Abstract
The clinical features of ring chromosome 6 include central nervous system anomalies, growth retardation, facial dysmorphism and other congenital anomalies. Ring chromosome 6 occurs rarely and manifests as various phenotypes. We report the case of mosaic ring chromosome 6 by conventional karyotyping in a 7-day-old male infant diagnosed with a large patent ductus arteriosus (PDA) with hypoplasia of aortic valve and aortic arch. These have not been previously reported with ring chromosome 6. He recovered from heart failure symptoms after ligation of the PDA. He showed infantile failure to thrive and delayed milestone in a follow-up evaluation. To the best of our knowledge, this is the first report of a Korean individual with ring chromosome 6 and hemodynamically significant PDA.
Keywords: Chromosome 6 Ring Syndrome; Developmental Delay Disorders; Hypoplasia of Aortic Arch; Hypoplasia of Aortic Valve; Ductus Arteriosus, Patent
INTRODUCTION
Ring chromosome 6 is a very rare chromosome abnormality that typically arises de novo (1). Only 25 ring chromosome cases had been reported up to 2005 (2). Ring chromosome 6 manifests as various phenotypes, ranging from normal to severe abnormalities such as mental and developmental retardation, facial dysmorphism, cardiac anomalies, seizure, limb anomalies, and hip joint malformations (1-3). To date, there have been few reports of ring chromosome 6 related to congenital heart defect (1, 4-7). One of these studies described a dilated aortic root in an 1-yr-old patient with this chromosome abnormality (7).
We report in detail the case of mosaic ring chromosome 6 by conventional karyotyping in a 7-day-old male infant diagnosed with a large patent ductus arteriosus (PDA) and mild hypoplasia of aortic valve and aortic arch, which has not been previously reported with ring chromosome 6.
CASE DESCRIPTION
A 7-day-old male infant was transferred to our hospital with tachypnea and poor oral feeding on 16 February 2011. The patient was born at 40 weeks of gestation by vaginal delivery with a birth weight of 3,390 grams (50-75 percentile), body length of 48.6 cm (10-25 percentile), and head circumference of 32.5 cm (5-10 percentile). The patient was the first baby of a 31-yr-old male and a 28-yr-old female. Both parents were healthy and there was no familial history of chromosomal abnormalities.
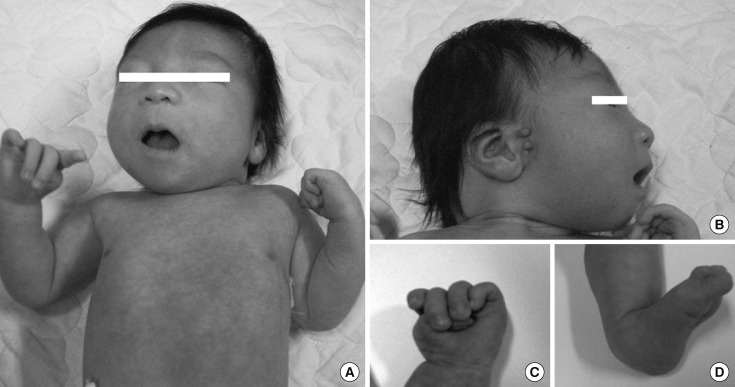
Physical examination of the newborn revealed microcephaly; dysmorphic facial features including micrognathia, microphthalmia, epicanthus, low-set and malformed ears; broad and flat nasal bridge; short neck and flat occiput; widely spaced nipples; thumb in palm hand deformity and both calcaneovalgus foot abnormality (Fig. 1A-C). Abnormal heart sound was detected and was revealed to be a continuous murmur and grade 1/6 systolic murmur at left sternal border.
Fig. 1.
Gross appearance of the patient. (A and B) Gross appearance showed for microcephaly, micrognathia, microphthalmia, epicanthus, short neck, low-set and malformed ears, broad and flat nasal bridge. (C) Thumb in palm deformity in hand. (D) Calcaneovalgus foot abnormality.
