Abstract
Angiotensin-converting enzyme 2 (ACE2) degrades angiotensin II to angiotensin-(1–7) and is expressed in podocytes. Here we overexpressed ACE2 in podocytes in experimental diabetic nephropathy using transgenic methods where a nephrin promoter drove the expression of human ACE2. Glomeruli from these mice had significantly increased mRNA, protein, and activity of ACE2 compared to wild-type mice. Male mice were treated with streptozotocin to induce diabetes. After 16 weeks, there was no significant difference in plasma glucose levels between wild-type and transgenic diabetic mice. Urinary albumin was significantly increased in wild-type diabetic mice at 4 weeks, whereas albuminuria in transgenic diabetic mice did not differ from wild-type nondiabetic mice. However, this effect was transient and by 16 weeks both transgenic and nontransgenic diabetic mice had similar rates of proteinuria. Compared to wild-type diabetic mice, transgenic diabetic mice had an attenuated increase in mesangial area, decreased glomerular area, and a blunted decrease in nephrin expression. Podocyte numbers decreased in wild-type diabetic mice at 16 weeks, but were unaffected in transgenic diabetic mice. At 8 weeks, kidney cortical expression of transforming growth factor-β1 was significantly inhibited in transgenic diabetic mice as compared to wild-type diabetic mice. Thus, the podocyte-specific overexpression of human ACE2 transiently attenuates the development of diabetic nephropathy.
Keywords: ACE2, albuminuria, angiotensin, apoptosis, diabetes, podocyte
Angiotensin-converting enzyme 2 (ACE2), a recently discovered member of the renin–angiotensin system, has a single catalytic domain and shares 40% homology with ACE.1, 2 Despite this similarity, ACE2 activity is not affected by ACE inhibitors, nor does it generate angiotensin (Ang) II. On the contrary, ACE2 degrades Ang II to Ang-(1–7), a peptide that antagonizes Ang II signaling.3, 4, 5 ACE2 also degrades Ang I to Ang-(1–9) that is subsequently converted to Ang-(1–7) by ACE.6 Because of these properties, ACE2 has been postulated to be an endogenous renoprotective enzyme.7
ACE2 may be involved in the pathogenesis of diabetic nephropathy. Pharmacological inhibition of ACE2 in streptozotocin (STZ)-induced diabetic mice causes increased albuminuria and expansion of the glomerular matrix.8 In Akita mice, a model of type 1 diabetes, deletion of the ACE2 gene has been reported to exacerbate albuminuria, associated with increased mesangial matrix deposition, glomerular basement membrane thickening, and glomerulosclerosis, without significant changes in blood pressure (BP).9 Recently, administration of human recombinant ACE2 to diabetic Akita mice significantly reduced albuminuria, associated with decreased BP.10 Similarly, intravenous administration of recombinant adenovirus carrying the mouse ACE2 gene to rats with STZ-diabetes was associated with diminished albuminuria and glomerulosclerosis, although systolic BP was also reduced.11 Accordingly, there is increasing interest in the potential therapeutic action of ACE2 amplification to treat diabetic nephropathy, as recently reviewed.12
Glomerular podocytes are terminally differentiated epithelial cells that are key components of the selective permeability barrier of the glomerular basement membrane.13 Podocyte apoptosis and detachment are directly correlated with the progression of diabetic nephropathy.14 ACE2 has been localized to the podocyte, and podocyte ACE2 expression decreases in experimental diabetes.15 However, there is no direct evidence regarding ACE2 overexpression in the podocyte as a protector against the development of diabetic nephropathy. In the current study, transgenic (TG) mice were generated that selectively overexpress human ACE2 in the podocyte to address this question. Specifically, we wished to examine the hypothesis that a podocyte-specific ACE2 transgene could protect against glomerular injury in the STZ model of diabetes, independent of changes in BP.
