Abstract
The purpose of this study was to investigate the clinical aspects of patients satisfying the Infectious Disease Society of America/American Thoracic Society (IDSA/ATS) minor severity criteria, focusing on their treatment response to empirical antibiotics. In total, 381 community-acquired pneumonia (CAP) patients who did not require mechanical ventilation or vasopressors at admission were enrolled, and 50 (13.1%) satisfied the minor severity criteria (i.e. , minor severe CAP [minor-SCAP]). The rates of new complication events and clinical treatment failure were significantly higher in the minor-SCAP group than in the control group (30.0% vs 2.1%, P < 0.001, and 42.0% vs 10.6%, P < 0.001, respectively), and the time to reach clinical stability was longer in the minor-SCAP group (8 days vs 3 days, P < 0.001). In a multivariate model, minor severity criteria (≥ 3) were significantly associated with treatment failure (odds ratio, 2.838; 95% confidence interval, 1.216 to 6.626), and for predicting treatment failure the value of the area under the receiver operating characteristic curve for minor criteria was 0.731, similar to other established scoring methods. The IDSA/ATS minor severity criteria can predict delayed treatment response and clinical treatment failure.
Keywords: Minor Criteria, Pneumonia, Severe, Treatment Response
INTRODUCTION
Severe community-acquired pneumonia (SCAP) is associated with a high mortality rate of about 30%, and treatment failure significantly increases the risk of complications, length of hospital stay, and death (1-3). In 2007, the Infectious Disease Society of America/American Thoracic Society (IDSA/ATS) adopted a new definition of SCAP, with the inclusion of a new set of minor criteria based on data of individual risks (4). Although it is clear that patients satisfying one of two major criteria usually present with severe illness and need intensive care unit (ICU) treatment, the clinical usefulness of minor criteria is not well known. Liapikou et al. (5) concluded that in the absence of major criteria, the value of the IDSA/ATS minor criteria is uncertain.
To date, there have been several studies on the utility of minor criteria for predicting hospital outcomes. Phua et al. (6) demonstrated that IDSA/ATS minor criteria were more effective for predicting hospital mortality than other established scores, and Chalmers et al. (7) showed that the minor criteria were equally predictive as other methods for adverse events, such as the need for mechanical ventilation/vasopressors, ICU admission, and 30-day mortality. However, it is still unclear which clinical characteristics are associated with patients that satisfy only the IDSA/ATS minor criteria. We hypothesized that CAP patients without mechanical ventilation or vasopressors, regardless of their hospital site of care (i.e., general ward or ICU), would present with different clinical aspects, depending on whether or not they satisfy the IDSA/ATS minor criteria.
Therefore, in the present study, we investigated the clinical characteristics of SCAP patients who satisfied the IDSA/ATS minor criteria (i.e., minor severe CAP [minor-SCAP]), focusing on their treatment response to empirical antibiotics.
MATERIALS AND METHODS
Study population
Anonymous data from electronic medical records were reviewed. We initially enrolled all adult patients (age ≥ 18 yr) admitted for CAP to Hallym University Sacred Heart Hospital between March 2007 and February 2009. CAP was defined as new pulmonary infiltrates on chest radiographs and symptoms and signs of lower respiratory tract infection (5). Exclusion criteria included immunosuppression (HIV infection, chemotherapy within the previous 6 months, hematologic malignancy), mycobacterial infection, discharge against medical advice, and transfer from or to other hospitals (8). We also excluded patients satisfying the IDSA/ATS major criteria; the major criteria were met when patients received mechanical ventilation or vasopressors.
Definitions
According to the 2007 IDSA/ATS guidelines, cases that met at least three of the nine minor severity criteria (i.e., respiratory rate ≥ 30 breaths/min, ratio of partial pressure of arterial oxygen to the fraction of inspired oxygen [PaO2/FiO2] ≤ 250, multilobar infiltrates, confusion and/or disorientation, blood urea nitrogen [BUN] ≥ 20 mg/dL, white blood cells [WBC] < 4,000 cells/µL, platelets < 100,000 cells/µL, core temperature < 36℃, and systolic blood pressure [BP] < 90 mmHg requiring aggressive fluid resuscitation) at hospital admission were defined as minor-SCAP. Pneumonia patients who did not meet the minor criteria were defined as the control group.
Clinical stability was defined as in Siegel (9); that is, when all of the following parameters were met: body temperature ≤ 37.2℃, heart rate ≤ 100 beats/min, respiratory rate ≤ 24/min, and saturation level of oxygen in hemoglobin (SaO2) ≥ 90% or PaO2 ≥ 60 mmHg (with no exogenous oxygen supply). Treatment failure was defined as clinical deterioration, as in Menendez et al. (10), when at least one of the following existed: hemodynamic instability with the need for aggressive fluid resuscitation (i.e., > 40 mL/kg colloid or crystalloids) and vasopressors or invasive procedures, respiratory failure (SaO2 < 90% or PaO2 < 60 mmHg with FiO2 = 0.21) or need for mechanical ventilation, or radiologic progression or new infection. Complications were classified as cardiac, renal, or respiratory failure (need of noninvasive ventilation or invasive mechanical ventilation) or shock.
Data collection and analysis
We collected data on demographics, co-morbid illnesses, clinical symptoms, laboratory findings, and severity of illness scores, such as CURB-65 (confusion, BUN ≥ 20 mg/dL, respiratory rate ≥ 30/min, systolic BP < 90 mmHg or diastolic BP ≤ 60 mmHg, and age ≥ 65) and pneumonia severity index (PSI) scores at hospital admission of all enrolled patients (11, 12). We also investigated the time to reach clinical stability and the rates of new complication events, clinical failure of empirical antibiotic treatment, and in-hospital mortality. We sought to identify independent factors for treatment failure by multivariate analysis and evaluated the performance of the IDSA/ATS minor criteria for predicting treatment failure, compared to CURB-65 and PSI scores, in CAP patients without mechanical ventilation or vasopressors.