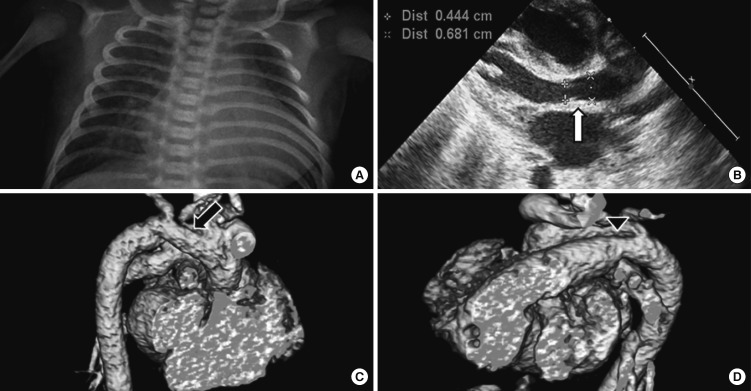
Routine laboratory data including complete blood count, liver function tests, serum electrolytes, glucose, blood urea nitrogen, and creatinine were within normal range. The metabolic screening tests were negative. The patient was examined by echocardiography and cardiac computed tomography for the evaluation of the continuous murmur. The examinations revealed large PDA with mild hypoplasia of aortic valve and aortic arch (Fig. 2). On 21 February 2011, the patient received ligation of the PDA due to congestive heart failure, which alleviated the heart failure symptoms. The patient did not present with neonatal seizure or neurologic symptoms, which have been noted in ring chromosome 6 cases.
Fig. 2.
Data from echocardiography and computed tomography examinations. (A) Cardiomegaly on chest X-ray. (B) Mild hypoplasia of aortic valve through the echocardiography (arrow). (C) Hypoplasia of arotic arch through the 3-dimensional cardiac computed tomography (arrow). (D) Large patent ductus arteriosus through the three-dimensional cardiac computed tomography (arrowhead).
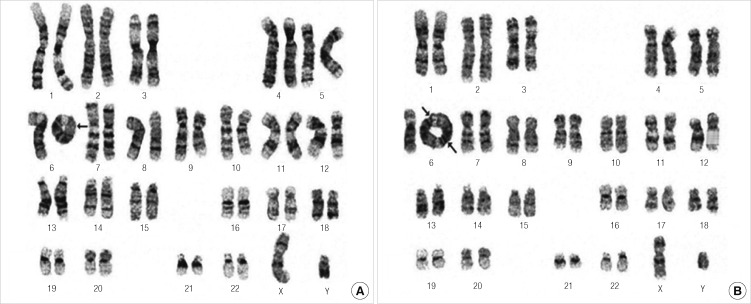
Because of the dysmorphic features, chromosome analysis was performed. Trypsin-giemsa banding of peripheral blood lymphocytes revealed a mosaic karyotype with abnormalities of chromosome 6 including a ring chromosome and a dicentric ring chromosome, 46, XY, r(6)(p25q27)/46, XY, dic r(6;6)(p25q27;p25q27). The total number of cells counted was 50. There were 46 monocentric 6 ring chromosomes and four dicentric 6 ring chromosomes (Fig. 3).
Fig. 3.
Karyotypes of the patient. (A) 46, XY, r(6)(p25q27), (B) 46, XY, dic (6;6)(p25q27;p25q27).
In the out-patient department examination at 3-months-of-age, the patient displayed a significant growth deficiency with vomiting, body weight of 4.0 kg (< 3 percentile = 5.6 kg), body length of 55.3 cm (< 3 percentile = 58.6 cm), and head circumference of 34.7 cm (< 3 percentile = 38.2 cm). The patient was checked for malformed lesions by an esophagography and abdominal ultrasonogram. No stenotic lesion and passage disturbance were evident, and there was no definite abnormal finding in the esophagus and abdomino-pelvic cavity. Concerning both foot and hand abnormalities, the patient received stretching exercise therapy comprised of plantaflexion and thumb abduction in the department of orthopedics of our institution.
We recommended the parents that the patient should receive an ophthalmologic examination and brain imaging study. Permission was not granted. A brainstem evoked response audiometry test revealed mild right hearing loss; our institution's ear-nose-and-throat department recommended a hearing aid.
At 9 months of age, the body weight of the patient was 5.5 kg (< 3 percentile = 7.5 kg), body length of 65.6 cm (< 3 percentile = 68.3 cm), and head circumference of 38.0 cm (< 3 percentile = 42.1 cm). The patient had global developmental delay including motor skills, personal-social interaction, and cognitive-adaptive development. A repeated echocardiogram revealed a closed ductus arteriosus with continued mild hypoplasia of the aortic valve and aortic arch.
