Abstract
A nationwide survey was conducted to determine the incidence of bronchopulmonary dysplasia (BPD) in Korea and the intercenter differences in survival and BPD rates among preterm infants. Questionnaires were sent to all registered neonatal intensive care units (NICUs). The questionnaires inquired about the survival and BPD rates of very low birth weight (VLBW, < 1,500 g) infants who had been admitted to each NICU from 2007 to 2008. BPD was defined as requiring oxygen at 36 weeks' postmenstrual age. Almost all level III NICUs replied. During the study period, 3,841 VLBW infants were born in the NICUs that responded to the survey. The survival rate was 81% and the BPD rate was 18%. Combined outcome of BPD or death rate was 37%. The BPD rate and combined outcome of BPD or death rate varied considerably from 5% to 50% and 11% to 73%, respectively across the centers. There was no significant correlation between the survival rate and the BPD rate across the centers. In conclusion, the incidence of BPD among VLBW infants in Korea during the study period was 18%, and a considerable intercenter difference in BPD rates was noted.
Keywords: Bronchopulmonary Dysplasia; Epidemiology; Infant, Very Low Birth Weight
INTRODUCTION
Recently, the quality of neonatal care in Korea has improved remarkably. The survival rate of very low birth weight (VLBW, < 1,500 g) infants has increased from 78% in 2002 to 86% in 2009. The survival rate of extremely low birth weight (ELBW, < 1,000 g) infants has also increased from 56% in 2002 to 72% in 2009 (1).
The improved survival of VLBW infants has presumably led to the increased occurrence of bronchopulmonary dysplasia (BPD). The incidence of BPD differs considerably from center to center and from country to country (2-4). Until recently, the incidence of BPD in Korea has been reported only by single-center, single area, or small multicenter studies (5-7). When BPD was defined as the need for oxygen at 36 weeks' postmenstrual age (PMA), these studies reported BPD incidences from 10% to 39%. These BPD incidences from small-scale studies do not represent the actual overall incidence of BPD in Korea. The need for a nationwide survey to determine the overall incidence of BPD in Korea has therefore been raised for planning national health policies for perinatal and neonatal health care services. Information about the nationwide BPD incidence in Korea is also necessary for academic exchanges with other international neonatology researchers and practitioners. For these purposes, the Statistical Research Committee of the Korean Society of Neonatology performed a nationwide survey of the incidence of BPD. The survey's objectives were to determine 1) the incidence of BPD in Korea, 2) the intercenter differences in survival rates and BPD rates among preterm infants, and 3) the clinical characteristics of BPD, based on disease severity.
MATERIALS AND METHODS
The survey questionnaires were sent to all 77 Korean hospitals with a registered neonatal intensive care unit (NICU). The surveys were mailed in April 2009 and collected three months later. The study subjects were VLBW infants with a birth weight of less than 1,500 g and preterm infants born before 32 weeks' gestational age (GA) who were admitted to each NICU during the two-year period between January 2007 and December 2008. In cases for which a preterm infant was transferred from his/her birth hospital to another hospital (e.g., for surgery) and two questionnaires were returned for a single preterm infant, the data from the two questionnaires were combined into a single entry. Only patient data collected during the initial admission was requested. We did not collect information about subsequent admissions.
The questionnaires consisted of two parts. The first part was a patient census for each NICU, and the second part was a demographic and clinical information sheet for each BPD patient. The patient census included total admissions, total deaths, total BPD occurrences, and total BPD deaths (total number of deaths among BPD patients) by birth weight and GA during the study period. The demographic and clinical information sheet included the hospital's name, the coded patient number, GA, as determined as the best obstetric estimate using ultrasound and/or the date of the mother's last menstrual period, birth weight, birth hospital, sex, multiplicity, the cause of preterm delivery, the presence of chorioamnionitis, antenatal steroids used, respiratory distress syndrome (RDS), the number of surfactant replacement treatments, patent ductus arteriosus (PDA), the use of prostaglandin synthase inhibitors, the outcome of PDA, the duration of mechanical ventilation, the nasal continuous positive airway pressure (NCPAP) and oxygen supplementation, BPD disease severity (mild/moderate/severe), pulmonary hypertension, other comorbidities, medications administered for BPD, the patient's outcome, and length of hospital stay. The data were entered into a computer using a spreadsheet program and subsequently analyzed.