Statistical analysis
To compare the results between groups, a one-way analysis of variance (ANOVA), Kruskal-Wallis test, or Student's t-test was used for continuous data, while the chi-square or Fisher's exact test was used for categorical data. Multivariate logistic regression analysis was performed using covariates associated with treatment failure (P < 0.05) in univariate analyses to examine the association between the minor criteria and treatment failure. A backward stepwise analysis (based on the likelihood ratio), with values of 0.05 to enter and 0.10 to stay in the model, was used. Receiver operating characteristic (ROC) curves for the IDSA/ATS minor criteria, PSI, and CURB-65 scores were also performed and compared. Finally, a Kaplan-Meier survival curve with a log rank test was performed to estimate and compare the time to reach clinical stability among the minor-SCAP and control groups. All reported P values were two-sided and P < 0.05 was considered statistically significant. Statistical analyses were conducted using SAS statistical software, EG version (SAS Institute Inc., Cary, NC, USA).
Ethics statement
The study protocol was approved by the institutional review board (IRB) of Hallym University Sacred Heart Hospital (IRB No. 2012 I016). Informed consent was waived by the IRB because of the retrospective nature of this study.
RESULTS
Patient characteristics
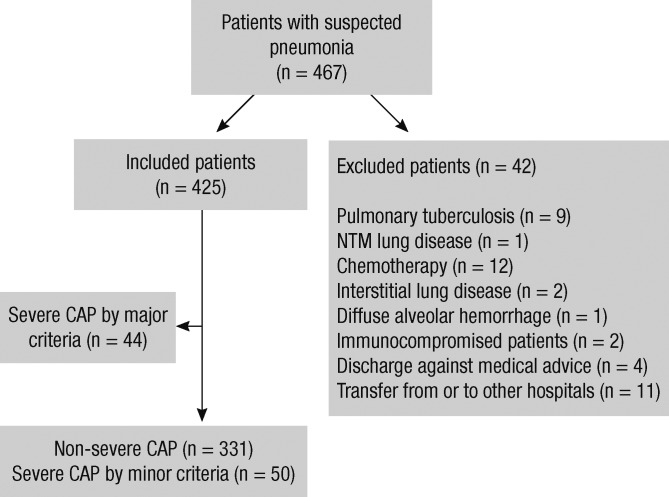
Of the 467 total patients diagnosed as having CAP, 425 were initially eligible for enrollment into our study. Among them, 44 patients with SCAP based on major criteria were excluded, and 381 patients who did not receive mechanical ventilation or vasopressors were enrolled (Fig. 1). The mean patient age was 61.2 ± 19.3 yr, and 55.1% of patients were male. Of the 381 patients, 13.1% (n = 50) satisfied the minor criteria of SCAP.
