Abstract
This study was conducted to review the clinical characteristics of parathyroid carcinoma (PC) and to evaluate potential preoperative predictive factors for PC in patients with primary hyperparathyroidism (PHPT). We performed a retrospective review of electronic medical records of 194 patients with pathologically confirmed PHPT in affiliated teaching hospitals of Seoul National University from January 2000 to March 2011. Adenoma was diagnosed in 171 patients, hyperplasia in 12, and carcinoma in 11. Several biochemical measurements were higher in patients with PC than in patients with benign disease, including serum total calcium (P < 0.001), intact parathyroid hormone (P = 0.003), and alkaline phosphatase (ALP) (P < 0.001). Tumors were larger in PC than in benign disease (P < 0.001). Multivariate analysis revealed that serum ALP level (P < 0.001) and tumor size were associated with PC (P = 0.03). Tumor size and serum ALP level were evaluated as preoperative predictive factors for PC using ROC analyses: a tumor size of 3.0 cm (sensitivity 90.9%, specificity 92.1%) and serum ALP level of 285 IU/L (83.3%, 97.0%) had predictive value for the diagnosis of PC in patients with PHPT. In conclusion, elevated serum ALP and a large parathyroid mass at the time of diagnosis can be helpful to predict PC in patients with PHPT.
Keywords: Hyperparathyroidism, Parathyroid Neoplasms, Alkaline Phosphatase
INTRODUCTION
Primary hyperparathyroidism (PHPT) is a common endocrine disorder that is being diagnosed more frequently due to the increasing use of routine serum total calcium tests as part of medical examinations (1). The clinical presentation of PHPT is changing from a severe disease with renal stones and metabolic bone disease to mild asymptomatic disease (2). Parathyroid carcinoma (PC) comprises a very small proportion of PHPT cases. A meta-analysis of 20,225 cases of PHPT in the USA reported that PC occurred only in 0.74% of cases (3). Nevertheless, PC requires early recognition and complete resection because its prognosis is quite variable (4-6). The average time to the first recurrence is approximately 3-yr and intervals of up to 20-yr have been reported (7).
The current definitive treatment of PC is en-bloc resection, which avoids rupture and spillage of the tumor. Once the tumor has recurred, curative treatment is unlikely and the surgical complication rate is high, although PC patients typically have a prolonged survival (8). Therefore, it is desirable to distinguish benign disease such as parathyroid adenoma and hyperplasia from PC prior to surgery. Unfortunately, PC is frequently only diagnosed after surgery for hyperparathyroidism because the clinical presentation of PC and benign disease are strikingly similar.
In this retrospective study, we compared the clinical presentation of PC with benign disease and reevaluated the biochemical parameters that have been used to differentiate PC from benign disease at the time of the first operation.
MATERIALS AND METHODS
We performed a retrospective review of electronic medical records in affiliated teaching hospitals of Seoul National University (Seoul National University Hospital, Seoul National University Bundang Hospital, and Seoul Metropolitan Government Borame Medical Center). The records were reviewed, over a 10-yr period from January 2000 to March 2011, of patients who had been diagnosed with hyperparathyroidism, parathyroid adenoma, parathyroid hyperplasia, PC, parathyroid tumor, or hypercalcemia. Patients with secondary or tertiary hyperparathyroidism, or other causes of hypercalcemia such as malignancy, vitamin D intoxication, immobilization, medications, hyperthyroidism, and other endocrine diseases, were excluded. Data collected from the records included the clinical features (hypercalcemia-related symptoms at the time of diagnosis, previous history of osteoporosis, fracture, and renal or urinary stone), laboratory data (serum levels of total calcium, phosphorous, intact parathyroid hormone [iPTH], creatinine, alkaline phosphatase [ALP], and 25-hydroxy vitamin D; 24 hr urinary calcium and phosphorus; and dual energy X-ray absorptiometry [DEXA]), and surgically proven pathology (adenoma, hyperplasia, and carcinoma). Serum iPTH was measured preoperatively by means of immunoradiometric assay (Cis Bio International, Gif-Sur-Yvette, Cedex, France). Follow-up duration, interval change of clinical features, and laboratory data after surgery were also included.