BPD was defined and graded using the National Institute of Child Health and Human Development (NICHD) consensus definition (8). Mild BPD was defined as the need for supplemental oxygen at or beyond 28 days but not at 36 weeks' PMA; moderate BPD was defined as the need for supplemental oxygen at 28 days, in addition to supplemental oxygen at FiO2 at or below 0.30 at 36 weeks' PMA; and severe BPD was defined as the need for supplemental oxygen at 28 days and, at 36 weeks' PMA, the need for mechanical ventilation and/or FiO2 above 0.30. RDS was defined clinically; only patients who received surfactant were considered to have RDS. During the study period, most cases of surfactant replacement therapy were therapeutic. Prophylactic use of surfactant was minimally practiced in the country during that period. PDA was diagnosed with an echocardiogram; the questionnaire asked whether the PDA was hemodynamically significant. Pulmonary hypertension was diagnosed clinically and/or echocardiographically.
Statistical analyses were performed using PASW Statistics 17.0. The trends of the individual demographic and clinical characteristics with increasing severity of BPD were tested using the Jonckheere-Terpstra test for continuous variables, and categorical variables were tested using linear by linear association. Statistical significance was defined as P < 0.05.
Ethics statement
The collection of these data was approved by the Institutional Review Boards of the individual centers that required ethical approval for retrospective medical record review (B-1107-132-105). In all of the participating centers, informed parental consent was waived due to retrospective nature of this study.
RESULTS
Patient census
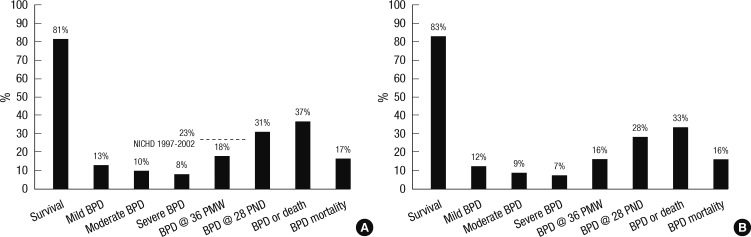
Fifty-two of 77 centers responded to the questionnaires. Based on the number of NICU beds, the response rate was 70% (935 of 1,332 beds). Nonreplies to the survey questionnaires were mostly from level I or II centers. Nearly all the level III centers replied to the questionnaires. During the study period, 3,841 VLBW infants were born and admitted to the NICUs that replied. Among these infants, 81% (3,117 infants) survived. The incidence of BPD, defined as needing oxygen at 36 weeks' PMA in VLBW infants was 18% (685 of 3,841 infants). Combined outcome of BPD or death rate among VLBW infants was 37%. Among the 4,312 preterm infants born before 32 weeks' GA, BPD occurred in 16% (692 infants of 4,312 infants). Combined outcome of BPD or death rate among preterm infants born before 32 weeks' GA was 33%. Among the 685 VLBW infants with BPD, 114 infants (17%) died; among the 692 preterm infants born before 32 weeks' GA, 112 infants (16%) died (Fig. 1).
Fig. 1.
The survival rates, overall incidences of bronchopulmonary dysplasia (BPD) of varying diagnostic criteria, combined outcome of BPD or death rates, and mortality rates from BPD. (A) For very low birth weight infants born with a birth weight less than 1,500 g. (B) For preterm infants born before 32 weeks' gestational age. The severity of BPD was defined according to the National Institute of Child Health and Human Development (NICHD) consensus definition (8). Survival, survival rate; BPD, bronchopulmonary dysplasia; PMW, postmenstrual weeks; PND, postnatal days; BPD or death, combined outcome of BPD or death; NICHD 1997-2002, the incidence of BPD in the National Institute of Child Health and Human Development (NICHD) Neonatal Network 1997-2002 data.