Fig. 1.
Flowchart of the patient population. CAP, community-acquired pneumonia; ICU, intensive care unit; NTM, nontuberculous mycobacteria.
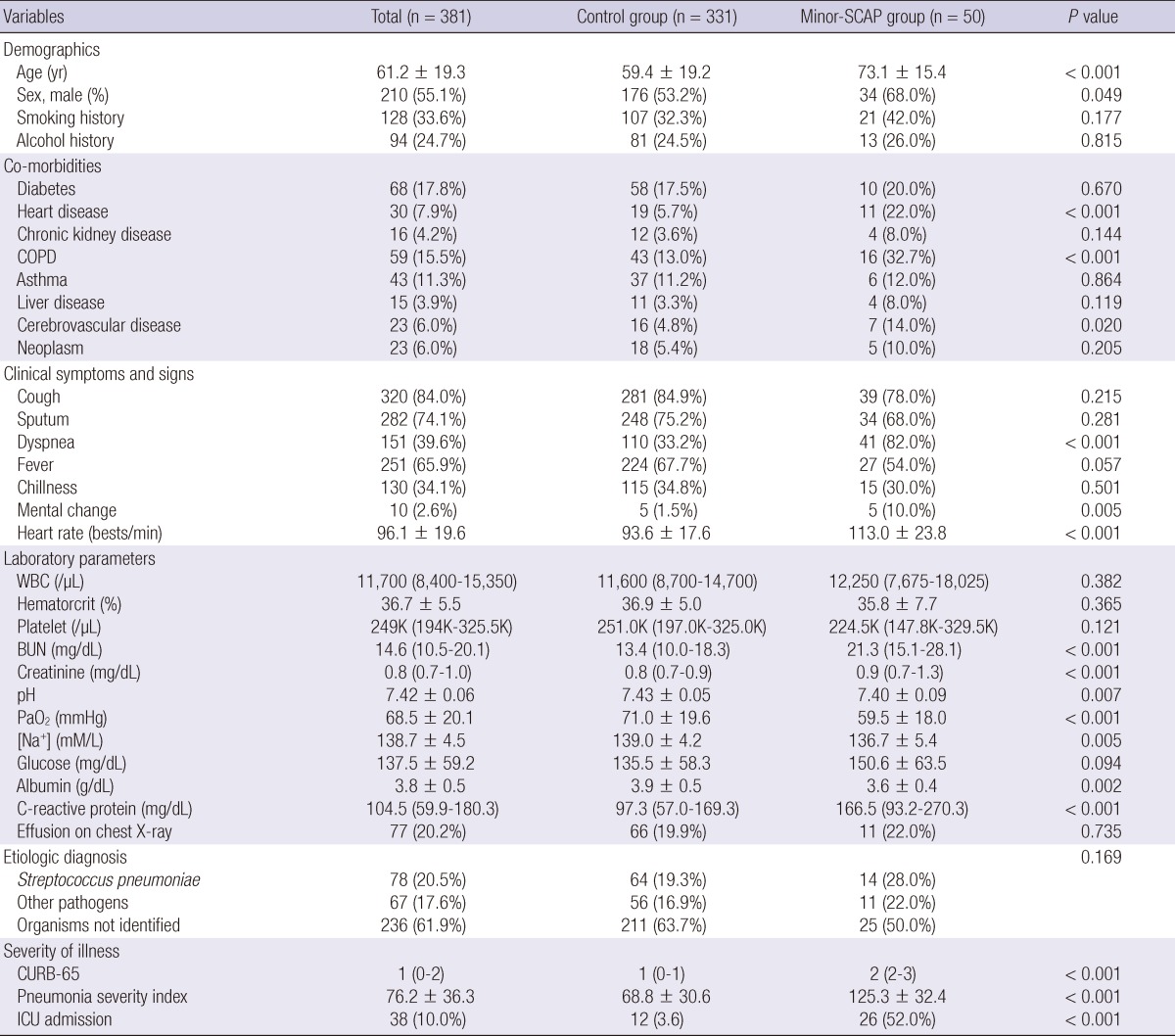
Diabetes and chronic obstructive pulmonary disease were the most common co-morbid illnesses (Table 1). Among the causal organisms, Streptococcus pneumoniae was the most frequent (20.5%, 78/381), and other organisms were identified in 67 patients (Mycoplasma pneumoniae, 37; Pseudomonas aeruginosa, 11; Klebsiella pneumoniae, 6; Staphylococcus aureus, 3; Moraxella catarrhalis, 1; Legionella spp., 1; Hemophilus influenzae, 2; Streptococcus viridians, 2; other organisms, 4). In terms of antibiotic treatment, adherence to the IDSA/ATS guidelines was achieved in 370 (97.1%) patients, and 38 (10.0%) were admitted to the ICU. Disease severity scores (CURB-65 and PSI scores) for all enrolled patients are included in Table 2.
Table 1.
Comparisons of clinical characteristics among the study population
BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; SCAP, severe community-acquired pneumonia; WBC, white blood cell; CURB-65 denotes confusion, blood urea nitrogen ≥ 20 mg/dL, respiratory rate ≥ 30/min, systolic blood pressure (BP) < 90 mmHg or diastolic BP ≤ 60 mmHg, and age ≥ 65 yr; Control group denotes patients with non-SCAP; Minor-SCAP denotes SCAP based on the IDSA/ATS minor criteria.
Table 2.
Pneumonia severity of the study population (n = 381)
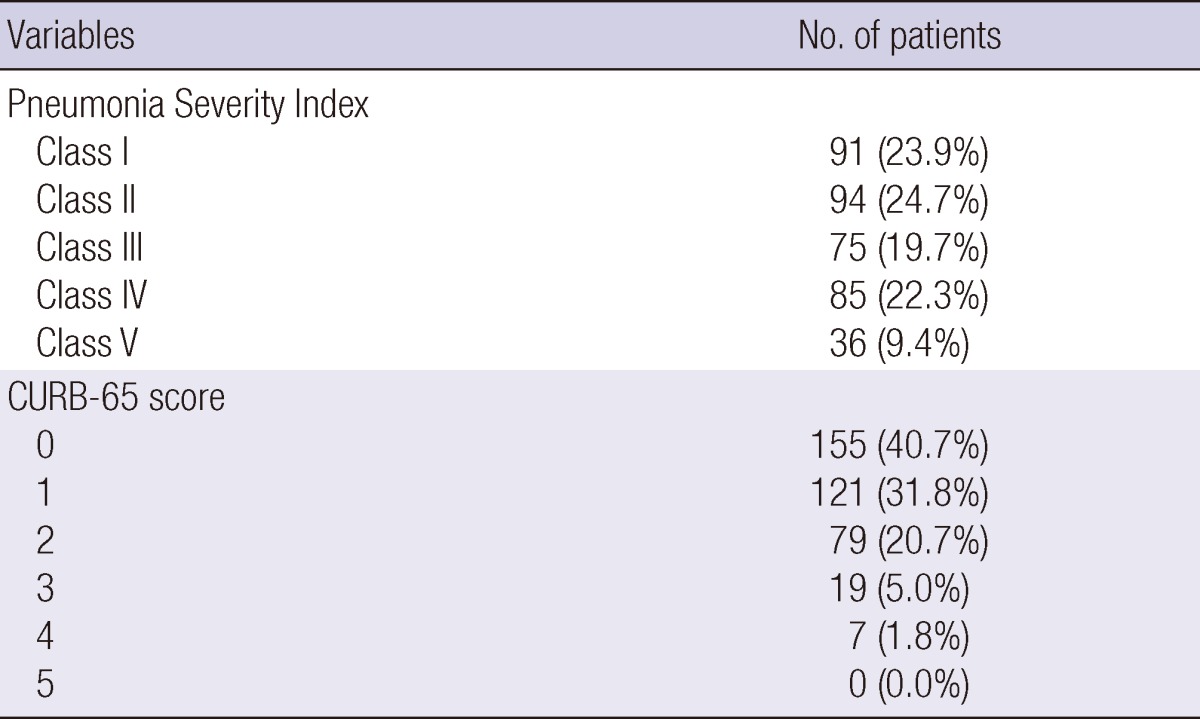
CURB-65 denotes confusion, blood urea nitrogen ≥ 20 mg/dL, respiratory rate ≥ 30/min, systolic blood pressure (BP) < 90 mmHg or diastolic BP ≤ 60 mmHg, and age ≥ 65 yr.