Descriptive statistics are reported as mean ± standard deviation, and confidence intervals were computed as two-tailed using 95% coverage. Categorical variables are reported as frequencies and proportions. Comparisons between continuous variables were performed using repeated measures ANOVA. Comparisons between categorical variables were performed using a chi-squared test. Univariate and multivariate analyses of variables were performed. Statistical analyses were done with SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). P values < 0.05 were accepted as significant.
Ethics statement
The study protocol was approved by the institutional review board (IRB) of Seoul National University Hospital (IRB No. H-1101-070-348). An informed consent was waived by the IRB.
RESULTS
Subjects and clinical characteristics of patients with PHPT according to the etiology
Two hundred and ninety-one patients with a diagnosis of PHPT were eligible for the study, and 194 of these patients with surgically proven parathyroid adenoma, hyperplasia, or carcinoma were included in the analysis. Adenoma was diagnosed in 171 patients, hyperplasia in 12 patients, and carcinoma in 11 patients. PC was diagnosed based on several characteristic pathologic findings (capsular penetration, vascular invasion, perineural space invasion, trabecular growth pattern, and mitotic figures) or definite evidence of metastasis (9, 10). Five patients revealed capsular penetration. Two patients revealed capsular invasion with extension into soft tissue, trabecular growth pattern, and partly cystic change. Two patients revealed capsular invasion with extension into soft tissue, mitotic figures, and partly cystic or hemorrhagic change. One patient revealed capsular penetration and vascular invasion. And the last one patient revealed vascular invasion with solid and spindle cell change.
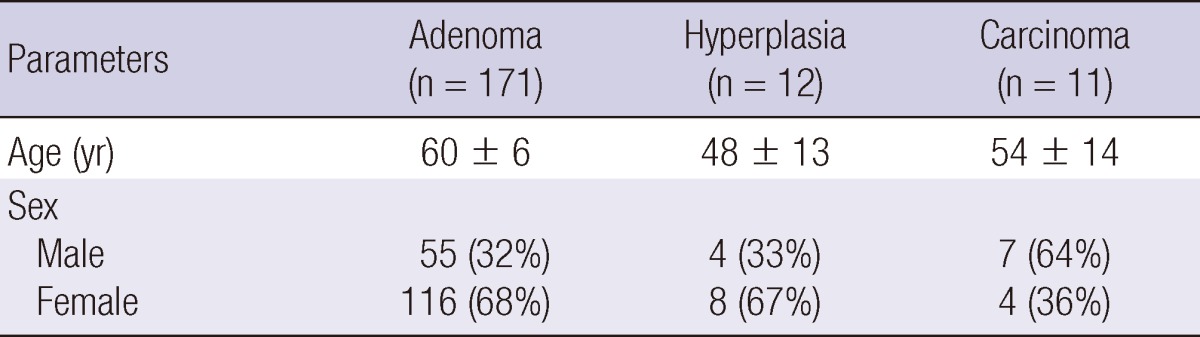
The mean age at diagnosis in the adenoma, hyperplasia and carcinoma groups were 60 ± 6 (range, 46-74), 48 ± 13 (35-61), and 54 ± 14 (40-68) yr old, respectively. The mean follow-up duration for each of the three groups was 46.5, 40.4, and 56.3 months, respectively. Female predominance was observed in the adenoma and hyperplasia groups, as is generally observed in patients with PHPT. In contrast, carcinoma predominantly occurred in males (7 males, 4 females). However, there were no significant differences in gender distribution in each of the three different etiological groups (Table 1).
Table 1.