The incidence of BPD by birth weight and gestational age
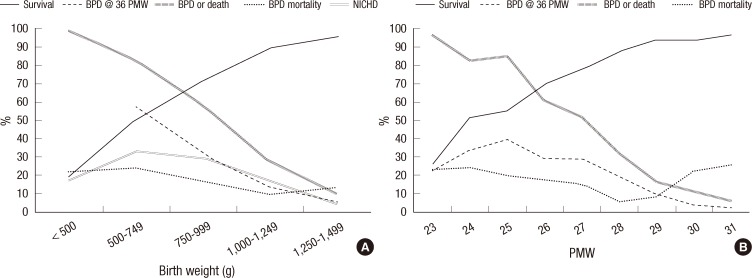
The incidence of BPD was 18% at less than 500 g, 33% at 500 to 749 g, 30% at 750 to 999 g, 14% at 1,000 to 1,249 g, and 6% at 1,250 to 1,499 g. The survival rates at each birth weight range were 22%, 51%, 71%, 89%, and 95%, respectively. Combined outcome of BPD or death rate was 98% at less than 500 g, 82% at 500 to 749 g, 58% at 750 to 999 g, 27% at 1,000 to 1,249 g, and 10% at 1,250 to 1,499 g. The incidence of BPD in NICHD Neonatal Network centers between 1997 and 2002 was higher than ours in the 500 to 749 g, 750 to 999 g, and 1,000 to 1,249 g weight ranges. At 1,250 to 1,499 g, our BPD incidence was higher (Fig. 2A). Based on GA, the BPD incidence was 23% at 23 postmenstrual weeks (PMW), 34% at 24 PMW, 39% at 25 PMW, 30% at 26 PMW, 29% at 27 PMW, 19% at 28 PMW, 10% at 29 PMW, 4% at 30 PMW, and 3% at 31 PMW. The survival rates for each gestational age were 26%, 51%, 55%, 69%, 77%, 87%, 93%, 93%, and 96%, respectively. Combined outcome of BPD or death rate was 96% at 23 PMW, 82% at 24 PMW, 85% at 25 PMW, 61% at 26 PMW, 51% at 27 PMW, 32% at 28 PMW, 17% at 29 PMW, 11% at 30 PMW, and 6% at 31 PMW (Fig. 2B).
Fig. 2.
Birth weight and gestational age-specific survival rates, bronchopulmonary dysplasia (BPD) rates, combined outcome of BPD or death rates, and mortality rates from BPD. (A) For very low birth weight infants with a birth weight less than 1,500 g. (B) For preterm infants born before 32 weeks' gestational age. The severity of BPD was defined according to the National Institute of Child Health and Human Development (NICHD) consensus definition (8). Survival, survival rate; BPD, bronchopulmonary dysplasia; PMW, postmenstrual weeks; BPD or Death, combined outcome of BPD or death rate; NICHD 1997-2002, the incidence of BPD in the National Institute of Child Health and Human Development (NICHD) Neonatal Network 1997-2002 data.
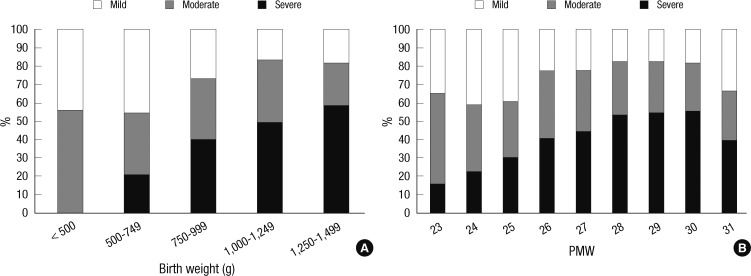
The proportion of the severity of BPD varied with increasing birth weight and GA. Generally as birth weight and GA increased, severe and moderate BPD decreased while mild BPD increased. Notably, severe BPD began to increase again in infants in the 1,000 to 1,249 g birth weight range or at 28 PMW (Fig. 3).
Fig. 3.
The proportions of the severity of bronchopulmonary dysplasia (BPD) according to birth weight and gestational age. (A) For very low birth weight infants with a birth weight less than 1,500 g. (B) For preterm infants born before 32 weeks' gestational age. The severity of BPD was defined according to the National Institute of Child Health and Human Development (NICHD) consensus definition (8).
Overall, the incidence of BPD was 18% in VLBW infants and 16% in preterm infants born before 32 weeks' GA. The NICHD Neonatal Network data reported a 23% BPD rate in VLBW infants, which is higher than ours. The mortality rate of the preterm infants with BPD was 17% for VLBW infants and 16% for preterm infants born before 32 weeks' GA. Combined outcome of BPD or death rate was 37% for VLBW infants and 33% for preterm infants born before 32 weeks' GA (Fig. 1).
Intercenter differences in survival rate, BPD rate, and BPD mortality
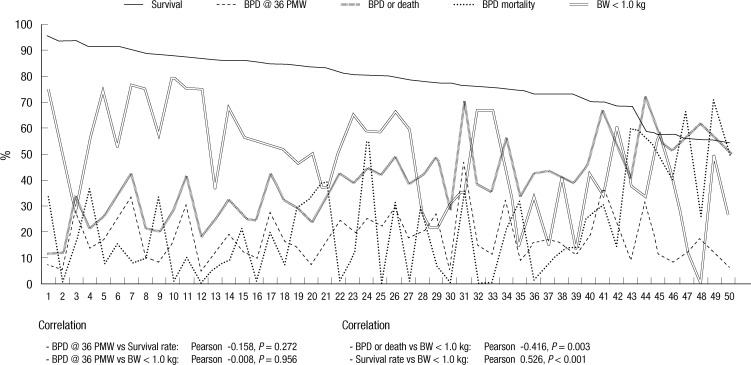
The survival rate, BPD rate, combined outcome of BPD or death rate and BPD mortality of the VLBW infant subjects differed greatly between the centers. In particular, the BPD rate varied from 5% to 50% and combined outcome of BPD or death rate varied from 11% to 73%. The proportions of ELBW infants who are at greatest risk for BPD in VLBW infants were also widely different from 0% to 80%. There was no significant correlation between the BPD rate and survival rate or the proportion of ELBW infants. Combined outcome of BPD or death rate was inversely correlated with the proportion of ELBW infants and survival rate was directly correlated with the proportion of ELBW infants (Fig. 4).