RESULTS
TG mouse characterization
TG mice with podocyte-specific overexpression of human ACE2 were characterized by reverse transcriptase–PCR, immunoblot analysis of isolated glomeruli, direct immunofluorescence, and measurement of glomerular ACE2 activity. By real-time reverse transcriptase–PCR, human ACE2 mRNA was readily detected in glomeruli from TG mice, at a level ∼50-fold higher than in kidney cortex (Figure 1a). In contrast, the levels of endogenous mouse ACE2 mRNA in TG mice were significantly lower in glomeruli than in kidney cortex (Figure 1b). In wild-type (WT) mice, levels of endogenous mouse ACE2 mRNA were also significantly lower in glomeruli than kidney cortex (n=4, not shown). In TG mice, expression of endogenous mouse ACE2 in kidney cortex was not significantly affected by the presence of the human ACE2 transgene (relative levels of mouse ACE2 mRNA, TG vs. WT mice: 1.8±1.1-fold, P=not significant, n=3). Similarly, glomerular expression of endogenous mouse ACE2 was not affected by the human ACE2 transgene (WT: 1.37±0.19 vs. TG: 1.65±0.33 arbitrary units, P=not significant, n=2–4). Expression of the human ACE2 transgene was also not detected at significant levels in the heart, brain, liver, spleen, or lung (Figure 1a).
Figure 1.
Characterization of transgenic (TG) mice. (a) Expression of mRNA for the human angiotensin-converting enzyme 2 (ACE2) transgene in tissues from TG mice. Graph shows relative levels of human ACE2 mRNA in various mouse tissues, determined by real-time reverse transcriptase–PCR (RT–PCR). *P<0.001, glomeruli vs. all other tissues; n=3 (glomeruli) and n=6–7 for other tissues. (b) Expression of endogenous mouse ACE2 mRNA in glomeruli and kidney cortex from TG mice. Graph depicts relative expression of mouse ACE2 mRNA in glomeruli and kidney cortex from TG mice, determined by real-time RT–PCR. *P<0.02; n=3. (c) Immunoblot analysis of ACE2 in glomeruli and cortex tissue. Shown is representative immunoblot of ACE2 protein in glomeruli and kidney cortex from wild-type (WT) and TG mice, with or without treatment with STZ, at 16 weeks (n=2). Human ACE2 transgenic protein is present as a single band at ∼120 kDa in glomeruli from TG or TG-STZ mice. Endogenous mouse ACE2 protein is observed in all lanes as a single band at ∼100 kDa. (d) Colocalization of human ACE2 transgene with podocyte protein synaptopodin. Dual-color immunofluorescence was performed on kidney sections from TG mice. Sections were stained with rabbit polyclonal antibody to hemagglutinin (HA; present in the transgene as a double epitope tag; left panel), and with rabbit polyclonal anti-synaptopodin antibody (middle panel). Merged view is shown in the panel on the right. Original magnification × 400. (e) Glomerular ACE2 activity. ACE2 activity was measured in isolated glomeruli from WT or TG mice, as described in Materials and Methods. *P<0.02, n=9 (WT), n=10 (TG).
In glomerular isolates from TG mice, the human ACE2 protein was detected by immunoblot as a single protein band of ∼120 kDa, compared with the endogenous mouse ACE2 at ∼100 kDa (Figure 1c). In TG mice, the human ACE2 protein was not readily detected in kidney cortical homogenates (Figure 1c). By immunofluorescence of kidney cortical tissue, TG mice demonstrated positive glomerular staining for the hemagglutinin epitope tag, which colocalized with the podocyte protein synaptopodin (Figure 1d). In freshly isolated glomeruli, ACE2 enzymatic activity was significantly increased in TG mice as compared with WT mice (Figure 1e: 2.82-fold increase in TG vs. WT mice, P<0.02, n=9–10).