Demographics of patients with pathologically confirmed primary hyperparathyroidism
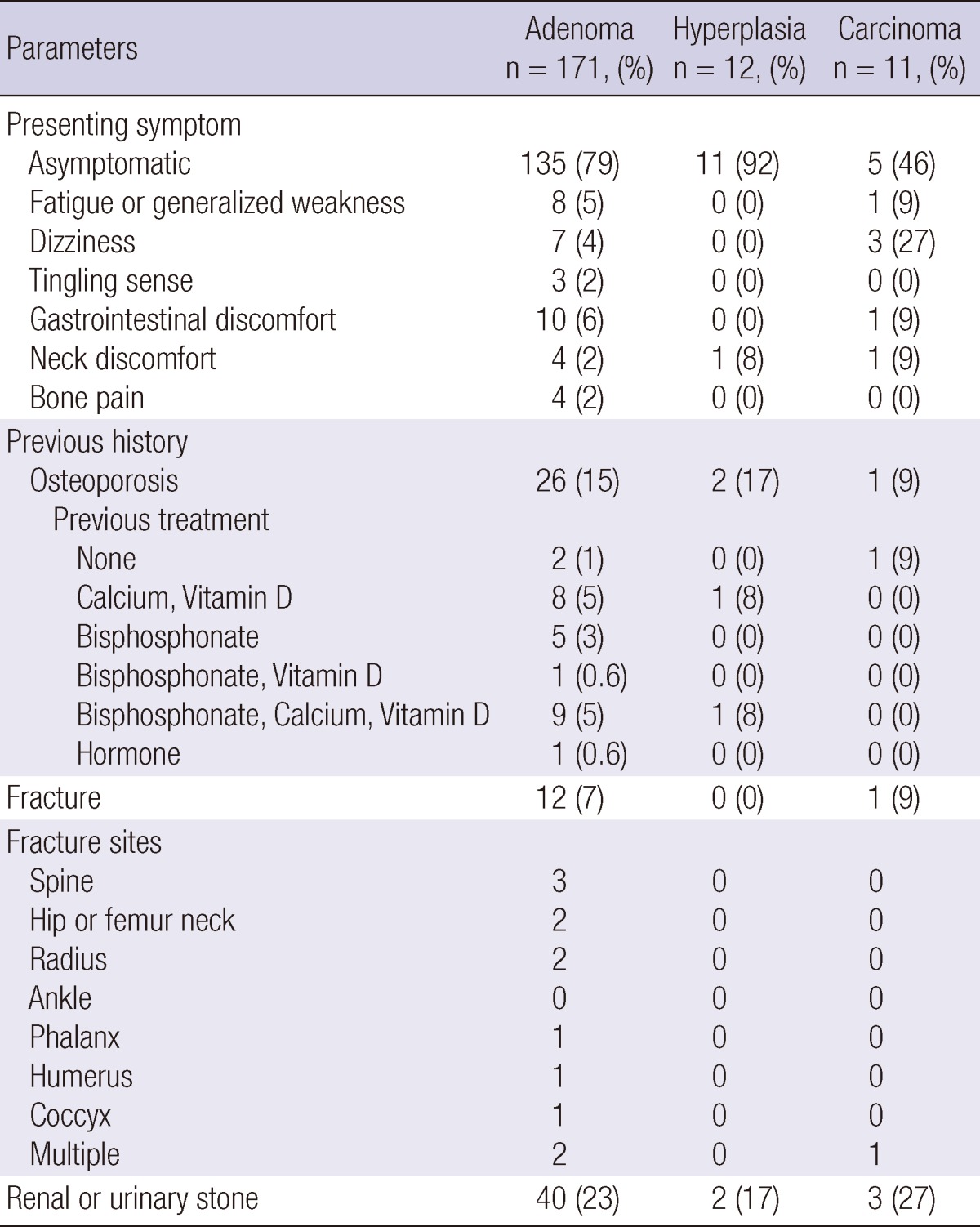
Patients with PHPT generally presented without any symptom or sign regardless of the etiology. Asymptomatic presentation was seen in 135 adenoma patients (79% of the PHPT patients with adenoma), 11 patients with hyperplasia (92%), and 5 patients with carcinoma (46%). In the remaining patients, presenting symptoms and signs at the time of diagnosis included fatigue or generalized weakness, dizziness, tingling sensation in the extremities, gastrointestinal discomfort, neck discomfort, and bone pain. Dizziness was observed in 3 patients with carcinoma (27%), but the number of patients was too small and this was not statistically significant compared to benign disease. There was no difference between the three etiological groups in the occurrence of other symptoms and signs. There was no significant difference in the frequency of a history of osteoporosis, fracture, or renal or urinary stone between the different etiological groups (Table 2).
Table 2.
Clinical characteristics of patients with pathologically confirmed hyperparathyroidism
Comparison of biochemical parameters in PHPT patients according to etiology
Measurements were obtained for serum total calcium, iPTH, creatinine, phosphorus, ALP, 25-hydroxy vitamin D, and 24 hr urine calcium and phosphorus. In the patients with PC, serum levels of total calcium, iPTH, creatinine, phosphorus and ALP were significantly higher than in patients with adenoma and hyperplasia. There were no significant differences between the three etiological groups in serum 25-hydroxy vitamin D, 24 hr urine calcium and phosphorous, and bone mineral density (BMD) measured by DEXA (Table 3).
