chlebova K, Lin P, Aklian A, Bryja V, Kozubik A, Wilcox WR. 2010. FGFR3 signaling induces a reversible senescence phenotype in chondrocytes similar to oncogene-induced premature senescence. Bone 47:102–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lindstrom TM, Robinson WH. 2010. Rheumatoid arthritis: a role for immunosenescence? J Am Geriatr Soc 58:1565–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Doran MF, Pond GR, Crowson CS, O'Fallon WM, Gabriel SE. 2002. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum 46:625–631 [DOI] [PubMed] [Google Scholar]
- 77. Pedersen JK, Svendsen AJ, Hørslev-Petersen K. 2007. Incidence of rheumatoid arthritis in the southern part of Denmark from 1995 to 2001. Open Rheumatol J 1:18–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Riise T, Jacobsen BK, Gran JT. 2000. Incidence and prevalence of rheumatoid arthritis in the county of Troms, northern Norway. J Rheumatol 27:1386–1389 [PubMed] [Google Scholar]
- 79. Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, Granath F, Catrina AI, Rosenquist R, Feltelius N, Sundström C, Klareskog L. 2006. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum 54:692–701 [DOI] [PubMed] [Google Scholar]
- 80. Colmegna I, Diaz-Borjon A, Fujii H, Schaefer L, Goronzy JJ, Weyand CM. 2008. Defective proliferative capacity and accelerated telomeric loss of hematopoietic progenitor cells in rheumatoid arthritis. Arthritis Rheum 58:990–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Koetz K, Bryl E, Spickschen K, O'Fallon WM, Goronzy JJ, Weyand CM. 2000. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci USA 97:9203–9208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Weng NP, Akbar AN, Goronzy J. 2009. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol 30:306–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vallejo AN. 2005. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev 205:158–169 [DOI] [PubMed] [Google Scholar]
- 84. Kannu P, Bateman JF, Randle S, Cowie S, du Sart D, McGrath S, Edwards M, Savarirayan R. 2010. Premature arthritis is a distinct type II collagen phenotype. Arthritis Rheum 62:1421–1430 [DOI] [PubMed] [Google Scholar]
- 85. Baker-LePain JC, Lane NE. 2010. Relationship between joint shape and the development of osteoarthritis. Curr Opin Rheumatol 22:538–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Andreas K, Lübke C, Häupl T, Dehne T, Morawietz L, Ringe J, Kaps C, Sittinger M. 2008. Key regulatory molecules of cartilage destruction in rheumatoid arthritis: an in vitro study. Arthritis Res Ther 10:R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Drissi H, Zuscik M, Rosier R, O'Keefe R. 2005. Transcriptional regulation of chondrocyte maturation: potential involvement of transcription factors in OA pathogenesis. Mol Aspects Med 26:169–179 [DOI] [PubMed] [Google Scholar]
- 88. Fuerst M, Bertrand J, Lammers L, Dreier R, Echtermeyer F, Nitschke Y, Rutsch F, Schäfer FK, Niggemeyer O, Steinhagen J, Lohmann CH, Pap T, Rüther W. 2009. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum 60:2694–2703 [DOI] [PubMed] [Google Scholar]
- 89. Kamekura S, Kawasaki Y, Hoshi K, Shimoaka T, Chikuda H, Maruyama Z, Komori T, Sato S, Takeda S, Karsenty G, Nakamura K, Chung UI, Kawaguchi H. 2006. Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum 54:2462–2470 [DOI] [PubMed] [Google Scholar]
- 90. Kawaguchi H. 2008. Endochondral ossification signals in cartilage degradation during osteoarthritis progression in experimental mouse models. Mol Cells 25:1–6 [PubMed] [Google Scholar]
- 91. Lee JH, Fitzgerald JB, Dimicco MA, Grodzinsky AJ. 2005. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum 52:2386–2395 [DOI] [PubMed] [Google Scholar]
- 92. Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, Setton LA, Weinberg JB. 2004. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res 423:17–26 [DOI] [PubMed] [Google Scholar]
- 93. Fitzgerald JB, Jin M, Dean D, Wood DJ, Zheng MH, Grodzinsky AJ. 2004. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J Biol Chem 279:19502–19511 [DOI] [PubMed] [Google Scholar]
- 94. Kurz B, Lemke AK, Fay J, Pufe T, Grodzinsky AJ, Schunke M. 2005. Pathomechanisms of cartilage destruction by mechanical injury. Ann Anat 187:473–485 [DOI] [PubMed] [Google Scholar]
- 95. Millward-Sadler SJ, Salter DM. 2004. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann Biomed Eng 32:435–446 [DOI] [PubMed] [Google Scholar]