Fig. 4.
The intercenter differences in the survival rates, severe bronchopulmonary dysplasia (BPD) rates, BPD rates at 36 weeks' postmenstrual age, combined outcome of BPD or death rates, the mortality rates from BPD, and the proportions of extremely low birth weight (ELBW, < 1.0 kg) infants in very low birth weight (VLBW, < 1.5 kg) infants. Each number on longitudinal axis represents individual centers which are arranged in order of survival rate. The BPD rates at 36 weeks' postmenstrual age were not significantly correlated with the BPD rates or the proportions of ELBW infants. The proportions of ELBW infants were inversely correlated with combined outcome of BPD or death rates and directly correlated with survival rates. The severity of BPD was defined according to the National Institute of Child Health and Human Development (NICHD) consensus definition (8). Survival, survival rate; BPD, bronchopulmonary dysplasia; PMW, postmenstrual weeks; BPD or Death, combined outcome of BPD or death rate; BW < 1.0 kg, the proportion of extremely low birth weight (< 1.0 kg) infants in very low birth weight (< 1.5 kg) infants; Pearson, Pearson coefficient.
Demographic and clinical characteristics based on BPD severity
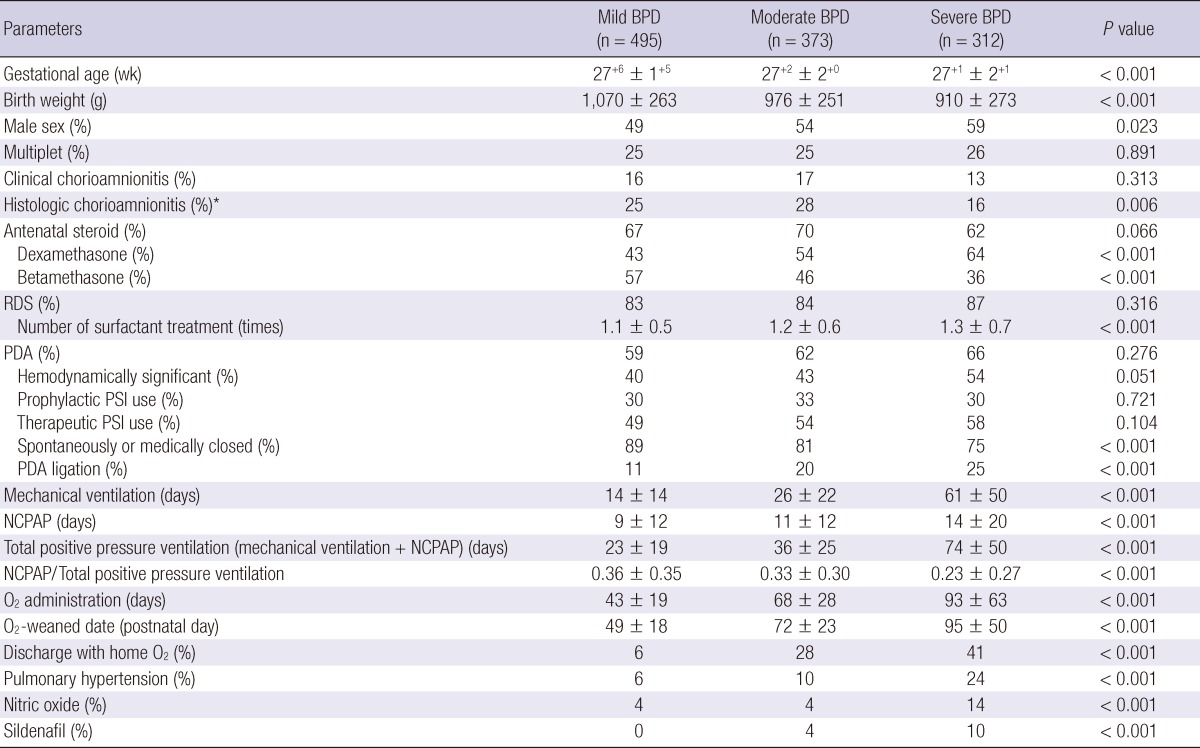
When the GA was younger and the birth weight was lower, BPD was significantly more severe. The proportion of males among the BPD patients increased as the BPD severity increased. In cases for which pathological examinations of the placenta were performed, the incidence of histologic chorioamnionitis was lower in severe BPD cases compared to mild and moderate BPD cases. The incidence of clinical chorioamnionitis did not differ according to BPD severity, nor did the frequency of antenatal steroid use. However, when antenatal steroids were used, dexamethasone was used more frequently than betamethasone as BPD severity increased. The incidence of RDS did not differ according to BPD severity. However, more surfactant was used as the BPD severity increased. The incidence of PDA, whether hemodynamically significant or not, and the use of prostaglandin synthase inhibitors, whether prophylactic or therapeutic, did not differ according to BPD severity. However, surgical ligation of PDA was more common as the BPD severity increased. As