Clinical characteristics at admission
Differences in the clinical characteristics at hospital admission between the minor-SCAP and control groups are shown in Table 1. Compared to the control group, males were more prevalent, the patients were older, and underlying diseases (heart disease, chronic obstructive pulmonary disease, and cerebrovascular accidents) were more common in the minor-SCAP group. In addition to several clinical symptoms (i.e., dyspnea and mental changes), there were also significant differences in laboratory parameters, such as renal function, pH, oxygenation, serum sodium concentration ([Na+]), albumin, and C-reactive protein. With regard to etiologic organisms, there were no significant differences between the two groups; however, disease severity was greater and the ICU admission rate was higher in the minor-SCAP group.
Treatment response and hospital outcomes
Table 3 compares the frequency of new complication events and treatment outcomes between the two groups. New complication events during the hospital stay, especially renal and respiratory failures, were significantly more common in the minor-SCAP group than in the control group (P < 0.001). In addition, the time to clinical stability was longer (P < 0.001), and the rates of both clinical failure of empirical antibiotic treatment and in-hospital mortality were higher in the minor-SCAP group (P < 0.001 and P < 0.001, respectively).
Table 3.
Comparisons of the treatment outcomes
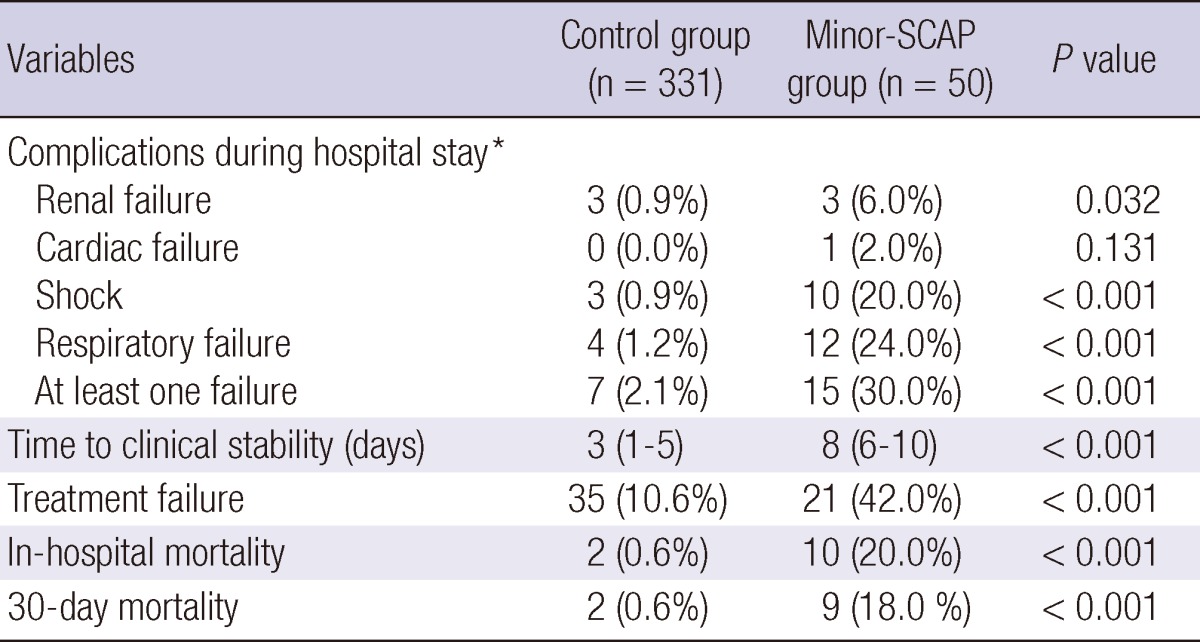
*Number of patients. ICU, intensive care unit; SCAP, severe community-acquired pneumonia; Control group denotes patients with non-SCAP; Minor-SCAP denotes SCAP based on the IDSA/ATS minor criteria.