- 96. Pulai JI, Chen H, Im HJ, Kumar S, Hanning C, Hegde PS, Loeser RF. 2005. NF-κB mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J Immunol 174:5781–5788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Abdulghani S, Caetano-Lopes J, Canhão H, Fonseca JE. 2009. Biomechanical effects of inflammatory diseases on bone-rheumatoid arthritis as a paradigm. Autoimmun Rev 8:668–671 [DOI] [PubMed] [Google Scholar]
- 98. Xu L, Peng H, Glasson S, Lee PL, Hu K, Ijiri K, Olsen BR, Goldring MB, Li Y. 2007. Increased expression of the collagen receptor discoidin domain receptor 2 in articular cartilage as a key event in the pathogenesis of osteoarthritis. Arthritis Rheum 56:2663–2673 [DOI] [PubMed] [Google Scholar]
- 99. Lee DM, Kiener HP, Agarwal SK, Noss EH, Watts GF, Chisaka O, Takeichi M, Brenner MB. 2007. Cadherin-11 in synovial lining formation and pathology in arthritis. Science 315:1006–1010 [DOI] [PubMed] [Google Scholar]
- 100. Vandooren B, Cantaert T, ter Borg M, Noordenbos T, Kuhlman R, Gerlag D, Bongartz T, Reedquist K, Tak PP, Baeten D. 2008. Tumor necrosis factor α drives cadherin 11 expression in rheumatoid inflammation. Arthritis Rheum 58:3051–3062 [DOI] [PubMed] [Google Scholar]
- 101. Bao JP, Chen WP, Wu LD. 2011. Lubricin: a novel potential biotherapeutic approaches for the treatment of osteoarthritis. Mol Biol Rep 38:2879–2885 [DOI] [PubMed] [Google Scholar]
- 102. Jüsten HP, Grünewald E, Totzke G, Gouni-Berthold I, Sachinidis A, Wessinghage D, Vetter H, Schulze-Osthoff K, Ko Y. 2000. Differential gene expression in synovium of rheumatoid arthritis and osteoarthritis. Mol Cell Biol Res Commun 3:165–172 [DOI] [PubMed] [Google Scholar]
- 103. Goldring MB, Goldring SR. 2010. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann NY Acad Sci 1192:230–237 [DOI] [PubMed] [Google Scholar]
- 104. Komuro H, Olee T, Kühn K, Quach J, Brinson DC, Shikhman A, Valbracht J, Creighton-Achermann L, Lotz M. 2001. The osteoprotegerin/receptor activator of nuclear factor κB/receptor activator of nuclear factor κB ligand system in cartilage. Arthritis Rheum 44:2768–2776 [DOI] [PubMed] [Google Scholar]
- 105. Schett G, Hayer S, Zwerina J, Redlich K, Smolen JS. 2005. Mechanisms of disease: the link between RANKL and arthritic bone disease. Nat Clin Pract Rheumatol 1:47–54 [DOI] [PubMed] [Google Scholar]
- 106. Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, van der Heide D, Landewe R, Lacey D, Richards WG, Schett G. 2007. Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13:156–163 [DOI] [PubMed] [Google Scholar]
- 107. Kishimoto K, Kitazawa R, Kurosaka M, Maeda S, Kitazawa S. 2006. Expression profile of genes related to osteoclastogenesis in mouse growth plate and articular cartilage. Histochem Cell Biol 125:593–602 [DOI] [PubMed] [Google Scholar]
- 108. Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, Rosier RN, O'Keefe RJ, Zuscik M, Chen D. 2009. Activation of β-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult β-catenin conditional activation mice. J Bone Miner Res 24:12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rousseau JC, Delmas PD. 2007. Biological markers in osteoarthritis. Nat Clin Pract Rheumatol 3:346–356 [DOI] [PubMed] [Google Scholar]
- 110. Sumer EU, Sondergaard BC, Rousseau JC, Delmas PD, Fosang AJ, Karsdal MA, Christiansen C, Qvist P. 2007. MMP and non-MMP-mediated release of aggrecan and its fragments from articular cartilage: a comparative study of three different aggrecan and glycosaminoglycan assays. Osteoarthritis Cartilage 15:212–221 [DOI] [PubMed] [Google Scholar]
- 111. Dayer E, Dayer JM, Roux-Lombard P. 2007. Primer: the practical use of biological markers of rheumatic and systemic inflammatory diseases. Nat Clin Pract Rheumatol 3:512–520 [DOI] [PubMed] [Google Scholar]
- 112. Lindqvist E, Eberhardt K, Bendtzen K, Heinegård D, Saxne T. 2005. Prognostic laboratory markers of joint damage in rheumatoid arthritis. Ann Rheum Dis 64:196–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Saito S, Kondo S, Mishima S, Ishiguro N, Hasegawa Y, Sandell LJ, Iwata H. 2002. Analysis of cartilage-derived retinoic-acid-sensitive protein (CD-RAP) in synovial fluid from patients with osteoarthritis and rheumatoid arthritis. J Bone Joint Surg Br 84:1066–1069 [DOI] [PubMed] [Google Scholar]
- 114. Clark AJ, Weber A. 1998. Adrenocorticotropin insensitivity syndromes. Endocr Rev 19:828–843 [DOI] [PubMed] [Google Scholar]
- 115. Chan LF, Clark AJ, Metherell LA. 2008. Familial glucocorticoid deficiency: advances in the molecular understanding of ACTH action. Horm Res 69:75–82 [DOI] [PubMed] [Google Scholar]