expected, the durations of mechanical ventilation, NCPAP, total positive pressure (mechanical ventilation plus NCPAP), and oxygen administration were longer as the BPD severity increased. The proportion of NCPAP in total positive pressure decreased as the BPD severity increased. The date of oxygen withdrawal was later and the need for home oxygen was greater as BPD severity increased. Pulmonary hypertension was more frequently diagnosed with the parallel increase of inhaled nitric oxide use as BPD severity increased (Table 1).
Table 1.
Demographic and clinical characteristics according to the severity of bronchopulmonary dysplasia
P is for score test for trend (Jonckheere-Terpstra test for continuous variables and linear by linear association for categorical variables). The severity of bronchopulmonary dysplasia was defined according to the NICHD consensus definition (8). *Available in 78% of all subject very low birth weight infants. BPD, bronchopulmonary dysplasia; RDS, respiratory distress syndrome; PDA, patent ductus arteriosus; PSI, prostaglandin synthase inhibitor; NCPAP, nasal continuous positive airway pressure; O2, oxygen.
Comorbidities, therapies for BPD, and outcomes based on BPD severity
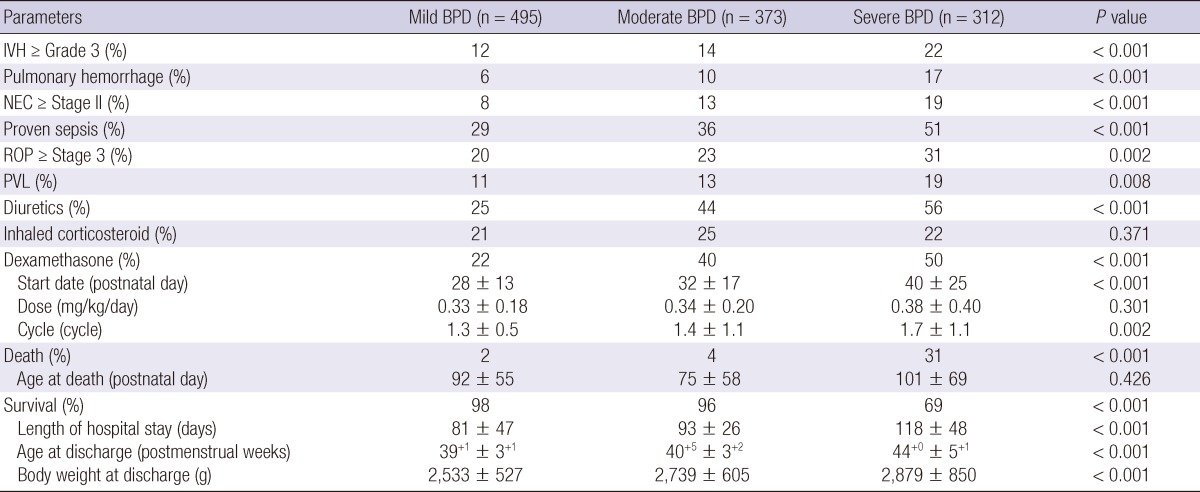
Regarding comorbidities, intraventricular hemorrhage (≥ Grade 3), pulmonary hemorrhage, necrotizing enterocolitis (≥ Stage II), culture-proven sepsis, retinopathy of prematurity (≥ Stage 3), and periventricular leukomalacia all occurred more frequently as the BPD severity increased. As expected, diuretics and dexamethasone were used more frequently as the BPD severity increased. Dexamethasone therapy was started later as the BPD severity increased. The dexamethasone dose did not differ according to the BPD severity, but dexamethasone therapy was used more often as the BPD severity increased. The use of inhaled corticosteroid did not differ according to BPD severity. As expected, the mortality rate increased as the BPD severity increased. Nearly one-third of VLBW infants with severe BPD died. However, the age at death did not differ according to BPD severity. For survivors, the hospital stay was longer as the BPD severity increased. The age and body weight at discharge were also greater as the BPD severity increased (Table 2).