DISCUSSION
The clinical presentation of patients with ring chromosome 6 abnormalities can be varied, ranging from growth delay and normal intellect (8, 9) to congenital anomalies and moderate intellectual impairment (4, 10). This is regarded as being dependent on the structure and size of the ring chromosome as well as the level of mosaicism. Correlations of the phenotype and karyotype have not proven reliable in predicting clinical presentation, and there does not seem to be a single distinguishing phenotype (5, 8). Also, even with identical breakpoints, different phenotypical outcomes have been noted (6).
The phenotypic variability associated with ring chromosome 6 may be influenced by a combination of factors including ring instability in maintaining its shape and structure, and the mechanism responsible for ring formation (11, 12). But, longitudinal studies of ring chromosome stability have not been carried out. Therefore, the long-term results of ring chromosome instability on clinical manifestation are not known (11, 12). Also, a loss of genetic material may be responsible for the clinical presentation of some cases, although not all ring chromosomes have been related to a detectable loss of genetic material (13).
Like other common findings of ring chromosome 6, our case showed clinical features similar to previously reported cases, such as microcephaly, postnatal growth retardation and developmental delay, dysmorphic facial features, short neck, and both hand and foot abnormalities. However, brain imaging was not done, so we could not rule out hydrocephalus, absent olfactory bulbs, corpus callosum. Also, since an ophthalmologic examination was also denied, we also could not rule out iris atrophy or anirida, which have been noted in ring chromosome 6 cases. Since intellectual functioning is extremely variable (4), longitudinal studies and follow-up evaluation of the present ring chromosome case needs to be performed. In addition, although ring chromosomes are mostly de novo (1), chromosomal studies of the phenotypically normal parents would have been crucially helpful. However, permission was not granted.
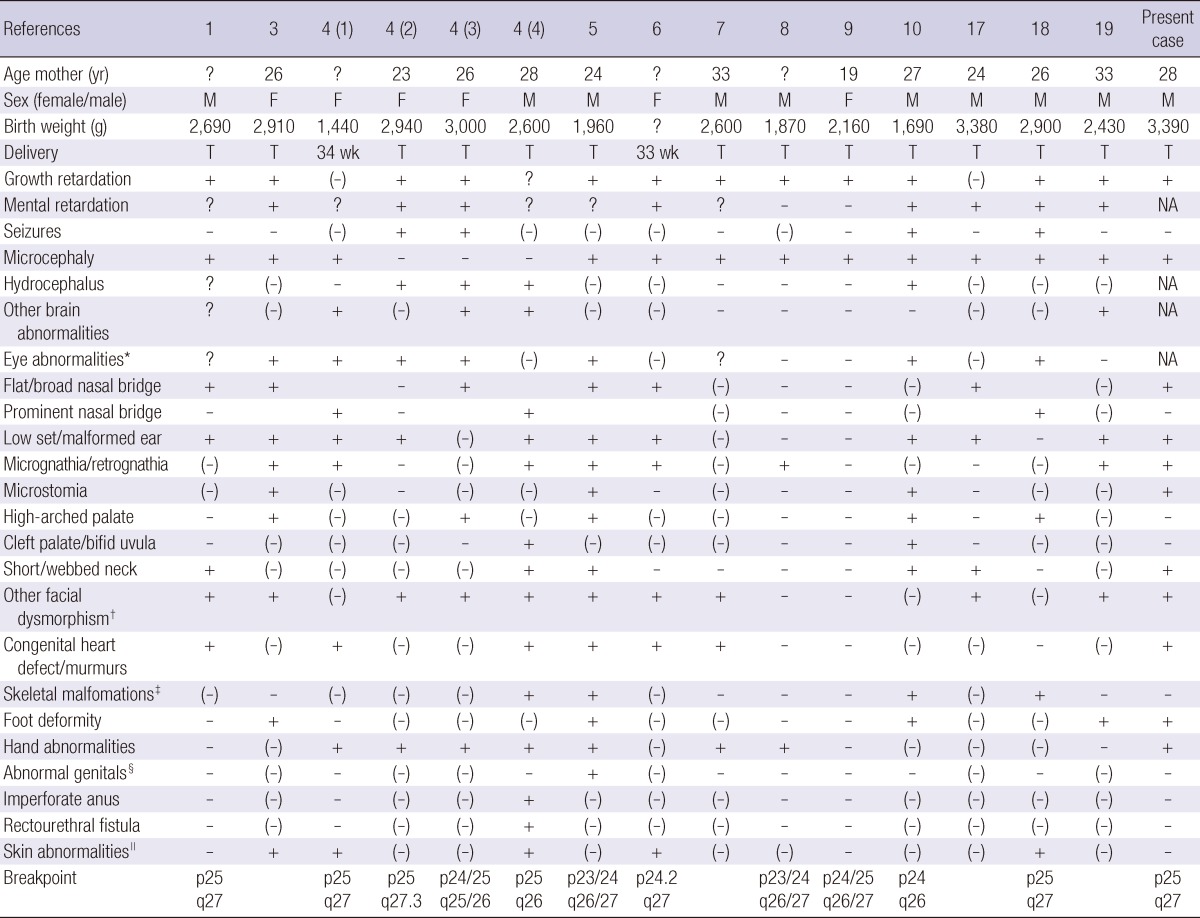
As ring chromosome 6 has a very low prevalence, little long-term developmental and medical follow-up information is available in the literature, especially concerning congenital heart defects. Different cases of ring chromosome 6 were reviewed and compared with the present case (Table 1). To date, no child with a ring chromosome 6 has been reported to have a hemodynamic significant PDA with mild hypoplasia of aortic valve and aortic arch, as shown in the present case. Although children with terminal deletions of both the p and q arms of chromosome 6 have been reported with various congenital heart defects (14, 15), only few congenital