- 116. Chan LF, Webb TR, Chung TT, Meimaridou E, Cooray SN, Guasti L, Chapple JP, Egertová M, Elphick MR, Cheetham ME, Metherell LA, Clark AJ. 2009. MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc Natl Acad Sci USA 106:6146–6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Migeon CJ, Kenny EM, Kowarski A, Snipes CA, Spaulding JS, Finkelstein JW, Blizzard RM. 1968. The syndrome of congenital adrenocortical unresponsiveness to ACTH. Report of six cases. Pediatr Res 2:501–513 [DOI] [PubMed] [Google Scholar]
- 118. Weber A, Toppari J, Harvey RD, Klann RC, Shaw NJ, Ricker AT, Näntö-Salonen K, Bevan JS, Clark AJ. 1995. Adrenocorticotropin receptor gene mutations in familial glucocorticoid deficiency: relationships with clinical features in four families. J Clin Endocrinol Metab 80:65–71 [DOI] [PubMed] [Google Scholar]
- 119. Kelch RP, Kaplan SL, Biglieri EG, Daniels GH, Epstein CJ, Grumbach MM. 1972. Hereditary adrenocortical unresponsiveness to adrenocorticotropic hormone. J Pediatr 81:726–736 [DOI] [PubMed] [Google Scholar]
- 120. Franks RC, Nance WE. 1970. Hereditary adrenocortical unresponsiveness to ACTH. Pediatrics 45:43–48 [PubMed] [Google Scholar]
- 121. Stempfel RS, Engel FL. 1960. A congenital, familial syndrome of adrenocortical insufficiency without hypoaldosteronism. J Pediatr 57:443–451 [Google Scholar]
- 122. Thistlethwaite D, Darling JA, Fraser R, Mason PA, Rees LH, Harkness RA. 1975. Familial glucocorticoid deficiency. Studies of diagnosis and pathogenesis. Arch Dis Child 50:291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Soltesz G, Dillon MJ, Jenkins PA, Moore A, Aynsley-Green A. 1985. Isolated glucocorticoid deficiency: metabolic and endocrine studies in a 5 year old boy. Eur J Pediatr 143:297–300 [DOI] [PubMed] [Google Scholar]
- 124. Shepard TH, Landing BH, Mason DG. 1959. Familial Addison's disease: case reports of two sisters with corticoid deficiency unassociated with hypoaldosteronism. Am J Dis Child 97:154–162 [PubMed] [Google Scholar]
- 125. Elias LL, Huebner A, Metherell LA, Canas A, Warne GL, Bitti ML, Cianfarani S, Clayton PE, Savage MO, Clark AJ. 2000. Tall stature in familial glucocorticoid deficiency. Clin Endocrinol (Oxf) 53:423–430 [DOI] [PubMed] [Google Scholar]
- 126. Imamine H, Mizuno H, Sugiyama Y, Ohro Y, Sugiura T, Togari H. 2005. Possible relationship between elevated plasma ACTH and tall stature in familial glucocorticoid deficiency. Tohoku J Exp Med 205:123–131 [DOI] [PubMed] [Google Scholar]
- 127. Elias LL, Clark AL. 2009. The molecular basis of adrenocorticotrophin resistance syndrome. Prog Mol Biol Transl Sci 88:155–171 [DOI] [PubMed] [Google Scholar]
- 128. Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, Cheetham T, O'Rahilly S. 2000. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest 106:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ahn JD, Dubern B, Lubrano-Berthelier C, Clement K, Karsenty G. 2006. Cart overexpression is the only identifiable cause of high bone mass in melanocortin 4 receptor deficiency. Endocrinology 147:3196–3202 [DOI] [PubMed] [Google Scholar]
- 130. Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. 2005. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520 [DOI] [PubMed] [Google Scholar]
- 131. Jarvis JL, Jenkins D, Sosman MC, Thorn GW. 1954. Roentgenologic observations in Addison's disease; a review of 120 cases. Radiology 62:16–29 [DOI] [PubMed] [Google Scholar]
- 132. Gordon DL. 1964. Calcification of auricular cartilage. Arch Intern Med 113:23–27 [DOI] [PubMed] [Google Scholar]
- 133. Bedsole A. 1966. Calcified auricular cartilages in Addison's disease. South Med J 59:1268–1270 [DOI] [PubMed] [Google Scholar]
- 134. Siebenmann RE. 1977. The ossification of the ear cartilage in Addison's disease. Schweiz Med Wochenschr 107:468–474 [PubMed] [Google Scholar]
- 135. Talmi YP, Cohen AM, Finkelstein Y, Fluro S, Zohar Y. 1989. Ossification of the auricular cartilages in patients with adrenal insufficiency. Arch Otolaryngol Head Neck Surg 115:1254–1255 [DOI] [PubMed] [Google Scholar]
- 136. Cohen AM, Talmi YP, Floru S, Bar Ziv J, Zohar Y, Djaldetti M. 1989. Ossification of the auricle in Addison's disease. J Laryngol Otol 103:885–886 [DOI] [PubMed] [Google Scholar]
- 137. Talmi YP, Cohen AM, Bar-Ziv J, Finkelstein Y, Floru S, Zohar Y. 1990. Ossified auricle in Addison's disease. Ann Otol Rhinol Laryngol 99:499–500 [DOI] [PubMed] [Google Scholar]