STZ-diabetes: whole animal data
Table 1 depicts whole animal data regarding body and organ weights, and plasma glucose. WT and TG mice treated with STZ (WT-STZ and TG-STZ, respectively) developed severe diabetes, with significantly increased plasma glucose compared with vehicle-treated mice, and with no significant difference between the two STZ groups. TG mice had lower body weights than WT mice matched for age. However, both WT-STZ and TG-STZ mice experienced weight loss, and renal hypertrophy, inferred from increased kidney weight to body weight ratio (P<0.001 vs. non-STZ mice). In contrast, there was no difference in cardiac weight to body weight ratios among the four groups. Despite the high levels of plasma glucose, diabetic mice did not develop ketosis, as determined by urine testing at 16 weeks, and absence of metabolic acidosis (not shown). Mortality rate was 12.2% (11/90) for all mice in the study, with no significant differences among the four groups. In TG-STZ mice, glomerular ACE2 activity remained significantly increased after 16 weeks as compared with WT-STZ (3.30±0.88-fold increase, P<0.01, n=5). By immunofluorescent staining, glomerular ACE2 expression was significantly increased in TG mice when compared with WT mice (P<0.001), and this increase persisted 16 weeks after STZ injection (Figure 2). Glomerular human ACE2 protein expression by immunoblot also remained robust at 16 weeks (Figure 1c). At this time point, in contrast, plasma ACE2 activity was actually decreased in TG-STZ mice compared with WT-STZ mice (TG-STZ: 0.25±0.03 vs. WT-STZ: 0.32±0.04 ng-eq/μl plasma, P<0.05, n=6; Figure 3). However, both diabetic groups had higher plasma ACE2 activity compared with non-STZ groups (P<0.001).
Table 1. Whole animal data.
| WT | TG | WT-STZ | TG-STZ | |
|---|---|---|---|---|
| BW (g) | 36.2±0.7 (6) | 31.1±1.1 (7)** | 27.2±0.5 (8)* | 25.9±0.5 (6)* |
| KW/BW (g/kg) | 11.4±0.4 (6) | 12.2±0.2 (7) | 20.8±0.7 (8)*** | 21.0±1.3 (6)*** |
| HW/BW (g/kg) | 3.9±0.3 (6) | 4.3±0.1 (7) | 4.3±0.2 (8) | 4.2±0.2 (6) |
| Plasma glucose (mmol/l) | 19.1±1.0 (11) | 18.3±1.3 (11) | 55.8±8.0 (11)† | 72.2±17.1 (11)† |
Abbreviations: BW, body weight; HW, heart weight; KW, kidney weight; TG, transgenic; TG-STZ, transgenic streptozotocin-treated; WT, wild type; WT-STZ, wild-type streptozotocin-treated.
Values are means±s.e. (n).
*P<0.001 vs. WT and *P<0.01 vs. TG.
**P<0.01 vs. WT.
***P<0.001 vs. WT and TG.
†P<0.05 vs. WT and TG.
Figure 2.
Glomerular expression of angiotensin-converting enzyme 2 (ACE2) by immunofluorescence at 16 weeks. Graph depicting semiquantitative analysis of ACE2 immunofluorescent staining in mouse glomeruli at 16 weeks. TG, podocyte-specific ACE2 transgenic; TG-STZ, transgenic streptozotocin-treated; WT, wild type; WT-STZ, wild-type streptozotocin-treated. Values are means±s.e. *P<0.001 vs. WT and vs. WT-STZ; n=100 glomeruli per group, from 6 to 11 mice per group. Representative images depicting ACE2 staining in glomeruli (red) at 16 weeks are shown above graph. Blue staining represents Hoechst nuclear stain. Original magnification × 640.
Figure 3.
Plasma angiotensin-converting enzyme 2 (ACE2) activity. Graph depicts plasma ACE2 enzymatic activity from wild-type (WT), transgenic (TG), wild-type streptozotocin-treated (WT-STZ), and transgenic streptozotocin-treated (TG-STZ) mice at 16 weeks. Values are means±s.e. *P<0.001 vs. WT and TG, #P<0.001 vs. WT and TG, $P<0.05 vs. TG-STZ; n=6.
At 16 weeks, there were no significant differences in plasma electrolytes, total protein, or total cholesterol between WT-STZ and TG-STZ mice (not shown). WT-STZ mice had significant elevations in serum triglycerides compared with all other groups (P<0.01).