heart defects associated with ring chromosome 6 have been reported (1, 4-7). Cardiac malformations present at birth in genetic alterations are associated with considerable mortality and morbidity in the short-term and later in life (16). Presently, as the patient had congestive heart failure due to hemodynamic significant PDA, detailed examination and echocardiography examination was performed to evaluate the need of early operation in this ring chromosome 6 case.
Table 1.
Phenotype comparison between reported cases and the present case with ring chromosome 6 abnormality
*Prominent eyes, microphthalmia, strabismus, nystagmus, glaucoma, iris hypoplasia, aniridia, optic atrophy, megalocornea; †Epicanthal folds, hypertelorism, ptosis; ‡Sacral abnormalities, hemivertebras, scoliosis; §Small penis, bilateral hydrocele, clitoromegaly; ∥Hemangioma, café-au-lait spots, nevus pigmentosus, hyperkeratosis of the soles, redundant skin. T, term; wk, week; +, feature present; -, feature absent; (-), not commented but likely to be absent; ?, unknown.
To the best of our knowledge, this is the first report of a Korean individual with ring chromosome 6 and hemodynamically significant PDA. This rare case report is added to the worldwide data of ring chromosome 6 abnormality.
References
- 1.Ahzad HA, Ramli SF, Loong TM, Salahshourifar I, Zilfalil BA, Yusoff NM. De novo ring chromosome 6 in a child with multiple congenital anomalies. Kobe J Med Sci. 2010;56:E79–E84. [PubMed] [Google Scholar]
- 2.Andrieux J, Devisme L, Valat AS, Robert Y, Frnka C, Savary JB. Prenatal diagnosis of ring chromosome 6 in a fetus with cerebellar hypoplasia and partial agenesis of corpus callosum: case report and review of the literature. Eur J Med Genet. 2005;48:199–206. doi: 10.1016/j.ejmg.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Moore CM, Heller RH, Thomas GH. Developmental abnormalities associated with a ring chromosome 6. J Med Genet. 1973;10:299–303. doi: 10.1136/jmg.10.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peeden JN, Scarbrough P, Taysi K, Wilroy RS, Finley S, Luthardt F, Martens P, Howard-Peebles PN. Ring chromosome 6: variability in phenotype expression. Am J Med Genet. 1983;16:563–573. doi: 10.1002/ajmg.1320160413. [DOI] [PubMed] [Google Scholar]
- 5.Salamanca-Gonez F, Nava S, Armendares S. Ring chromosome 6 in a malformed boy. Clin Genet. 1975;8:370–375. doi: 10.1111/j.1399-0004.1975.tb01516.x. [DOI] [PubMed] [Google Scholar]
- 6.Römke C, Heyne K, Stewens J, Schwinger E. Erroneous diagnosis of fetal alcohol syndrome in a patient with ring chromosome 6. Eur J Pediatr. 1987;146:443. doi: 10.1007/BF00444963. [DOI] [PubMed] [Google Scholar]