- 138. Rodríguez Rodríguez R, Ontanilla López A, Rodríguez Rodríguez C, Herrera Silva J, Echevarría Moreno M, Martínez Diestre MD. 1991. [Bilateral ossification of the earlobes. Addisonian crisis in the postoperative period]. Rev Esp Anestesiol Reanim 38:391–392 (Spanish) [PubMed] [Google Scholar]
- 139. Cohen AM, Talmi YP, Floru S, Tsigelman R, Kalmanovitz M, Zohar Y, Djaldetti M. 1991. X-ray microanalysis of ossified auricles in Addison's disease. Calcif Tissue Int 48:88–92 [DOI] [PubMed] [Google Scholar]
- 140. High WA, Larson MJ, Hoang MP. 2004. Idiopathic bilateral auricular ossificans: a case report and review of the literature. Arch Pathol Lab Med 128:1432–1434 [DOI] [PubMed] [Google Scholar]
- 141. Agut Fuster MA, Agulles Fornés MJ, Ferrer Rodríguez A, Ramos Martínez MJ, del Campo Biosca J, Viel Martínez JM. 2007. [Calcification of auricular cartilages in adrenal insufficiency]. Acta Otorrinolaringol Esp 58:167–168 (Spanish) [PubMed] [Google Scholar]
- 142. Fukushima K, Sato S, Yamazaki M, Kaneko A, Oofusa H, Yahikozawa H. 2007. Shock and stony hard ears. Lancet 369:856. [DOI] [PubMed] [Google Scholar]
- 143. Machado A, Lopes M, Ferreira C. 2009. Petrified auricular cartilages pointing the diagnosis of post-partum hypopituitarism in an encephalopathic patient. Eur Arch Otorhinolaryngol 266:305–307 [DOI] [PubMed] [Google Scholar]
- 144. Richter JC, Chappuis B. 2009. Ears “made of stone” - unusual secondary phenomenon of undersubstituted adrenal insufficiency. Dtsch Med Wochenschr 134:1869–1871 [DOI] [PubMed] [Google Scholar]
- 145. DiBartolomeo JR. 1985. The petrified auricle: comments on ossification, calcification and exostoses of the external ear. Laryngoscope 95:566–576 [PubMed] [Google Scholar]
- 146. Montoli A, Colussi G, Minetti L. 1992. Hypercalcaemia in Addison's disease: calciotropic hormone profile and bone histology. J Intern Med 232:535–540 [DOI] [PubMed] [Google Scholar]
- 147. Walser M, Robinson BH, Duckett JW., Jr 1963. The hypercalcemia of adrenal insufficiency. J Clin Invest 42:456–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Downie WW, Gunn A, Paterson CR, Howie GF. 1977. Hypercalcaemic crisis as presentation of Addison's disease. Br Med J 1:145–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. De Rosa G, Corsello SM, Cecchini L, Della Casa S, Testa A. 1987. A clinical study of Addison's disease. Exp Clin Endocrinol 90:232–242 [DOI] [PubMed] [Google Scholar]
- 150. Muls E, Bouillon R, Boelaert J, Lamberigts G, Van Imschoot S, Daneels R, De Moor P. 1982. Etiology of hypercalcemia in a patient with Addison's disease. Calcif Tissue Int 34:523–526 [DOI] [PubMed] [Google Scholar]
- 151. Hoch M, Hirzel E, Lindinger P, Eberle AN, Linscheid P, Martin I, Peters T, Peterli R. 2008. Weak functional coupling of the melanocortin-1 receptor expressed in human adipocytes. J Recept Signal Transduct Res 28:485–504 [DOI] [PubMed] [Google Scholar]
- 152. Smith SR, Gawronska-Kozak B, Janderová L, Nguyen T, Murrell A, Stephens JM, Mynatt RL. 2003. Agouti expression in human adipose tissue: functional consequences and increased expression in type 2 diabetes. Diabetes 52:2914–2922 [DOI] [PubMed] [Google Scholar]
- 153. Hench PS, Kendall EC, Slocumb CH, Polley HE. 1949. The effect of adrenal cortex (17-hydroxy-11-dehydrocortisone. Compound E) and of pituitary adrenorticotropin on rheumatoid arthritis. Proc Staff Meet Mayo Clin 24:181–197 [PubMed] [Google Scholar]
- 154. Spies TD, Stone 1949. Relief of the symptoms of acute and rheumatoid arthritis by means of pituitary adrenocorticotropic hormone (ACTH). South M J 42:720–722 [DOI] [PubMed] [Google Scholar]
- 155. Wolfson WQ, Cohn C, Levine R. 1949. Rapid treatment of acute gouty arthritis by the current administration of pituitary adrenocorticotropic hormone (ACTH) and colchicine. J Lab Clin Med 34:1766 [Google Scholar]
- 156. Gutman AB, Yü TF. 1950. Effects of adrenocorticotropic hormone (ACTH) in gout. Am J Med 9:24–30 [DOI] [PubMed] [Google Scholar]
- 157. Margolis HM, Caplan PS. 1950. Treatment of acute gouty arthritis with pituitary adrenocorticotropic hormone (ACTH). J A M A 142:256–258 [DOI] [PubMed] [Google Scholar]
- 158. Muzzolini M, Rosetto G, Compagnoni A. 1970. Therapy of gout attacks with synthetic delayed-action ACTH. Policlinico Med 77:322–332 [PubMed] [Google Scholar]
- 159. Axelrod D, Preston S. 1988. Comparison of parenteral adrenocorticotropic hormone with oral indomethacin in the treatment of acute gout. Arthritis Rheum 31:803–805 [DOI] [PubMed] [Google Scholar]