Systolic BP
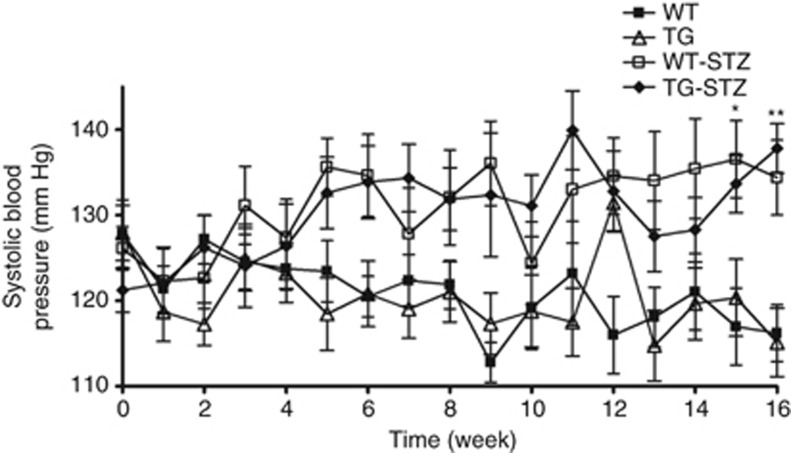
Systolic BP was measured weekly throughout the study and tended to be higher in both groups of STZ mice when compared with non-STZ mice, but the difference was not significant until the 15-week time point (Figure 4). Systolic BP did not significantly differ between WT-STZ and TG-STZ mice at any point in the study, nor did it differ between WT and TG mice (Figure 4).
Figure 4.
Systolic blood pressure (BP). Systolic BP was measured weekly by tail-cuff plethysmography. TG, transgenic; TG-STZ, transgenic streptozotocin-treated; WT, wild type; WT-STZ, wild-type streptozotocin-treated. Values are means±s.e. *P<0.01 vs. WT and TG, **P<0.05 vs. WT and TG. There was no significant difference between WT-STZ and TG-STZ mice; n=14 mice per group.
Glomerular filtration rate
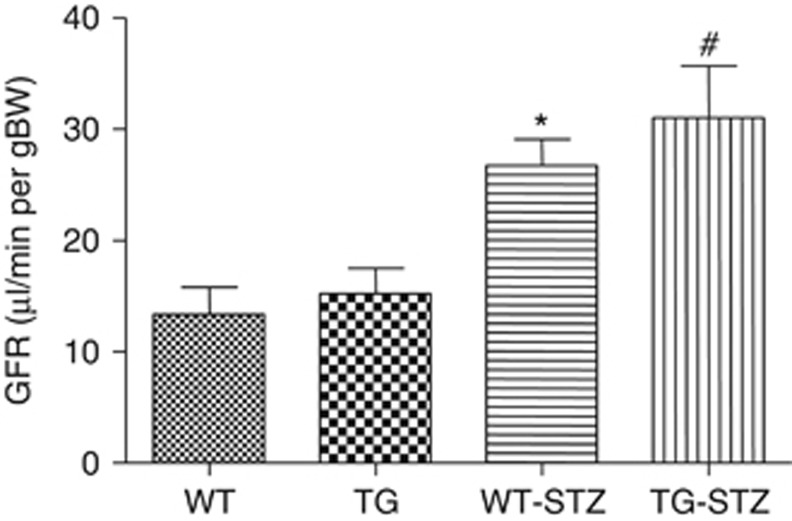
At 16 weeks, glomerular filtration rate was significantly higher in WT-STZ and TG-STZ mice when compared with WT and TG mice, but there was no difference between the two STZ groups (Figure 5).
Figure 5.
Glomerular filtration rate (GFR). Graph shows GFR measurements in wild-type (WT), transgenic (TG), wild-type streptozotocin-treated (WT-STZ), and transgenic streptozotocin-treated (TG-STZ) mice after 16 weeks. Values are means±s.e. *P<0.05 vs. WT and TG, #P<0.01 vs. WT and TG; n=4–6.