Table 2.
Clinical outcomes and therapies employed according to the severity of bronchopulmonary dysplasia
P is for score test for trend (Jonckheere-Terpstra test for continuous variables and linear by linear association for categorical variables). The severity of bronchopulmonary dysplasia was defined according to the NICHD consensus definition (8). BPD, bronchopulmonary dysplasia; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; ROP, retinopathy of prematurity; PVL, periventricular leukomalacia.
DISCUSSION
The present study is the first nationwide study to investigate the incidence of BPD in Korea. Almost all of the level III centers in Korea participated in this study, and the NICHD consensus definition of BPD was employed. This study revealed an 18% incidence of BPD at 36 weeks' PMA in VLBW infants and a 16% incidence of BPD in preterm infants born at less than 32 weeks' gestation. The incidence of BPD at 36 weeks' PMA in VLBW infants in NICHD Neonatal Network sites in the United States between 1997 and 2002 was 23% (11). The Vermont Oxford Network (VON), another major neonatal network based in the United States, reported a 29% incidence of BPD in 2003 (12). A recent European population-based cohort study enrolling 4,185 preterm infants born at less than 32 weeks' gestation in 2003 in 10 European regions reported a 17% incidence of BPD at 36 weeks' PMA (13). For Asian countries, recent literature on the nationwide incidence of BPD is not available from a PubMed search. The difference in the BPD incidence among countries may be attributable to different population characteristics, different care practices, and different survival rates. In particular, the difference in survival rates may have a great influence on the incidence of BPD because BPD develops in surviving extremely preterm infants (14). Data from the NICHD Neonatal Network suggests a close relationship between the BPD incidence and the survival of ELBW infants (15). The incidence of BPD in our study was lower than that of the NICHD Neonatal Network (18% vs 23%, respectively; Fig. 1A) (11). The gap between our BPD incidence and that of the NICHD Neonatal Network was noticed in extremely preterm infants with a birth weight below 750 g. In preterm infants with a birth weight within or above the range of 750 g to 999 g, there was no discernible difference in the BPD incidence between our data and NICHD Neonatal Network's (Fig. 2A). The low incidence of BPD in our data in compared with that of the NICHD Neonatal Network may result primarily from the low survival rate of extremely preterm infants (those infants with birth weights below 750 g). However, the survival rate of these very immature infants born below a birth weight of 750 g does not differ greatly between our study and NICHD Neonatal Network's (51% vs 53%, respectively) (11). Therefore, other factors including different population characteristics and different care practices are more likely to have contributed to the difference in BPD rates. It is noteworthy that the BPD rates at less than 500 g and at 23 weeks' GA were irrelevantly low (18% and 23%, respectively). These low BPD rates are attributable to extremely high mortality at these birth weight range and GA. Combined outcome of BPD or death rates which incorporate mortality in these birth weight range and GA were 98% and 96%, respectively as expected.
In the present study, the BPD rate at 36 weeks' PMA among centers varied from 5% to 50%. This great difference in the BPD rate among centers is not likely to be attributable to different patient population among centers, in that the BPD rate in each center was not correlated with the proportion of ELBW infants who are at greatest risk for BPD in VLBW infants in each center. Intercenter variability in the incidence of BPD is observed internationally. In a multicenter study in which eight major academic medical centers in the United States participated, the incidence of BPD in preterm infants with birth weights of 700 to 1,500 g ranged from 6% to 33% (16). Data from the VON in the United States also revealed wide variance in the BPD incidence among VLBW infants: their data show an incidence from 4% to 58% in 2003, even after a quality improvement initiative (12). The European data referred to above also demonstrated intercenter variation (from 11% to 22%) in the BPD incidence in preterm infants born at less than 32 weeks' GA (13). Thus, intercenter variability in the BPD rate is a universal finding. Intercenter variability in the BPD rate may be attributable to population characteristics, different care practices, or different survival rates among centers. Because racial differences are minimal in Korea compared with the United States, population characteristics may not have contributed greatly to the intercenter differences in our study. Different survival rates among centers can introduce a selection bias to the BPD rate. However, there was no significant correlation between the survival rates and BPD rates in our study. Therefore, the most reasonable explanation for the intercenter variability in the BPD rate in our study is different care practices among centers. Potentially better care practices to reduce the occurrence of BPD have not yet been discovered. Our next step should be identifying care practices that are associated with lower BPD rates. Applying such care practices could reduce the BPD rate in the underperforming centers. However, in VON sites in the United States, the intercenter differences in the BPD rate have not abolished, even after a quality improvement initiative was implemented (12). This finding demonstrates the complicated nature of identifying potentially beneficial practices and applying such practices to reduce the BPD rate in individual centers.