Table 3.
Biochemi cal parameters of patients with pathologically confirmed hyperparathyroidism
*Log-transformed values were used for analysis. †Median (interquartile range) values were shown. ‡Bone mineral density. §P < 0.001, ∥P = 0.03. SE β, standard error of β coefficient; WalS, Wald statistic; OR, odds ratio; CI, confidence interval.
Univariate analysis was performed to compare carcinoma and benign disease (adenoma and hyperplasia). Serum total calcium, iPTH, creatinine, phosphorous, and ALP were included in the analysis. All biochemical parameters in the univariate analysis were significantly higher in carcinoma than in benign disease (Table 3).
Multivariate analysis was done by logistic regression analysis. The same biochemical parameters were included in the analysis as independent variables comparing carcinoma and benign disease. When all variables were adjusted, only serum ALP level was significantly higher in carcinoma than in benign disease (P < 0.001) (Table 3).
Tumor size
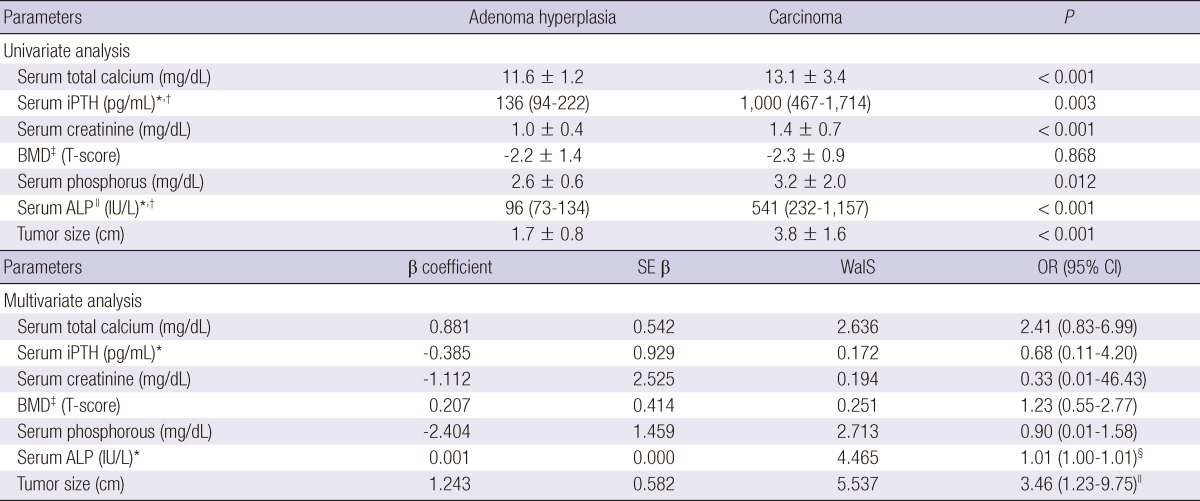
The mean tumor (or hyperplasic gland) size was 3.8 ± 1.6 cm for carcinoma and 1.7 ± 0.8 cm for benign disease. The carcinomas were significantly larger in univariate (P < 0.001) and multivariate analysis (P = 0.03). The locations of the carcinomas were intrathyroid (5 patients), right lower (2 patients), ectopic (2 patients), right upper (1 patient), and left upper (1 patient). The number of carcinomas in the parathyroid gland was only one for all patients. The location and number of involved parathyroid glands was not statistically significant between carcinoma and benign diseases.