Albuminuria
At 4 weeks, WT-STZ mice had significantly increased levels of albuminuria as compared with vehicle-treated mice (Figure 6a). In contrast, TG-STZ mice had no increase in albuminuria at 4 weeks (P<0.05 vs. WT-STZ, n=5). However, there was no significant difference in albuminuria between WT-STZ and TG-STZ mice at 8 weeks (WT-STZ: 1312±527 vs. TG-STZ: 1233±380 μg per 24 h; P=not significant, n=6). Similarly, at 16 weeks, both WT-STZ and TG-STZ mice had markedly increased levels of albuminuria, with no significant difference between the two groups (Figure 6b). Coomassie blue staining of sodium dodecyl sulfate gels revealed generalized proteinuria in STZ-treated mice compared with nondiabetic mice (Figure 6c).
Figure 6.
Urinary protein excretion. (a) The 24 h urinary albumin excretion in wild-type (WT), transgenic (TG), wild-type streptozotocin-treated (WT-STZ), and transgenic streptozotocin-treated (TG-STZ) mice at 4 weeks. *P<0.05 vs. all other groups (WT, TG, and TG-STZ mice); n=5. (b) The 24 h urinary albumin excretion at 16 weeks. *P<0.01 vs. WT and TG, #P<0.01 vs. WT and TG. There was no significant difference between WT-STZ and TG-STZ mice; n=14. (c) Representative Coomassie blue-stained sodium dodecyl sulfate (SDS) gel of urine samples from WT, TG, WT-STZ, and TG-STZ mice at 8 weeks. Multiple protein bands are evident in urine samples from WT-STZ and TG-STZ mice. Molecular weight marker is depicted in the first and last lanes.
Histological analysis and expression of podocyte proteins
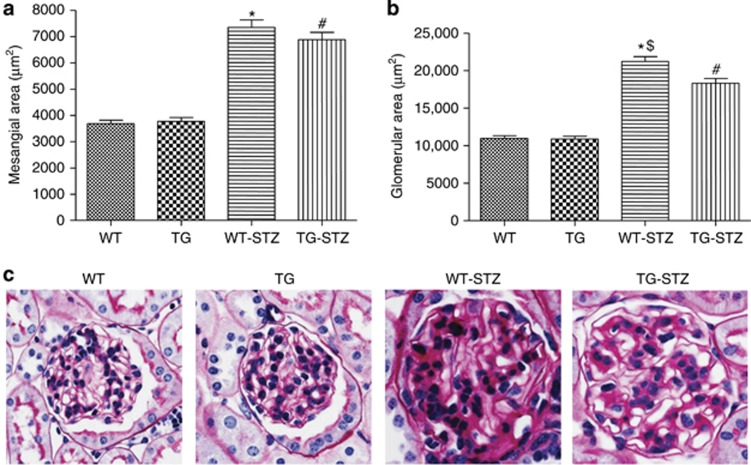
Histological analysis was performed on kidney sections at 8 and 16 weeks. At 8 weeks, WT-STZ mice and TG-STZ mice demonstrated increases in mesangial and glomerular areas compared with vehicle-treated mice (Figure 7). The increase in mesangial area, however, was significantly attenuated in TG-STZ mice. At 16 weeks, mesangial and glomerular areas were significantly increased in WT-STZ and TG-STZ groups as compared with nondiabetic mice (Figure 8). Glomerular diameter was significantly diminished in TG-STZ mice when compared with WT-STZ mice (Figure 8).
Figure 7.
Effect of podocyte angiotensin-converting enzyme 2 (ACE2) overexpression on mesangial and glomerular area at 8 weeks. Graphs depict (a) mesangial and (b) glomerular areas at 8 weeks in wild-type (WT), transgenic (TG), wild-type streptozotocin-treated (WT-STZ), and transgenic streptozotocin-treated (TG-STZ) mice. (a) Mesangial area at 8 weeks. Values are means±s.e. (n=40 glomeruli per group of 4 mice). *P<0.001 vs. WT and TG, #P<0.02 vs. WT and TG, $P<0.03 vs. TG-STZ. (b) Glomerular area at 8 weeks. Values are means±s.e. (n=40 glomeruli per group of 4 mice). *P<0.001 vs. WT and TG, #P<0.001 vs. WT and TG. There was no significant difference between WT-STZ and TG-STZ mice. (c) Representative photomicrographs depicting periodic acid–Schiff (PAS)-stained glomeruli in WT, TG, WT-STZ, and TG-STZ mice at 8 weeks. Glomerular hypertrophy and increased mesangial staining are evident in STZ groups as compared with non-STZ mice. Original magnification × 640.