Association between the IDSA/ATS minor criteria and treatment outcomes
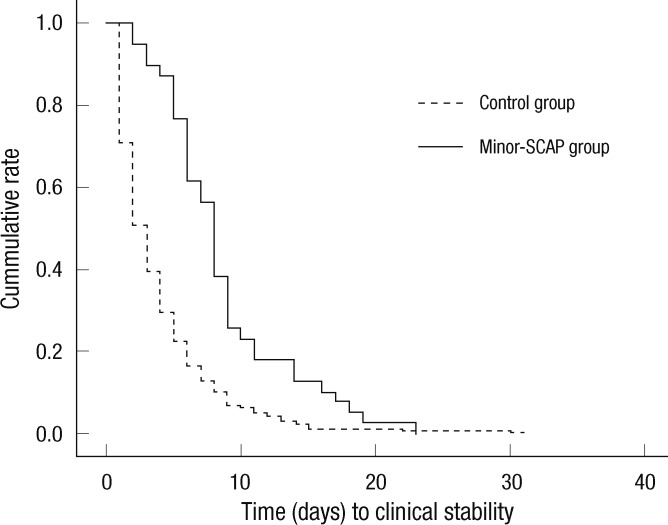
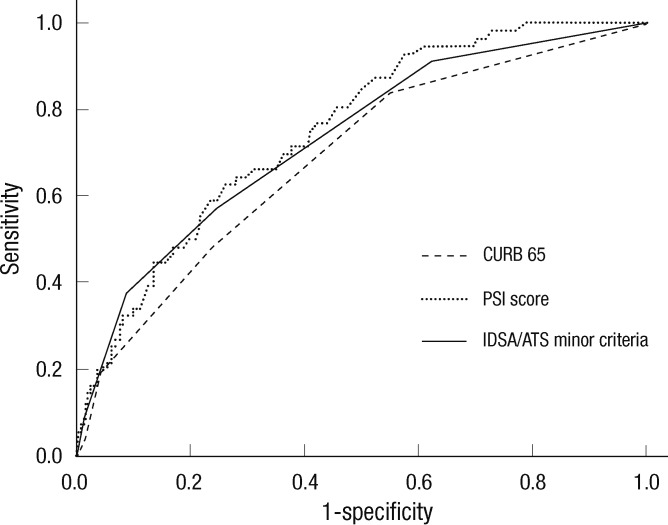
We analyzed the association of clinical parameters, including the IDSA/ATS minor criteria for SCAP, with the response to empirical antibiotic treatment in patients without mechanical ventilation or vasopressors (Table 4). In univariate analyses, 16 independent variables were significantly associated with treatment failure, including age, dyspnea, fever, mental change, cerebrovascular accident, pleural effusion, heart rate, WBC, BUN, [Na+] (i.e., hyponatremia), glucose, albumin, PaO2, IDSA/ATS minor criteria ≥ 3, and CURB-65 and PSI scores. Among these, pleural effusion, PSI score, and IDSA/ATS minor criteria ≥ 3 were significantly associated with treatment failure in the multivariate model (Hosmer-Lemeshow goodness-of-fit test, P = 0.111; Table 5); PaO2 was excluded from the multivariate model due to many missing data (n = 101). The odds ratio (OR) of the IDSA/ATS minor criteria ≥ 3 for treatment failure was 2.838 (95% confidence interval [CI], 1.216 to 6.626). The time to reach clinical stability was significantly longer in the minor-SCAP group than in the control group by Kaplan-Meier survival curve analysis (log rank = 28.6, P < 0.001; Fig. 2). The value of the area under the ROC curve for the IDSA/ATS minor criteria for the prediction of treatment failure was 0.731 (95% CI, 0.660 to 0.802), similar to the PSI (0.751; 95% CI, 0.688 to 0.814) and CURB-65 scores (0.688; 95% CI, 0.614 to 0.762; Fig. 3).
Table 4.
Univariate analyses of predictors for treatment failure to empirical antibiotics
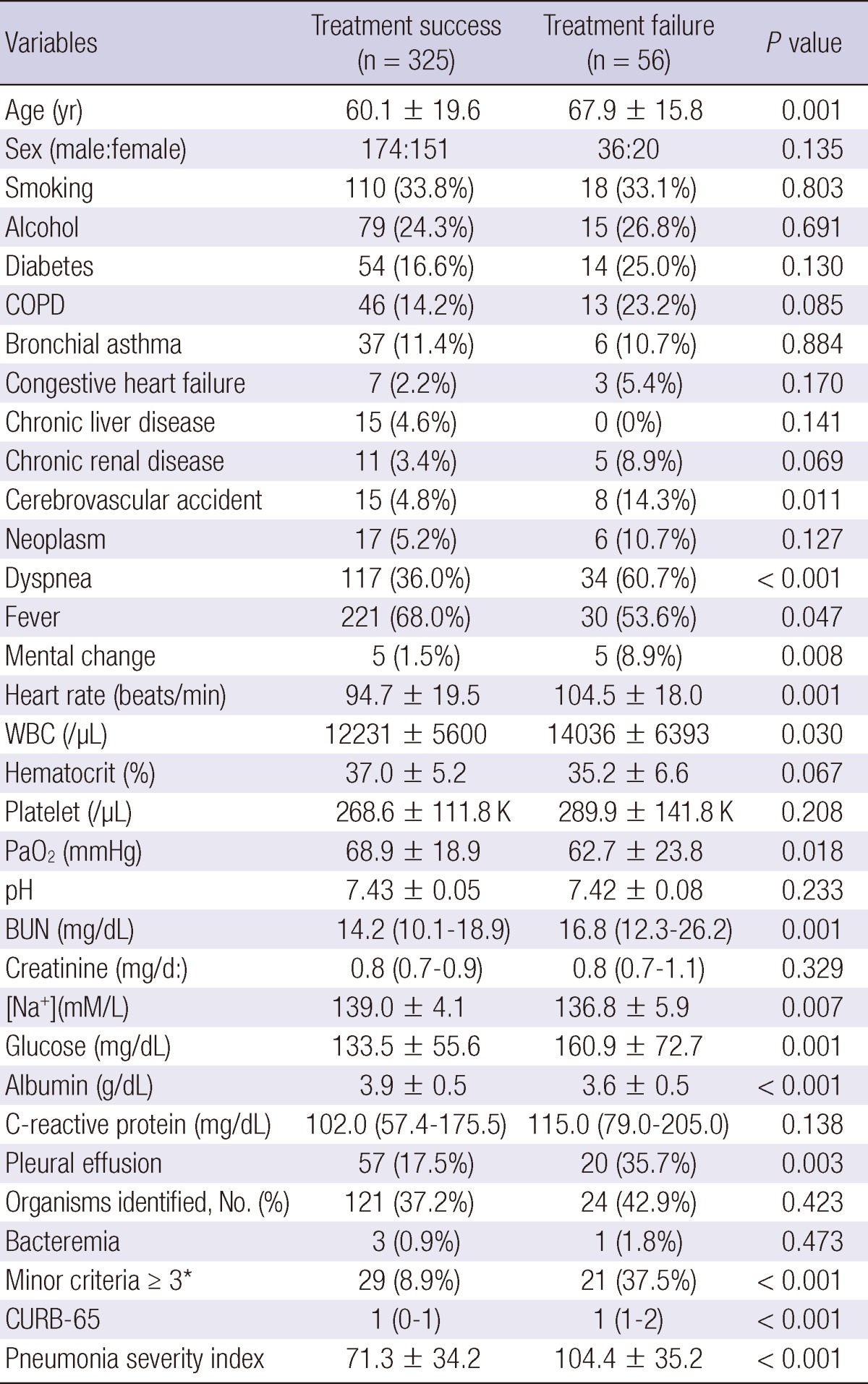
*Minor criteria for severe community-acquired pneumonia by IDSA/ATS. BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; WBC, white blood cell; CURB-65 denotes confusion, blood urea nitrogen ≥ 20 mg/dL, respiratory rate ≥ 30/min, systolic blood pressure (BP) < 90 mmHg or diastolic BP ≤ 60 mmHg, and age ≥ 65 yr.