- 160. Ritter J, Kerr LD, Valeriano-Marcet J, Spiera H. 1994. ACTH revisited: effective treatment for acute crystal induced synovitis in patients with multiple medical problems. J Rheumatol 21:696–699 [PubMed] [Google Scholar]
- 161. Hellman L. 1949. Production of acute gouty arthritis by adrenocorticotropin. Science 109:280–281 [DOI] [PubMed] [Google Scholar]
- 162. Schlesinger N. 2008. Overview of the management of acute gout and the role of pituitary adrenocorticotropic hormone. Drugs 68:407–415 [DOI] [PubMed] [Google Scholar]
- 163. Stein C, Comisel K, Haimerl E, Yassouridis A, Lehrberger K, Herz A, Peter K. 1991. Analgesic effect of intraarticular morphine after arthroscopic knee surgery. N Engl J Med 325:1123–1126 [DOI] [PubMed] [Google Scholar]
- 164. Stein C, Millan MJ, Shippenberg TS, Herz A. 1988. Peripheral effect of fentanyl upon nociception of inflamed tissue in the rat. Neurosci Lett 84:225–228 [DOI] [PubMed] [Google Scholar]
- 165. Stein C, Millan MJ, Shippenberg TS, Peter K, Herz A. 1989. Peripheral opioid receptors mediating antinociception in inflammation: evidence for involvement of μ, δ and κ receptors. J Pharmacol Exp Ther 248:1269–1275 [PubMed] [Google Scholar]
- 166. Denko CW, Aponte J, Gabriel P, Petricevic M. 1986. β-Endorphin, immunological and biochemical changes in synovial fluid in rheumatic disorders. Clin Rheumatol 5:25–32 [DOI] [PubMed] [Google Scholar]
- 167. Toth K, Barna I, Nagy G, Wellinger K, Horvath G, Bender T. 2011. Synovial fluid β-endorphin level in avascular necrosis, rheumatoid arthritis, and osteoarthritis of the femoral head and knee. A controlled pilot study. Clin Rheumatol 30:537–540 [DOI] [PubMed] [Google Scholar]
- 168. Suzuki N, Yoshino S, Nakamura H. 1992. [A study of opioid peptides in synovial fluid and synovial tissue in patients with rheumatoid arthritis]. Arerugi 41:615–620 (Japanese) [PubMed] [Google Scholar]
- 169. Catania A, Gerloni V, Procaccia S, Airaghi L, Manfredi MG, Lomater C, Grossi L, Lipton JM. 1994. The anticytokine neuropeptide α-melanocyte-stimulating hormone in synovial fluid of patients with rheumatic diseases: comparisons with other anticytokine molecules. Neuroimmunomodulation 1:321–328 [DOI] [PubMed] [Google Scholar]
- 170. Koiwa M, Shiga H, Nakamura H, Yoshino S. 1992. [Role of opioid peptide in rheumatoid arthritis: detection of β-endorphin in synovial tissue]. Arerugi 41:1423–1429 (Japanese) [PubMed] [Google Scholar]
- 171. Mousa SA, Straub RH, Schäfer M, Stein C. 2007. β-Endorphin, met-enkephalin and corresponding opioid receptors within synovium of patients with joint trauma, osteoarthritis and rheumatoid arthritis. Ann Rheum Dis 66:871–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Karahan S, Kincaid SA, Baird AN, Kammermann JR. 2002. Distribution of β-endorphin and substance P in the shoulder joint of the dog before and after a low impact exercise programme. Anat Histol Embryol 31:72–77 [DOI] [PubMed] [Google Scholar]
- 173. Takeba Y, Suzuki N, Kaneko A, Asai T, Sakane T. 2001. Endorphin and enkephalin ameliorate excessive synovial cell functions in patients with rheumatoid arthritis. J Rheumatol 28:2176–2183 [PubMed] [Google Scholar]
- 174. Shen H, Aeschlimann A, Reisch N, Gay RE, Simmen BR, Michel BA, Gay S, Sprott H. 2005. κ and δ opioid receptors are expressed but down-regulated in fibroblast-like synoviocytes of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum 52:1402–1410 [DOI] [PubMed] [Google Scholar]
- 175. McDougall JJ, Barin AK, McDougall CM. 2004. Loss of vasomotor responsiveness to the μ-opioid receptor ligand endomorphin-1 in adjuvant monoarthritic rat knee joints. Am J Physiol Regul Integr Comp Physiol 286:R634–R641 [DOI] [PubMed] [Google Scholar]
- 176. Andjelkov N, Elvenes J, Figenschau Y, Bjorkoy G, Knutsen G, Seternes T, Johansen O. 2006. Detection of mRNA transcripts of truncated opiate precursor (POMC) in human cartilage. Cell Biochem Funct 24:229–235 [DOI] [PubMed] [Google Scholar]
- 177. Grässel S, Opolka A, Anders S, Straub RH, Grifka J, Luger TA, Böhm M. 2009. The melanocortin system in articular chondrocytes: detection of melanocortin receptors, proopiomelanocortin and precursor proteases. Evidence for a regulatory effect on pro-inflammatory cytokines and extracellular matrix components. Arthritis Rheum 60:3017–3027 [DOI] [PubMed] [Google Scholar]
- 178. Castaño MT, Freire-Garabal M, Giraldez M, Núñez MJ, Belmonte A, Couceiro J, Jorge J. 1991. Autoradiographic evidence of 125I-β-endorphin binding sites in the articular cartilage of the rat. Life Sci 49:PL103–PL105 [DOI] [PubMed] [Google Scholar]