Figure 8.
Effect of podocyte angiotensin-converting enzyme 2 (ACE2) overexpression on mesangial and glomerular area at 16 weeks. Graphs depict (a) mesangial and (b) glomerular areas at 16 weeks in wild-type (WT), transgenic (TG), wild-type streptozotocin-treated (WT-STZ), and transgenic streptozotocin-treated (TG-STZ) mice. (a) Mesangial area at 16 weeks. Values are means±s.e. (n=60 glomeruli per group of 6 mice). *P<0.001 vs. WT and TG, #P<0.001 vs. WT and TG. There was no significant difference between WT-STZ and TG-STZ mice. (b) Glomerular area at 16 weeks. Values are means±s.e. (n=60 glomeruli per group of 6 mice). *P<0.001 vs. WT and TG, #P<0.001 vs. WT and TG, $P<0.001 vs. TG-STZ. (c) Representative photomicrographs depicting periodic acid–Schiff (PAS)-stained glomeruli in WT, TG, WT-STZ, and TG-STZ mice at 16 weeks. Glomerular hypertrophy and increased mesangial staining is evident in STZ groups as compared with non-STZ mice. Original magnification × 640.
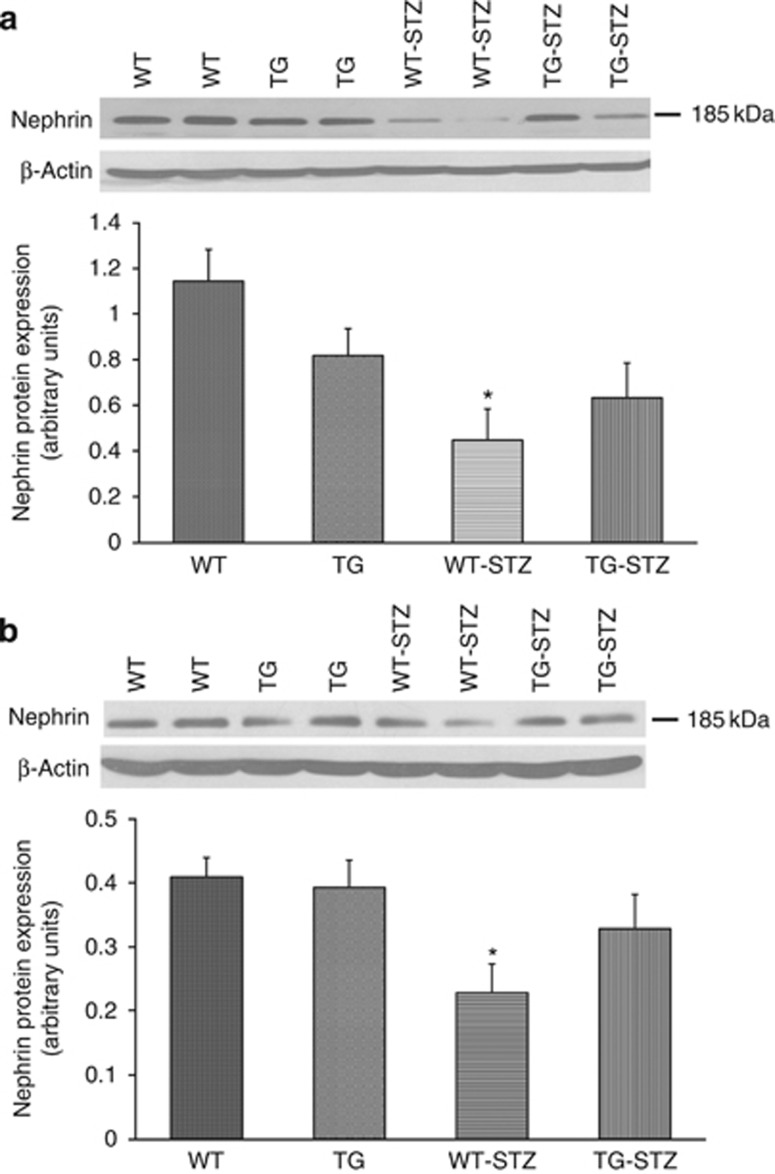
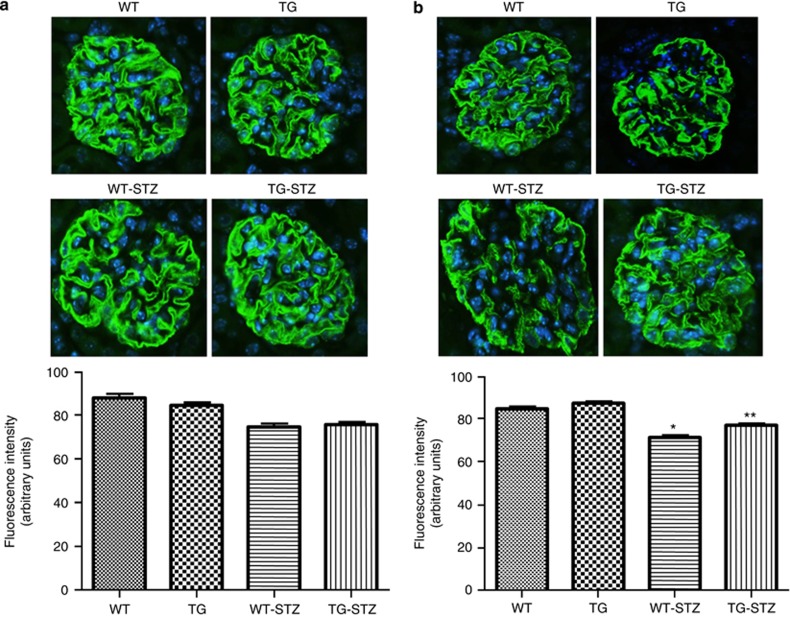
Expression of the podocyte protein nephrin was examined by immunoblots and immunofluorescence. In kidney cortical lysates from WT-STZ mice, nephrin expression by immunoblot was significantly decreased at 8 weeks as compared with WT mice (Figure 9a). TG-STZ mice, in contrast, exhibited no significant difference in nephrin expression compared with their TG counterparts, although baseline levels were lower. Similarly, at 16 weeks, nephrin