Table 5.
Multivariate analysis of predictors for treatment failure
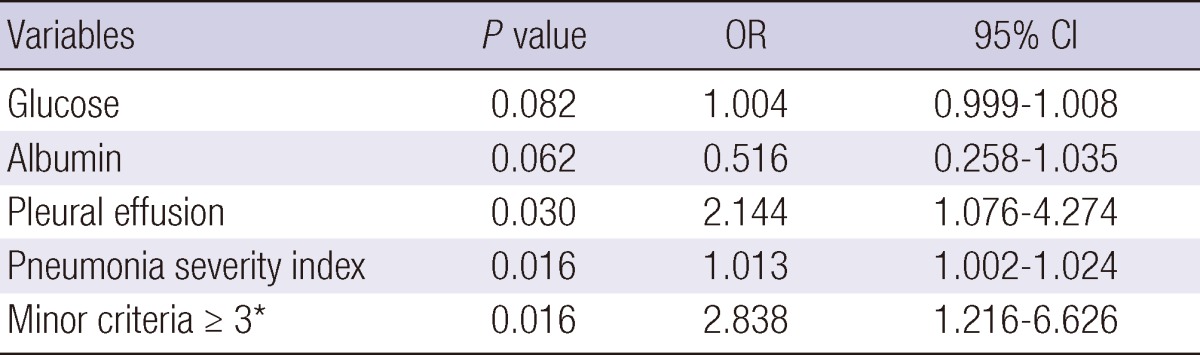
*Minor criteria for severe community-acquired pneumonia by IDSA/ATS. CI, confidence interval; OR, odds ratio; PSI, pneumonia severity index.
Fig. 2.
Kaplan-Meier survival curves for the time to reach clinical stability. The time to reach clinical stability was longer in the minor-SCAP than in the control (i.e., non-SCAP) groups (log rank = 28.6, P < 0.001).
Fig. 3.
ROC curves for prediction of clinical treatment failure. The AUC values for the minor criteria, CURB-65 and PSI scores were 0.731 (95% CI, 0.660-0.802), 0.688 (95% CI, 0.614-0.762), and 0.751 (95% CI, 0.688-0.814), respectively.
DISCUSSION
We found that among hospitalized CAP patients who did not require mechanical ventilation or vasopressors, those who satisfied the minor IDSA/ATS criteria for SCAP had different clinical characteristics, both at hospital admission and during treatment, compared with those who did not satisfy these criteria. Patients who met the minor criteria were more likely to have higher rates of new complications and clinical treatment failure, and their time to clinical stability was much longer. In addition, IDSA/ATS minor criteria were independent factors for treatment failure, and their predictive value was not inferior to that of PSI or CURB-65 scores.
The IDSA/ATS minor criteria for SCAP include variables that are also included in the CURB-65 and ATS minor criteria (4), and the usefulness of the IDSA/ATS minor criteria has been investigated by several authors. Liapikou et al. (5) and Phua et al. (6) investigated the association with the IDSA/ATS minor criteria and hospital mortality and then focused on its prediction rule for ICU admission. However, in the present study, we investigated the clinical response to empirical antibiotics and found that the IDSA/ATS minor criteria were associated with delayed treatment response and a higher clinical failure rate, which are the most striking features of our study.