- 179. Freire-Garabal M, Castaño MT, Belmonte A, Jorge J, Couceiro J, Núñez MJ. 1992. Autoradiographic evidence of opioid binding sites in rat growth plate chondrocytes. Neuroendocrinology 55:357–359 [DOI] [PubMed] [Google Scholar]
- 180. Keates HL, Cramond T, Smith MT. 1999. Intraarticular and periarticular opioid binding in inflamed tissue in experimental canine arthritis. Anesth Analg 89:409–415 [DOI] [PubMed] [Google Scholar]
- 181. Elvenes J, Andjelkov N, Figenschau Y, Seternes T, Bjørkøy G, Johansen O. 2003. Expression of functional μ-opioid receptors in human osteoarthritic cartilage and chondrocytes. Biochem Biophys Res Commun 311:202–207 [DOI] [PubMed] [Google Scholar]
- 182. Evans JF, Niu QT, Canas JA, Shen CL, Aloia JF, Yeh JK. 2004. ACTH enhances chondrogenesis in multipotential progenitor cells and matrix production in chondrocytes. Bone 35:96–107 [DOI] [PubMed] [Google Scholar]
- 183. Yoon SW, Chun JS, Sung MH, Kim JY, Poo H. 2008. α-MSH inhibits TNF-α-induced matrix metalloproteinase-13 expression by modulating p38 kinase and nuclear factor κB signaling in human chondrosarcoma HTB-94 cells. Osteoarthritis Cartilage 16:115–124 [DOI] [PubMed] [Google Scholar]
- 184. Dumont LM, Wu CS, Tatnell MA, Cornish J, Mountjoy KG. 2005. Evidence for direct actions of melanocortin peptides on bone metabolism. Peptides 26:1929–1935 [DOI] [PubMed] [Google Scholar]
- 185. Pérez-Castrillón JL, Olmos JM, Gómez JJ, Barrallo A, Riancho JA, Perera L, Valero C, Amado JA, González-Macías J. 2000. Expression of opioid receptors in osteoblast-like MG-63 cells, and effects of different opioid agonists on alkaline phosphatase and osteocalcin secretion by these cells. Neuroendocrinology 72:187–194 [DOI] [PubMed] [Google Scholar]
- 186. Yeh JK, Evans JF, Niu QT, Aloia JF. 2006. A possible role for melanocortin peptides in longitudinal growth. J Endocrinol 191:677–686 [DOI] [PubMed] [Google Scholar]
- 187. Stenström A, Hansson LI, Thorngren KG. 1979. Influence of α-MSH and ACTH on cortical bone remodelling in hypophysectomized rats. Experientia 35:132–133 [DOI] [PubMed] [Google Scholar]
- 188. Cornish J, Callon KE, Mountjoy KG, Bava U, Lin JM, Myers DE, Naot D, Reid IR. 2003. α-Melanocyte-stimulating hormone is a novel regulator of bone. Am J Physiol Endocrinol Metab 284:E1181–E1190 [DOI] [PubMed] [Google Scholar]
- 189. Aspenberg P, Hansson LI, Thorngren KG. 1985. Modification of bone formation rate by growth hormone, melanocyte-stimulating hormone, and cortisone in the normal rat. Acta Anat 121:84–88 [DOI] [PubMed] [Google Scholar]
- 190. Isales CM, Zaidi M, Blair HC. 2010. ACTH is a novel regulator of bone mass. Ann NY Acad Sci 1192:110–116 [DOI] [PubMed] [Google Scholar]
- 191. Andjelkov N, Elvenes J, Martin J, Johansen O. 2005. Opiate regulation of IL-1β and TNF-α in cultured human articular chondrocytes. Biochem Biophys Res Commun 333:1295–1299 [DOI] [PubMed] [Google Scholar]
- 192. Andjelkov N, Elvenes J, Knutsen G, Johansen O. 2007. β-Endorphin regulation of MAPK in cultured human articular chondrocytes: MAPK inhibitors prevent the increase of IL-1β protein levels during β-endorphin stimulation. Cell Commun Adhes 14:1–8 [DOI] [PubMed] [Google Scholar]
- 193. Raap T, Jüsten HP, Miller LE, Cutolo M, Schölmerich J, Straub RH. 2000. Neurotransmitter modulation of interleukin 6 (IL-6) and IL-8 secretion of synovial fibroblasts in patients with rheumatoid arthritis compared to osteoarthritis. J Rheumatol 27:2558–2565 [PubMed] [Google Scholar]
- 194. Miller LE, Jüsten HP, Schölmerich J, Straub RH. 2000. The loss of sympathetic nerve fibers in the synovial tissue of patients with rheumatoid arthritis is accompanied by increased norepinephrine release from synovial macrophages. FASEB J 14:2097–2107 [DOI] [PubMed] [Google Scholar]
- 195. Yin H, Zhang F, Yu M, Cheng H, Lin J, Gao Y, Han B, Zhu L. 2005. β-Endorphin ameliorates synovial cell hyperfunction in the collagen-induced arthritis rat model by specific downregulation of NF-κB activity. Neuroendocrinology 81:10–18 [DOI] [PubMed] [Google Scholar]
- 196. Zaidi M, Sun L, Robinson LJ, Tourkova IL, Liu L, Wang Y, Zhu LL, Liu X, Li J, Peng Y, Yang G, Shi X, Levine A, Iqbal J, Yaroslavskiy BB, Isales C, Blair HC. 2010. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad Sci USA 107:8782–8787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. 1984. Endogenous opioids biology and function. Annu Rev Neurosci 7:223–225 [DOI] [PubMed] [Google Scholar]