Receiver operating characteristic (ROC) curve analysis of serum ALP level and tumor size for prediction of PC
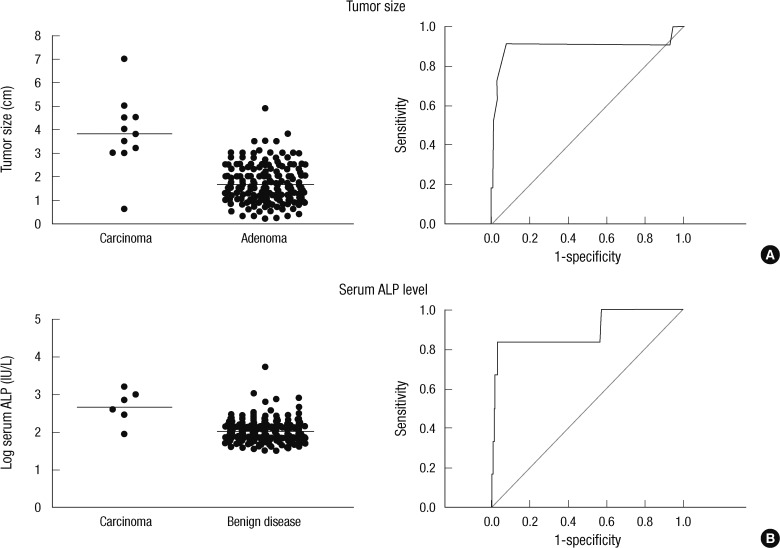
To evaluate the diagnostic values of serum ALP level and tumor size for PC, ROC were calculated for each parameters. ROC curve compared true positive rate, or sensitivity with false positive rate, or 1-specificity. Cut-off value was determined at the highest points of sensitivity and specificity. Confidential interval was 95%. When the cut-off value of tumor size was 3.0 cm, sensitivity and specificity for prediction of carcinoma were 90.9% and 92.1%, respectively. Serum ALP level was 540.5 (232.3-1,156.8) (median, [interquartile range]) IU/L in carcinoma and 96.0 (73.0-133.8) IU/L in benign disease (P < 0.001). When the cut-off value of serum ALP was 285 IU/L, sensitivity and specificity for prediction of carcinoma were 83.3% and 97.0%, respectively (Fig. 1).
Fig. 1.
Receiver operating curve analyses of tumor size and serum ALP level. Tumor size and serum ALP level were evaluated for preoperative predictive factors for PC. Tumor size (A) 3.0 cm had 90.9% of sensitivity, 92.1% of specificity and 43.4% of positive predictive values (PPV), 99.9% of negative predictive value (NPV). Serum ALP level (B) 285 IU/L had 83.3% of sensitivity, 97.0% of specificity and 33.3% of PPV, 99.9% of NPV.
Correlation of tumor size with biochemical parameters in patients with PHPT
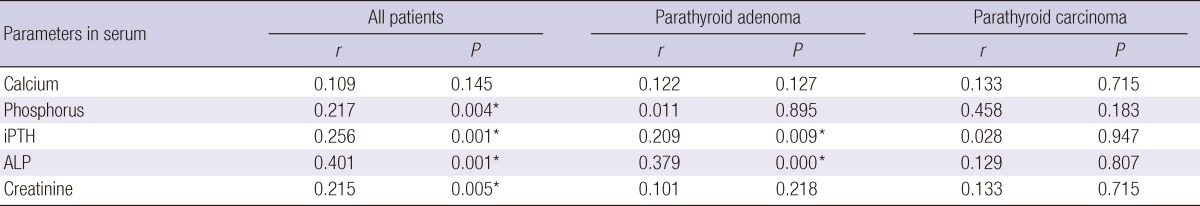
The tumor size was correlated with serum ALP and iPTH levels in patients with parathyroid adenoma (P = 0.009, and P < 0.001). However, correlation between tumor size and biochemical parameters was not significant in PC patients (Table 4).
Table 4.
Correlation of tumor size with biochemical parameters in patients with primary hyperparathyroidism
*P < 0.01.
DISCUSSION
In this retrospective study of 194 patients with pathologically confirmed PHPT, the tumor size and serum ALP level were predictors of PC before the first operation. PC is an extremely rare tumor. In this study, only 11 patients were diagnosed with PC in Seoul National University's three affiliated teaching hospitals during a 10-yr period. The proportion of PC in patients with PHPT was relatively high in our study, 5.7% considering that previous studies reported that PC accounts for only 0.5%-5% of all patients with PHPT (3, 5, 11). This discrepancy could result from referral bias since the hospitals involved in the present study are referral centers.