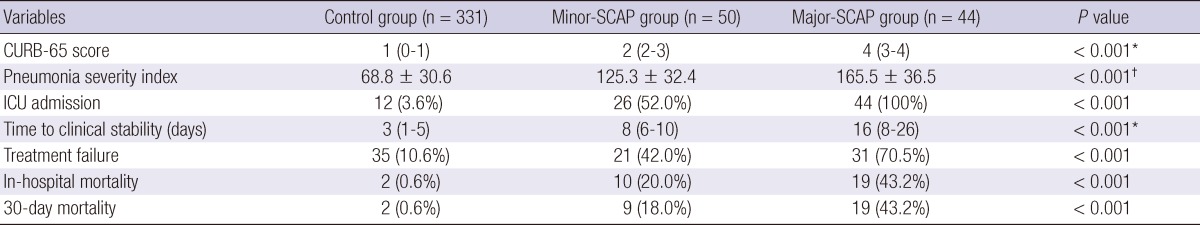
In univariate analyses (Table 3), the rates of new complications, treatment failure, and in-hospital mortality were significantly higher in the minor-SCAP group than in the control group. Therefore, our results indicate that among CAP patients without mechanical ventilation or vasopressors, those who meet the IDSA/ATS minor criteria are at high risk of poor outcomes. As shown in Table 6, the severity of illness scores and the outcome parameters of the minor-SCAP group fell between those of the control group and major-SCAP patients that had been excluded based on major criteria. Thus, we can say that the minor-SCAP group in the present study had intermediate disease severity among all CAP patients. In addition, although it is reasonable to assume that more severe pneumonia is associated with a higher incidence of treatment failure, few studies have demonstrated the relationship between severity scores and treatment response. Menendez and Torres (13) reported that the median time to clinical stability in CAP patients was 4 days, and PSI score, treatment failure, and ICU admission were all associated with an increased time to reach clinical stability. In the present study, the median time to reach clinical stability was 3.0 days (interquartile range [IQR], 1-6 days) for all enrolled (n = 381) patients, and it was significantly longer in the minor-SCAP group than in the control group (8 days [6-10 days] vs 3 days [1-5 days]).
Table 6.
Comparisons of the disease severity and hospital outcomes (n = 425)
*Kruskal-Wallis and †one-way ANOVA were performed to compare values among the three groups. ICU, intensive care unit; SCAP, severe community-acquired pneumonia; CURB-65 denotes confusion, blood urea nitrogen ≥ 20 mg/dL, respiratory rate ≥ 30/min, systolic blood pressure (BP) < 90 mmHg or diastolic BP ≤ 60 mmHg, and age ≥ 65; Control group denotes patients with non-SCAP; Minor-SCAP denotes SCAP based on the IDSA/ATS minor criteria; Major-SCAP denotes SCAP based on the IDSA/ATS major criteria.
The in-hospital mortality rate of the enrolled patients was just 3.1%. Therefore, we used empirical antibiotic treatment failure as the outcome variable in multivariate analysis. In these analyses, pleural effusion, PSI score, and minor criteria ≥ 3 were significantly associated with treatment failure. In particular, patients satisfying ≥ 3 minor criteria for SCAP had a greater than two-fold risk of treatment failure. CURB-65 scores were not significantly associated with treatment failure in the multivariate model. This may be due to its incorporation of few variables and/or the fact that it does not include some important parameters, such as hypoxemia (4, 14, 15). Hypoxemia, which is included in the IDSA/ATS minor criteria, is considered very important to the hospitalization decision for CAP patients, and the implementation of oxygenation assessment immediately improves the prognosis of CAP patients (16). In 2006, Espana et al. (14) and Yandiola et al. (17) developed another prediction rule for SCAP (PS-CURXO80), and the hypoxemia variable was included as one of their six minor criteria. Recently, Guo et al. (18) reported that among the individual minor criteria, PaO2/FiO2 ≤ 250 was correlated with the length of hospital stay, sequential organ failure assessment (SOFA) score, and treatment costs.
As shown in Table 2, patients with PSI class IV and V and CURB-65 scores ≥ 3 comprised only 31.8% and 6.8% of all patients in the present study, respectively. Therefore, the patients enrolled in our study mostly represented the low-to-moderate risk group. Although the PSI score was designed to identify and assess low-risk pneumonia patients, the OR of the IDSA/ATS minor criteria ≥ 3 for treatment failure in the present study was higher than that of the PSI score. Furthermore, the PSI score is fairly complex and time-consuming to use. Therefore, based on the results of our study, the IDSA/ATS minor criteria for SCAP could be more useful for disease severity assessment and the prediction of clinical response of hospitalized CAP patients who do not require mechanical ventilation or vasopressors, compared with established scoring systems. However, we did not evaluate the predictive value of using ≥ 4 minor criteria, as did Brown et al. (19), due to the limited number of patients; only 10 (2.6%) patients with ≥ 4 minor criteria were included in our study. Therefore, care should be taken when interpreting our results.
With regard to the analysis of ROC curves for predicting treatment failure, minor criteria were not inferior to PSI or CURB65 scores in this study. Brown et al. (19) also reported that using four minor criteria may be superior to the PSI or CURB-65 scores for predicting severe pneumonia. They used the receipt of intensive therapy in the ICU as the reference value rather than the inhospital mortality rate. In the present study, we did not use ICU treatment as the reference because it may be highly dependent on local guidelines and individual physicians' practices.
Our study has several limitations. First, this was a single-center study, and the number of patients was limited. Second, due to the retrospective design of our study, there might be unidentified selection bias. Finally, as mentioned above, because the mortality rate of all enrolled patients was low, we could not assess the relationship between the minor criteria and in-hospital mortality. However, because few studies have focused on the clinical aspects of the IDSA/ATS minor criteria for SCAP in hospitalized patients without mechanical ventilation or vasopressors, our results are meaningful and can add to the previously reported body of work. We believe that patients who satisfy the IDSA/ATS minor criteria have different clinical characteristics among those without mechanical ventilation or vasopressors, and that these minor criteria will be very helpful for physicians to identify patients at high-risk for delayed treatment response and empirical antibiotic treatment failure.
In conclusion, satisfying IDSA/ATS minor criteria for SCAP (i.e., ≥ 3 minor criteria) can predict delayed time to reach clinical stability and high rates of clinical failure of empirical antibiotics in hospitalized patients who do not require mechanical ventilation or vasopressors.
ACKNOWLEDGMENT
We thank Dr. Young-Soo Ju, Department of Occupational and Environmental Medicine, Hallym University Sacred Heart Hospital for statistical contribution to this study.