- 198. Napal J, Amado JA, Riancho JA, Olmos JM, González-Macías J. 1993. Stress decreases the serum level of osteocalcin. Bone Miner 21:113–118 [DOI] [PubMed] [Google Scholar]
- 199. Marczak ED, Jinsmaa Y, Myers PH, Blankenship T, Wilson R, Balboni G, Salvadori S, Lazarus LH. 2009. Orally administered H-Dmt-Tic-Lys-NH-CH2-Ph (MZ-2), a potent μ/δ-opioid receptor antagonist, regulates obese-related factors in mice. Eur J Pharmacol 616:115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. 1997. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88:131–141 [DOI] [PubMed] [Google Scholar]
- 201. Cawley NX, Yanik T, Woronowicz A, Chang W, Marini JC, Loh YP. 2010. Obese carboxypeptidase E knockout mice exhibit multiple defects in peptide hormone processing contributing to low bone mineral density. Am J Physiol Endocrinol Metab 299:E189–E197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Ceriani G, Diaz J, Murphree S, Catania A, Lipton JM. 1994. The neuropeptide α-melanocyte-stimulating hormone inhibits experimental arthritis in rats. Neuroimmunomodulation 1:28–32 [DOI] [PubMed] [Google Scholar]
- 203. Getting SJ, Christian HC, Flower RJ, Perretti M. 2002. Activation of melanocortin type 3 receptor as a molecular mechanism for adrenocorticotropic hormone efficacy in gouty arthritis. Arthritis Rheum 46:2765–2775 [DOI] [PubMed] [Google Scholar]
- 204. Getting SJ, Allcock GH, Flower R, Perretti M. 2001. Natural and synthetic agonists of the melanocortin receptor type 3 possess anti-inflammatory properties. J Leukoc Biol 69:98–104 [PubMed] [Google Scholar]
- 205. Patel HB, Bombardieri M, Sampaio AL, D'Acquisto F, Gray M, Grieco P, Getting SJ, Pitzalis C, Perretti M. 2010. Anti-inflammatory and antiosteoclastogenesis properties of endogenous melanocortin receptor type 3 in experimental arthritis. FASEB J 24:4835–4843 [DOI] [PubMed] [Google Scholar]
- 206. Shen PC, Shiau Al, Jou IM, Lee CH, Tai MH, Juan HY, Lin RR, Liu GS, Wu CL, Hsieh JL. 2011. Inhibition of cartilage damage by pro-opiomelanocortin prohormone overexpression in a rat model of osteoarthritis. Exp Biol Med (Maywood) 236:334–340 [DOI] [PubMed] [Google Scholar]
- 207. Martinez JH, Mondragón CE, Céspedes A. 1996. An evaluation of the antiinflammatory effects of intraarticular synthetic β-endorphin in the canine model. Anesth Analg 82:177–181 [DOI] [PubMed] [Google Scholar]
- 208. Yin H, Yu M, Cheng H, Zhang F, Gao Y, Lin J, Han B, Zhu L. 2005. β-Endorphin prevents collagen induced arthritis by neuroimmuno-regulation pathway. Neuro Endocrinol Lett 26:739–744 [PubMed] [Google Scholar]
- 209. Zadina JE, Hackler L, Ge LJ, Kastin AJ. 1997. A potent and selective endogenous agonist for the μ-opiate receptor. Nature 386:499–502 [DOI] [PubMed] [Google Scholar]
- 210. Straub RH, Wolff C, Fassold A, Hofbauer R, Chover-Gonzalez A, Richards LJ, Jessop DS. 2008. Antiinflammatory role of endomorphins in osteoarthritis, rheumatoid arthritis, and adjuvant-induced polyarthritis. Arthritis Rheum 58:456–466 [DOI] [PubMed] [Google Scholar]
- 211. Jessop DS, Fassold A, Wolff C, Hofbauer R, Chover-Gonzalez A, Richards LJ, Straub RH. 2010. Endomorphins in rheumatoid arthritis, osteoarthritis and experimental arthritis. Ann NY Acad Sci 1193:117–122 [DOI] [PubMed] [Google Scholar]
- 212. Pelletier J-P, Boileau C, Altman RA, Martel-Pelletier J. 2010. Animal models of osteoarthritis. In: Hochberg M, Silman A, Smolen JS, Weinblatt M, Weisman M, eds. Rheumatology. 5th ed London: Elsevier; 1723–1730 [Google Scholar]
- 213. Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G. 1991. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J 10:4025–4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Zwerina J, Redlich K, Polzer K, Joosten L, Krönke G, Distler J, Hess A, Pundt N, Pap T, Hoffmann O, Gasser J, Scheinecker C, Smolen JS, van den Berg W, Schett G. 2007. TNF-induced structural joint damage is mediated by IL-1. Proc Natl Acad Sci USA 104:11742–11777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Abdollahi-Roodsaz S, Joosten LA, Koenders MI, Devesa I, Roelofs MF, Radstake TR, Heuvelmans-Jacobs M, Akira S, Nicklin MJ, Ribeiro-Dias F, van den Berg WB. 2008. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest 118:205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Geyer M, Müller-Ladner U. 2010. Actual status of antiinterleukin-1 therapies in rheumatic diseases. Curr Opin Rheumatol 22:246–251 [DOI] [PubMed] [Google Scholar]