There were no significant differences between PC and the benign disease in terms of clinical symptoms, bone loss or fracture, or nephrolithiasis. PC affects the classical target organs, such as bone and kidney, more severely than benign PHPT, since patients with PC commonly have profound hypercalcemia (8, 12-14). Although most PHPT patients in contemporary clinical practice are asymptomatic, some patients with benign PHPT present with more severe manifestations (2, 15, 16). In such patients, it may be extremely difficult to differentiate the clinical manifestations of malignancy from those of benign diseases. As the number of benign, symptomatic PHPT patients is progressively decreasing, the sample size of 194 patients in the present study would be unlikely to find a statistical difference between the clinical manifestations of malignant and benign PHPT.
Serum ALP level was higher in patients with PC than in those with benign disease (17), and our results suggest that a patient with serum ALP level less than 300 IU/L is unlikely to have carcinoma. Elevated serum ALP level in patients with PHPT may reflect the longstanding effect of PTH on the bone. This result is supported by the previous study that serum levels of PTH and ALP are useful for the prediction of PC (18). In this study, serum iPTH levels in patients with benign PHPT showed relatively wide distribution compared to serum ALP levels. This result suggests that serum ALP rather than serum iPTH could be predictive factors of PC. Persistent excess of PTH in the circulation causes an increase in the RANKL/OPG ratio, which is the main mechanism by which PTH stimulates osteoclastogenesis and bone resorption (19, 20). Histomorphometric studies have shown that PHPT commonly causes preferential cortical bone loss with preservation of cancellous bone (21, 22). Therefore, serum ALP might reflect the effects of PTH on the bone earlier than skeletal phenotypes such as bone mineral density or fractures.
The size of a parathyroid tumor may be an important factor in predicting PC in a patient with PHPT. In this study, 10 of 11 patients with PC had a parathyroid mass larger than 3.0 cm, and ROC analysis showed that the sensitivity and specificity for PC was 90.9% and 92.1%, respectively. This result is in agreement with previous findings that PC are usually larger than 3 cm and may be palpable (23). Interestingly, the median tumor size at the time of diagnosis in 286 cases of PC treated in the USA from 1985 to 1995 was approximately 3.3 cm (8). A palpable mass in the neck has been suggested as a strong predictive factor for PC (7, 11, 24), and particular attention should be paid to a hyperparathyroid patient with a parathyroid mass larger than 3.0 cm.
We also examined PTH as a potential factor to distinguish PC from benign causes of PHPT. In multivariate analysis, patients with PC did not show a statistically significant difference in serum levels of iPTH compared to those with benign PHPT. The relationship between preoperative serum PTH levels and the size of a parathyroid adenoma or carcinoma is controversial (25, 26). In the present study, serum iPTH levels were correlated with tumor size in patients with parathyroid adenoma but not in patients with PC. This difference may be resulted from the small number of patients with carcinoma. A recent report suggested that an N-terminal PTH distinct from iPTH can be recognized in a 3rd generation assay of whole PTH and that this form is overproduced in some patients with PC (27). Oncogenes and tumor suppressor genes involved in the control of the cell cycle have been linked to the pathogenesis of PC, including retinoblastoma (Rb), p53, breast carcinoma susceptibility (BRCA2), and cyclin D l/parathyroid adenomatosis gene 1 (CCND1/PRAD1) genes (28, 29). The excessive expression of CCND1 by the highly active PTH gene promoter in the parathyroid cell may contribute to excess cellular proliferation and development of PC. This result indicates that PC is frequently functional. Although CCND1 is overexpressed in more than 90% of cases of PC, its role in pathogenesis remains to be determined (30).
In conclusion, the results of this study suggest that the size of tumor and serum level of ALP may be helpful to predict PC in patients with PHPT before the primary operation.
Footnotes
This study was supported by a grant from Seoul National University Hospital (No.04-2005-0200).