References
- 1.Hoogewerf M, Oosterheert JJ, Hak E, Hoepelman IM, Bonten MJ. Prognostic factors for early clinical failure in patients with severe community-acquired pneumonia. Clin Microbiol Infect. 2006;12:1097–1104. doi: 10.1111/j.1469-0691.2006.01535.x. [DOI] [PubMed] [Google Scholar]
- 2.Menendez R, Torres A, Zalacain R, Aspa J, Martin Villasclaras JJ, Borderias L, Benitez Moya JM, Ruiz-Manzano J, Rodriguez de Castro F, Blanquer J, et al. Risk factors of treatment failure in community acquired pneumonia: implications for disease outcome. Thorax. 2004;59:960–965. doi: 10.1136/thx.2003.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Restrepo MI, Mortensen EM, Velez JA, Frei C, Anzueto A. A comparative study of community-acquired pneumonia patients admitted to the ward and the ICU. Chest. 2008;133:610–617. doi: 10.1378/chest.07-1456. [DOI] [PubMed] [Google Scholar]
- 4.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liapikou A, Ferrer M, Polverino E, Balasso V, Esperatti M, Piner R, Mensa J, Luque N, Ewig S, Menendez R, et al. Severe community-acquired pneumonia: validation of the Infectious Diseases Society of America/American Thoracic Society guidelines to predict an intensive care unit admission. Clin Infect Dis. 2009;48:377–385. doi: 10.1086/596307. [DOI] [PubMed] [Google Scholar]
- 6.Phua J, See KC, Chan YH, Widjaja LS, Aung NW, Ngerng WJ, Lim TK. Validation and clinical implications of the IDSA/ATS minor criteria for severe community-acquired pneumonia. Thorax. 2009;64:598–603. doi: 10.1136/thx.2009.113795. [DOI] [PubMed] [Google Scholar]
- 7.Chalmers JD, Taylor JK, Mandal P, Choudhury G, Singanayagam A, Akram AR, Hill AT. Validation of the Infectious Diseases Society of America/American Thoratic Society minor criteria for intensive care unit admission in community-acquired pneumonia patients without major criteria or contraindications to intensive care unit care. Clin Infect Dis. 2011;53:503–511. doi: 10.1093/cid/cir463. [DOI] [PubMed] [Google Scholar]
- 8.Hilbert G, Gruson D, Vargas F, Valentino R, Gbikpi-Benissan G, Dupon M, Reiffers J, Cardinaud JP. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344:481–487. doi: 10.1056/NEJM200102153440703. [DOI] [PubMed] [Google Scholar]
- 9.Siegel RE. Time to clinical stability for patients with community-acquired pneumonia. JAMA. 1999;281:231–232. doi: 10.1001/jama.281.3.231. [DOI] [PubMed] [Google Scholar]
- 10.Menendez R, Torres A, Rodriguez de Castro F, Zalacain R, Aspa J, Martin Villasclaras JJ, Borderias L, BenitezMoya JM, Ruiz-Manzano J, Blanquer J, et al. Reaching stability in community-acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis. 2004;39:1783–1790. doi: 10.1086/426028. [DOI] [PubMed] [Google Scholar]
- 11.Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, Lewis SA, Macfarlane JT. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, Coley CM, Marrie TJ, Kapoor WN. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 13.Menendez R, Torres A. Treatment failure in community-acquired pneumonia. Chest. 2007;132:1348–1355. doi: 10.1378/chest.06-1995. [DOI] [PubMed] [Google Scholar]
- 14.Espana PP, Capelastegui A, Gorordo I, Esteban C, Oribe M, Ortega M, Bilbao A, Quintana JM. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med. 2006;174:1249–1256. doi: 10.1164/rccm.200602-177OC. [DOI] [PubMed] [Google Scholar]
- 15.Levin KP, Hanusa BH, Rotondi A, Singer DE, Coley CM, Marrie TJ, Kapoor WN, Fine MJ. Arterial blood gas and pulse oximetry in initial management of patients with community-acquired pneumonia. J Gen Intern Med. 2001;16:590–598. doi: 10.1046/j.1525-1497.2001.016009590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blot SI, Rodriguez A, Sole-Violan J, Blanquer J, Almirall J, Rello J. Effects of delayed oxygenation assessment on time to antibiotic delivery and mortality in patients with severe community-acquired pneumonia. Crit Care Med. 2007;35:2509–2514. doi: 10.1097/01.CCM.0000287587.43801.9C. [DOI] [PubMed] [Google Scholar]
- 17.Yandiola PP, Capelastegui A, Quintana J, Diez R, Gorordo I, Bilbao A, Zalacain R, Menendez R, Torres A. Prospective comparison of severity scores for predicting clinically relevant outcomes for patients hospitalized with community-acquired pneumonia. Chest. 2009;135:1572–1579. doi: 10.1378/chest.08-2179. [DOI] [PubMed] [Google Scholar]
- 18.Guo Q, Li HY, Zhou YP, Li M, Chen XK, Liu H, Peng HL, Yu HQ, Chen X, Liu N, et al. Weight of the IDSA/ATS minor criteria for severe community-acquired pneumonia. Respir Med. 2011;105:1543–1549. doi: 10.1016/j.rmed.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Brown SM, Jones BE, Jephson AR, Dean NC. Validation of the Infectious Disease Society of America/American Thoracic Society 2007 guidelines for severe community-acquired pneumonia. Crit Care Med. 2009;37:3010–3016. doi: 10.1097/CCM.0b013e3181b030d9. [DOI] [PMC free article] [PubMed] [Google Scholar]