- 217. Geiler J, Buch M, McDermott MF. 2011. Anti-TNF treatment in rheumatoid arthritis. Curr Pharm 17:3141–3154 [DOI] [PubMed] [Google Scholar]
- 218. Catania A. 2007. The melanocortin system in leukocyte biology. J Leukoc Biol 81:383–392 [DOI] [PubMed] [Google Scholar]
- 219. Gaston 2010. Cellular immunity in rheumatoid arthritis. In: Hochberg M, Silman A, Smolen JS, Weinblatt M, Weisman M, eds. Rheumatology. 5th ed London: Elsevier; 897–903 [Google Scholar]
- 220. Edling AE, Gomes D, Weeden T, Dzuris J, Stefano J, Pan C, Williams J, Kaplan J, Perricone MA. 2011. Immunosuppressive activity of a novel peptide analog of α-melanocyte stimulating hormone (α-MSH) in experimental autoimmune uveitis. J Neuroimmunol 236:1–9 [DOI] [PubMed] [Google Scholar]
- 221. Taylor AW, Kitaichi N. 2008. The diminishment of experimental autoimmune encephalomyelitis (EAE) by neuropeptide α-melanocyte stimulating hormone. Brain Behav Immun 22:639–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Cutuli M, Cristiani S, Lipton JM, Catania A. 2000. Antimicrobial effects of α-MSH peptides. J Leukoc Biol 67:233–239 [DOI] [PubMed] [Google Scholar]
- 223. Lipton JM. 1990. Modulation of host defense by the neuropeptide α-MSH. Yale J Biol Med 63:173–182 [PMC free article] [PubMed] [Google Scholar]
- 224. Potaman VN, Alfeeva LY, Kamensky AA, Nezavibatko VN. 1993. Degradation of ACTH/MSH(4–10) and its synthetic analog semax by rat serum enzymes: an inhibitor study. Peptides 14:491–495 [DOI] [PubMed] [Google Scholar]
- 225. Castrucci AM, Hadley ME, Sawyer TK, Hruby VJ. 1984. Enzymological studies of melanotropins. Comp Biochem Physiol B 78:519–524 [DOI] [PubMed] [Google Scholar]
- 226. Marks N, Stern F, Kastin AJ. 1976. Biodegradation of α-MSH and derived peptides by rat brain extracts, and by rat and human serum. Brain Res Bull 1:591–593 [DOI] [PubMed] [Google Scholar]
- 227. Trochard MC, Vaudry H, Leboulenger F, Usategui R, Vaillant R. 1976. The degradation of radioiodinated α MSH in vitro: effect of some inhibitors of proteolytic enzymes. C R Seances Soc Biol Fil 170:1103–1109 [PubMed] [Google Scholar]
- 228. Deschodt-Lanckman M, Vanneste Y, Loir B, Michel A, Libert A, Ghanem G, Lejeune F. 1990. Degradation of α-melanocyte stimulating hormone (α-MSH) by CALLA/endopeptidase 24.11 expressed by human melanoma cells in culture. Int J Cancer 46:1124–1130 [DOI] [PubMed] [Google Scholar]
- 229. Wallingford N, Perroud B, Gao Q, Coppola A, Gyengesi E, Liu ZW, Gao XB, Diament A, Haus KA, Shariat-Madar Z, Mahdi F, Wardlaw SL, Schmaier AH, Warden CH, Diano S. 2009. Prolylcarboxypeptidase regulates food intake by inactivating α-MSH in rodents. J Clin Invest 119:2291–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Diano S. 2011. New aspects of melanocortin signaling: a role for PRCP in α-MSH degradation. Front Neuroendocrinol 32:70–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Kumamoto K, Stewart TA, Johnson AR, Erdös EG. 1981. Prolylcarboxypeptidase (angiotensinase C) in human lung and cultured cells. J Clin Invest 67:210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Harms J, Lautenschlager S, Minder CE, Minder EI. 2009. An α-melanocyte-stimulating hormone analogue in erythropoietic protoporphyria. N Engl J Med 360:306–307 [DOI] [PubMed] [Google Scholar]
- 233. Vessillier S, Adams G, Montero-Melendez T, Jones R, Seed M, Perretti M, Chernajovsky Y. 2012. Molecular engineering of short half-life small peptides (VIP, αMSH and γ3MSH) fused to latency-associated peptide results in improved anti-inflammatory therapeutics. Ann Rheum Dis 71:143–149 [DOI] [PubMed] [Google Scholar]
- 234. Mastrofrancesco A, Kokot A, Eberle A, Gibbons NC, Schallreuter KU, Strozyk E, Picardo M, Zouboulis CC, Luger TA, Böhm M. 2010. KdPT, a tripeptide derivative of α-melanocyte-stimulating hormone, suppresses IL-1β-mediated cytokine expression and signaling in human sebocytes. J Immunol 185:1903–1911 [DOI] [PubMed] [Google Scholar]
- 235. Bettenworth D, Buyse M, Böhm M, Mennigen R, Czorniak I, Kannengiesser K, Brzoska T, Luger TA, Kucharzik T, Domschke W, Maaser C, Lügering A. 2011. The tripeptide KdPT protects from intestinal inflammation and maintains intestinal barrier function. Am J Pathol 179:1230–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Grässel S, Dreier R. 2011. Effect of cellular microenvironment and paracrine signals on chondrogenic differentiation of mesenchymal progenitor cells. In: Singh SR, ed. Stem cells, regenerative medicine and cancer. New York: Nova Science Publishers; 441–467 [Google Scholar]