Another important factor that may have influenced the intercenter differences in the BPD rate is the diagnostic criteria used for BPD. In the present study, the need for oxygen at 36 weeks' PMA was used as the diagnostic criteria for BPD. However, oxygen requirements can be subject to the caregiver's practice style. It is a general practice to target oxygen saturation to 92% to 94% as the preterm infants approaches discharge from the NICU to prevent pulmonary hypertension from chronic hypoxia. Furthermore, unnecessary oxygen supplementation is often given to more than a few preterm infants who genuinely do not need oxygen to keep their oxygen saturation at 92% to 94%. This practice can cause the BPD rate to appear greater than it really is in each center. Walsh et al. (17) proposed a physiological test for oxygen requirements at 36 weeks' PMA to resolve the problem of diagnostic inaccuracy in the NICHD consensus definition of BPD. After the authors applied a physiological definition of BPD, they found an approximately 30% reduction in the BPD rate and the intercenter variability in BPD rate (18). During our study period, some centers performed a physiological test to determine the need for oxygen requirement at 36 weeks' PMA before a BPD diagnosis was made, but others did not. This inconsistent method for determining the need for oxygen at 36 weeks' PMA may have also influenced the intercenter differences observed in our study.
It is worth noting that the proportion of ELBW infants was inversely correlated with combined outcome of BPD or death and directly correlated with survival rate. This result indicates that the centers which accommodate more extremely immature infants have better neonatal intensive care performance. As expected, the incidence of BPD and BPD mortality decreased as GA increased from 23 weeks' PMA to 28 weeks' PMA; thereafter, however, the BPD mortality began to increase again until 31 weeks' PMA. Similarly, the proportion of severe BPD decreased as the GA increased to 29 weeks' PMA; afterward, however, the proportion of severe BPD increased again. Because BPD is relatively rare at these late GAs, when it does occur, it may be associated with a different genetic predisposition, etiology or pathophysiology; thus, its clinical course could be unusually difficult and eventually result in worse outcomes. This unusual type of BPD occurring in relatively mature preterm infants warrants further study.
The strengths of our study are that it included 52 NICUs, which represents nearly all the level III centers in Korea and that uniform diagnostic criteria for BPD was used. Nonreplies to the survey questionnaire (30%) were mostly from primary or secondary level centers, where neonatal services are provided mainly for more mature preterm infants who are not likely to develop BPD.
The limitations of our study include its retrospective nature and lack of information on the clinical practices of the individual NICUs. The data were collected retrospectively, and this study was noninterventional. The oxygen saturation target may have differed from center to center, but the differences may not be significant. Determining the need for oxygen was subject to the individual practice patterns of the caregivers in each NICU because physiological tests for oxygen dependency were not uniformly performed. In addition, the differences in other clinical practices among centers, such as delivery room care, indications for mechanical ventilation, surfactant replacement therapy, the use of noninvasive ventilation, and fluid management, were not explored in this study. The differences in these various clinical practices, which are presumably not small, may have exerted a significant influence on the BPD rate in each NICU.
In summary, the incidence of BPD in VLBW infants in Korea between January 2007 and December 2008 was 18%, and considerable intercenter variability in the BPD rate was found. Further studies are warranted to determine whether this intercenter variability in the BPD rate can be explained by different care practices.