expression was significantly reduced in WT-STZ mice compared with WT mice, whereas nephrin levels did not significantly differ between TG and TG-STZ mice (Figure 9b). Semiquantitative immunofluorescence analysis at 8 and 16 weeks revealed a similar pattern of glomerular nephrin expression as the immunoblots (Figure 10). In particular, whereas nephrin expression was significantly reduced in WT-STZ mice, there was no significant difference in nephrin expression between TG and TG-STZ mice, although TG mice tended to have lower expression than WT mice.
Figure 9.
Nephrin expression at 8 and 16 weeks by immunoblot. (a) Graph depicting nephrin expression by immunoblot analysis of cortical lysates in wild-type (WT), transgenic (TG), wild-type streptozotocin-treated (WT-STZ), and transgenic streptozotocin-treated (TG-STZ) mice at 8 weeks. Representative immunoblot is shown above graph, with nephrin at ∼185 kDa, and β-actin signal below, as control for protein loading. Values are mean±s.e., n=6 per group. *P<0.02 vs. WT. There was no significant difference between TG and TG-STZ mice or between WT-STZ and TG-STZ mice. (b) Graph depicting nephrin expression by immunoblot analysis of cortical lysates in WT, TG, WT-STZ, and TG-STZ mice at 16 weeks. Representative immunoblot is shown above graph, with nephrin at ∼185 kDa, and β-actin signal below, as control for protein loading. Values are mean±s.e., n=6 per group. *P<0.05 vs. WT. There was no significant difference between TG and TG-STZ mice.