References
- 1.Wermers RA, Khosla S, Atkinson EJ, Achenbach SJ, Oberg AL, Grant CS, Melton LJ., 3rd Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993-2001: an update on the changing epidemiology of the disease. J Bone Miner Res. 2006;21:171–177. doi: 10.1359/JBMR.050910. [DOI] [PubMed] [Google Scholar]
- 2.Fraser WD. Hyperparathyroidism. Lancet. 2009;374:145–158. doi: 10.1016/S0140-6736(09)60507-9. [DOI] [PubMed] [Google Scholar]
- 3.Ruda JM, Hollenbeak CS, Stack BC., Jr A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg. 2005;132:359–372. doi: 10.1016/j.otohns.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Iacobone M, Lumachi F, Favia G. Up-to-date on parathyroid carcinoma: analysis of an experience of 19 cases. J Surg Oncol. 2004;88:223–228. doi: 10.1002/jso.20152. [DOI] [PubMed] [Google Scholar]
- 5.Iihara M, Okamoto T, Suzuki R, Kawamata A, Nishikawa T, Kobayashi M, Obara T. Functional parathyroid carcinoma: long-term treatment outcome and risk factor analysis. Surgery. 2007;142:936–943. doi: 10.1016/j.surg.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Kleinpeter KP, Lovato JF, Clark PB, Wooldridge T, Norman ES, Bergman S, Perrier ND. Is parathyroid carcinoma indeed a lethal disease? Ann Surg Oncol. 2005;12:260–266. doi: 10.1245/ASO.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 7.Shane E. Clinical review 122: Parathyroid carcinoma. J Clin Endocrinol Metab. 2001;86:485–493. doi: 10.1210/jcem.86.2.7207. [DOI] [PubMed] [Google Scholar]
- 8.Hundahl SA, Fleming ID, Fremgen AM, Menck HR The American College of Surgeons Commission on Cancer and the American Cancer Society. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. between 1985-1995: a National Cancer Data Base Report. Cancer. 1999;86:538–544. doi: 10.1002/(sici)1097-0142(19990801)86:3<538::aid-cncr25>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto T, Iihara M, Obara T, Tsukada T. Parathyroid carcinoma: etiology, diagnosis, and treatment. World J Surg. 2009;33:2343–2354. doi: 10.1007/s00268-009-9999-0. [DOI] [PubMed] [Google Scholar]
- 10.Owen RP, Silver CE, Pellitteri PK, Shaha AR, Devaney KO, Werner JA, Rinaldo A, Ferlito A. Parathyroid carcinoma: a review. Head Neck. 2011;33:429–436. doi: 10.1002/hed.21376. [DOI] [PubMed] [Google Scholar]
- 11.Schaapveld M, Jorna FH, Aben KK, Haak HR, Plukker JT, Links TP. Incidence and prognosis of parathyroid gland carcinoma: a population based study in The Netherlands estimating the preoperative diagnosis. Am J Surg. 2011;202:590–597. doi: 10.1016/j.amjsurg.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Obara T, Okamoto T, Kanbe M, Iihara M. Functioning parathyroid carcinoma: clinicopathologic features and rational treatment. Semin Surg Oncol. 1997;13:134–141. doi: 10.1002/(sici)1098-2388(199703/04)13:2<134::aid-ssu9>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.Wang CA, Gaz RD. Natural history of parathyroid carcinoma. Diagnosis, treatment, and results. Am J Surg. 1985;149:522–527. doi: 10.1016/s0002-9610(85)80050-7. [DOI] [PubMed] [Google Scholar]
- 14.Wynne AG, van Heerden J, Carney JA, Fitzpatrick LA. Parathyroid carcinoma: clinical and pathologic features in 43 patients. Medicine (Baltimore) 1992;71:197–205. [PubMed] [Google Scholar]
- 15.Chon S, Kim YH, Park JY, Ko KP, Park CY, Kim DY, Woo JT, Kim SW, Kim JW, Kim YS, et al. A case of cystic parathyroid adenoma presenting as severe bony lesion. J Korean Endocr Soc. 2003;18:214–220. [Google Scholar]
- 16.Chung JO, Jeong GH, Hong SE, Cho DH, Chung DJ, Chung MY. A case of primary hyperparathyroidism due to cystic parathyroid adenoma presenting as hypercalcemic crisis associated with intracranial hemorrhage. J Korean Soc Endocrinol. 2007;22:292–298. [Google Scholar]