Footnotes
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0021644). We thank the representatives of participating centers that contributed data for this article. The names of the participating centers and their representatives are listed at http://www.neonatology.or.kr
References
- 1.Hahn WH, Chang JY, Chang YS, Shim KS, Bae CW. Recent trends in neonatal mortality in very low birth weight Korean infants: in comparison with Japan and the USA. J Korean Med Sci. 2011;26:467–473. doi: 10.3346/jkms.2011.26.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vohr BR, Wright LL, Dusick AM, Perritt R, Poole WK, Tyson JE, Steichen JJ, Bauer CR, Wilson-Costello DE, Mayes LC Neonatal Research Network. Center differences and outcomes of extremely low birth weight infants. Pediatrics. 2004;113:781–789. doi: 10.1542/peds.113.4.781. [DOI] [PubMed] [Google Scholar]
- 3.Cotten CM, Oh W, McDonald S, Carlo W, Fanaroff AA, Duara S, Stoll B, Laptook A, Poole K, Wright LL, Goldberg RN NICHD Neonatal Research Network. Prolonged hospital stay for extremely premature infants: risk factors, center differences, and the impact of mortality on selecting a best-performing center. J Perinatol. 2005;25:650–655. doi: 10.1038/sj.jp.7211369. [DOI] [PubMed] [Google Scholar]
- 4.Payne NR, Lacorte M, Sun S, Karna P, Lewis-Hunstiger M, Goldsmith JP. Evaluation and development of potentially better practices to reduce bronchopulmonary dysplasia in very low birth weight infants. Pediatrics. 2006;118:S65–S72. doi: 10.1542/peds.2006-0913B. [DOI] [PubMed] [Google Scholar]
- 5.Lim IS, Choi CW, Kim BI, Kim DH, Sim SY, Kim EK, Kim HS, Choi JH. Clinical usefulness of the new definition of bronchopulmonary dysplasia. J Korean Soc Neonatol. 2006;13:9–16. [Google Scholar]
- 6.Na BM, Kim MJ, Kim WH. Recent outcomes of very low birth weight infants at Cheongju area. J Korean Soc Neonatol. 2006;13:128–138. [Google Scholar]
- 7.Sung KH, Kim MH, Kim ER, Shim JW, Lee JJ, Im JW, Jin HS. Epidemiology of bronchopulmonary dysplasia in Korea: multi-center study. Korean J Perinatol. 2009;20:225–233. [Google Scholar]
- 8.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 9.Wang SW, Lee YA, Park SE, Shin JB, Hong YR, Park JJ, Lee JA, Son SH, Byun SO, Kim JP. Changes in the outcomes of very low birth weight infants in Busan Area. J Korean Soc Neonatol. 2007;14:206–214. [Google Scholar]
- 10.Kim SH, Lee KH, Lee SH, You DK, Choi SJ, Hwang JH, Choi CW, Shim JW, Yoon HK, Yang SH, et al. The comparison of severity according to preceding causes of bronchopulmonary dysplasia in very low birth weight infants. J Korean Soc Neonatol. 2003;10:47–54. [Google Scholar]
- 11.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147.e1–147.e8. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Payne NR, LaCorte M, Karna P, Chen S, Finkelstein M, Goldsmith JP, Carpenter JH Breathsavers Group, Vermont Oxford Network Neonatal Intensive Care Quality Improvement Collaborative. Reduction of bronchopulmonary dysplasia after participation in the Breathsavers Group of the Vermont Oxford Network Neonatal Intensive Care Quality Improvement Collaborative. Pediatrics. 2006;118:S73–S77. doi: 10.1542/peds.2006-0913C. [DOI] [PubMed] [Google Scholar]
- 13.Zeitlin J, Draper ES, Kollée L, Milligan D, Boerch K, Agostino R, Gortner L, Van Reempts P, Chabernaud JL, Gadzinowski J, et al. Differences in rates and short-term outcome of live births before 32 weeks of gestation in Europe in 2003: results from the MOSAIC cohort. Pediatrics. 2008;121:e936–e944. doi: 10.1542/peds.2007-1620. [DOI] [PubMed] [Google Scholar]
- 14.de Kleine MJ, den Ouden AL, Kollée LA, Ilsen A, van Wassenaer AG, Brand R, Verloove-Vanhorick SP. Lower mortality but higher neonatal morbidity over a decade in very preterm infants. Paediatr Perinat Epidemiol. 2007;21:15–25. doi: 10.1111/j.1365-3016.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- 15.van Marter LJ. Epidemiology of bronchopulmonary dysplasia. In: Abman SH, editor. Bronchopulmonary dysplasia. New York: Informa Healthcare USA Inc; 2010. pp. 223–266. [Google Scholar]
- 16.Avery ME, Tooley WH, Keller JB, Hurd SS, Bryan MH, Cotton RB, Epstein MF, Fitzhardinge PM, Hansen CB, Hansen TN, et al. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics. 1987;79:26–30. [PubMed] [Google Scholar]
- 17.Walsh MC, Wilson-Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol. 2003;23:451–456. doi: 10.1038/sj.jp.7210963. [DOI] [PubMed] [Google Scholar]
- 18.Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, Everette R, Peters N, Miller N, Muran G, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114:1305–1311. doi: 10.1542/peds.2004-0204. [DOI] [PubMed] [Google Scholar]