- 7.Ivanovich JL, Watson MS, Whelan AJ. An 11-year-old boy with mosaic ring chromosome 6 and dilated aortic root. Am J Med Genet. 2001;98:182–184. doi: 10.1002/1096-8628(20010115)98:2<182::aid-ajmg1028>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Carnevale A, Blanco B, Castillo J, del Castillo V, Dominguez D. Ring chromosome 6 in a child with minimal abnormalities. Am J Med Genet. 1979;4:271–277. doi: 10.1002/ajmg.1320040310. [DOI] [PubMed] [Google Scholar]
- 9.Nishi Y, Yoshimura O, Ohama K, Usui T. Ring chromosome 6: case report and review. Am J Med Genet. 1982;12:109–114. doi: 10.1002/ajmg.1320120115. [DOI] [PubMed] [Google Scholar]
- 10.Chitayat D, Hahm SY, Iqbal M, Nitowsky H. Ring chromosome 6: report of a patient and literature review. Am J Med Genet. 1987;26:145–151. doi: 10.1002/ajmg.1320260122. [DOI] [PubMed] [Google Scholar]
- 11.Callen DF, Eyre HJ, Ringenbergs ML, Freemantle CJ, Woodroffe P, Haan EA. Chromosomal origin of small ring marker chromosomes in man: characterization by molecular genetics. Am J Hum Genet. 1991;48:769–782. [PMC free article] [PubMed] [Google Scholar]
- 12.Pezzolo A, Gimelli G, Cohen A, Lavaggetto A, Romano C, Fogu G, Zuffardi O. Presence of telomeric and subtelomeric sequences at the fusion points of ring chromosomes indicates that the ring syndrome is caused by ring instability. Hum Genet. 1993;92:23–27. doi: 10.1007/BF00216140. [DOI] [PubMed] [Google Scholar]
- 13.Kosztolányi G. Does "ring syndrome" exist? An analysis of 207 case reports on patients with a ring autosome. Hum Genet. 1987;75:174–179. doi: 10.1007/BF00591082. [DOI] [PubMed] [Google Scholar]
- 14.Davies AF, Mirza G, Sekhon G, Turnpenny P, Leroy F, Speleman F, Law C, van Regemorter N, Vamos E, Flinter F, et al. Delineation of two distinct 6p deletion syndromes. Hum Genet. 1999;104:64–72. doi: 10.1007/s004390050911. [DOI] [PubMed] [Google Scholar]
- 15.Hopkin RJ, Schorry E, Bofinger M, Milatovich A, Stern HJ, Jayne C, Saal HM. New insights into the phenotype of 6q deletions. Am J Med Genet. 1997;70:377–386. [PubMed] [Google Scholar]
- 16.Pierpont ME, Basson CT, Benson DW, Jr, Gelb BD, Giglia TM, Goldmuntz E, McGee G, Sable CA, Srivastava D, Webb CL. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:3015–3038. doi: 10.1161/CIRCULATIONAHA.106.183056. [DOI] [PubMed] [Google Scholar]
- 17.Fried K, Rosenblatt M, Mundel G, Krikler R. Mental retardation and congenital malformations associated with a ring chromosome 6. Clin Genet. 1975;7:192–196. doi: 10.1111/j.1399-0004.1975.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 18.Van den Berghe H, Fryns JP, Cassiman JJ, David G. Ring chromosome 6. Karotype 46, XY, r(6)-45, XY,-6. Ann Genet. 1974;17:29–35. [PubMed] [Google Scholar]
- 19.Wurster D, Pomeroy J, Benirschke K, Hoefnagel D. Mental deficiency and malformations in a boy with a group-C ring chromosome: 46,XY, Cr. J Ment Defic Res. 1969;13:184–190. doi: 10.1111/j.1365-2788.1969.tb01078.x. [DOI] [PubMed] [Google Scholar]