- 17.Silverberg SJ, Shane E, Jacobs TP, Siris ES, Gartenberg F, Seldin D, Clemens TL, Bilezikian JP. Nephrolithiasis and bone involvement in primary hyperparathyroidism. Am J Med. 1990;89:327–334. doi: 10.1016/0002-9343(90)90346-f. [DOI] [PubMed] [Google Scholar]
- 18.Chen Q, Kaji H, Nomura R, Sowa H, Yamauchi M, Tsukamoto T, Yamaguchi T, Kobayashi A, Sugimoto T, Chihara K. Trial to predict malignancy of affected parathyroid glands in primary hyperparathyroidism. Endocr J. 2003;50:527–534. doi: 10.1507/endocrj.50.527. [DOI] [PubMed] [Google Scholar]
- 19.Huang JC, Sakata T, Pfleger LL, Bencsik M, Halloran BP, Bikle DD, Nissenson RA. PTH differentially regulates expression of RANKL and OPG. J Bone Miner Res. 2004;19:235–244. doi: 10.1359/JBMR.0301226. [DOI] [PubMed] [Google Scholar]
- 20.Ma YL, Cain RL, Halladay DL, Yang X, Zeng Q, Miles RR, Chandrasekhar S, Martin TJ, Onyia JE. Catabolic effects of continuous human PTH (1--38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology. 2001;142:4047–4054. doi: 10.1210/endo.142.9.8356. [DOI] [PubMed] [Google Scholar]
- 21.Parisien M, Mellish RW, Silverberg SJ, Shane E, Lindsay R, Bilezikian JP, Dempster DW. Maintenance of cancellous bone connectivity in primary hyperparathyroidism: trabecular strut analysis. J Bone Miner Res. 1992;7:913–919. doi: 10.1002/jbmr.5650070808. [DOI] [PubMed] [Google Scholar]
- 22.Parisien M, Silverberg SJ, Shane E, de la Cruz L, Lindsay R, Bilezikian JP, Dempster DW. The histomorphometry of bone in primary hyperparathyroidism: preservation of cancellous bone structure. J Clin Endocrinol Metab. 1990;70:930–938. doi: 10.1210/jcem-70-4-930. [DOI] [PubMed] [Google Scholar]
- 23.Marcocci C, Cetani F, Rubin MR, Silverberg SJ, Pinchera A, Bilezikian JP. Parathyroid carcinoma. J Bone Miner Res. 2008;23:1869–1880. doi: 10.1359/jbmr.081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robert JH, Trombetti A, Garcia A, Pache JC, Herrmann F, Spiliopoulos A, Rizzoli R. Primary hyperparathyroidism: can parathyroid carcinoma be anticipated on clinical and biochemical grounds? Report of nine cases and review of the literature. Ann Surg Oncol. 2005;12:526–532. doi: 10.1245/ASO.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Randhawa PS, Mace AD, Nouraei SA, Stearns MP. Primary hyperparathyroidism: do perioperative biochemical variables correlate with parathyroid adenoma weight or volume? Clin Otolaryngol. 2007;32:179–184. doi: 10.1111/j.1365-2273.2007.01447.x. [DOI] [PubMed] [Google Scholar]
- 26.Williams JG, Wheeler MH, Aston JP, Brown RC, Woodhead JS. The relationship between adenoma weight and intact (1-84) parathyroid hormone level in primary hyperparathyroidism. Am J Surg. 1992;163:301–304. doi: 10.1016/0002-9610(92)90007-e. [DOI] [PubMed] [Google Scholar]
- 27.Rubin MR, Silverberg SJ, D'Amour P, Brossard JH, Rousseau L, Sliney J, Jr, Cantor T, Bilezikian JP. An N-terminal molecular form of parathyroid hormone (PTH) distinct from hPTH(1 84) is overproduced in parathyroid carcinoma. Clin Chem. 2007;53:1470–1476. doi: 10.1373/clinchem.2007.085506. [DOI] [PubMed] [Google Scholar]
- 28.Arnold A, Shattuck TM, Mallya SM, Krebs LJ, Costa J, Gallagher J, Wild Y, Saucier K. Molecular pathogenesis of primary hyperparathyroidism. J Bone Miner Res. 2002;17:N30–N36. [PubMed] [Google Scholar]
- 29.Cryns VL, Rubio MP, Thor AD, Louis DN, Arnold A. p53 abnormalities in human parathyroid carcinoma. J Clin Endocrinol Metab. 1994;78:1320–1324. doi: 10.1210/jcem.78.6.8200932. [DOI] [PubMed] [Google Scholar]
- 30.Vasef MA, Brynes RK, Sturm M, Bromley C, Robinson RA. Expression of cyclin D1 in parathyroid carcinomas, adenomas, and hyperplasias: a paraffin immunohistochemical study. Mod Pathol. 1999;12:412–416. [PubMed] [Google Scholar]