Figure 10.
Nephrin expression at 8 and 16 weeks by immunofluorescence. (a) Graph depicting semiquantitative analysis of nephrin expression by immunofluorescence in wild-type (WT), transgenic (TG), wild-type streptozotocin-treated (WT-STZ), and transgenic streptozotocin-treated (TG-STZ) mice at 8 weeks. Values are means±s.e., n=100 glomeruli per group, from 7 to 8 mice per group. *P<0.001 vs. WT. There was no significant difference between TG and TG-STZ mice. Representative images depicting glomerular nephrin expression (red) at 8 weeks are shown above graph. Nuclear Hoechst staining (blue) is also shown. Original magnification × 640. (b) Graph depicting nephrin expression by immunofluorescence at 16 weeks. Values are means±s.e., n=100 glomeruli per group, from 6 to 11 mice per group. *P<0.001 vs. WT. Representative images depicting glomerular nephrin expression (red) at 16 weeks are shown above graph. Nuclear Hoechst staining (blue) is also shown. Original magnification × 640.
By immunofluorescence, glomerular synaptopodin expression was not significantly affected at 8 weeks, although there was a tendency for reduced expression in WT-STZ and TG-STZ mice (Figure 11). However, at 16 weeks, expression of synaptopodin was significantly reduced in WT-STZ mice when compared with WT, TG, or TG-STZ mice (Figure 11).
Figure 11.
Synaptopodin expression at 8 and 16 weeks by immunofluorescence. (a) Graph depicting semiquantitative analysis of synaptopodin expression by immunofluorescence in wild-type (WT), transgenic (TG), wild-type streptozotocin-treated (WT-STZ), and transgenic streptozotocin-treated (TG-STZ) mice at 8 weeks. Values are means±s.e., n=100 glomeruli per group, from 7 to 8 mice per group. There was no significant difference among the groups. Representative images depicting glomerular synaptopodin expression (green) at 8 weeks are shown above graph. Nuclear Hoechst staining (blue) is also shown. Original magnification × 640. (b) Graph depicting synaptopodin expression at 16 weeks. Values are means±s.e., n=100 glomeruli per group, from 6 to 11 mice per group. *P<0.001 vs. WT and TG, **P<0.001 vs. WT-STZ. There was no significant difference between TG and TG-STZ mice. Representative images depicting glomerular synaptopodin expression (green) at 16 weeks are shown above graph. Nuclear Hoechst staining (blue) is also shown. Original magnification × 640.
Podocyte numbers were assayed at 8 and 16 weeks by counting glomerular nuclei staining positively for WT-1. At 8 weeks, both WT-STZ and TG-STZ mice had significantly decreased podocyte number per glomerulus compared with non-STZ mice (Figure 12a). In TG mice, podocyte number was lower than in WT mice, and the decrease with STZ was less pronounced compared with WT-STZ mice. At 16 weeks, WT-STZ mice showed a significant decrease in average podocyte number compared with vehicle-treated mice. In contrast, TG-STZ mice did not exhibit a significant decrease in podocytes as compared with nondiabetic mice (Figure 12b).
Figure 12.
Podocyte number at 8 and 16 weeks. (a) Graph shows average number of podocytes per glomerulus in wild-type (WT), transgenic (TG), wild-type streptozotocin-treated (WT-STZ), and transgenic streptozotocin-treated (TG-STZ) mice at 8 weeks, as determined by counting of WT-1-positive nuclei in kidney sections. Values are means±s.e., n=78–106 glomeruli per 7 to 8 mice in each group. *P<0.001 vs. WT and TG, #P<0.01 vs. TG, $P<0.01 vs. WT. (b) Podocyte numbers at 16 weeks. Values are means±s.e., n=120 glomeruli per 6 mice in each group. *P<0.01 vs. WT and TG. There was no significant difference between TG-STZ and WT, TG, or WT-STZ mice. (c) Representative photomicrograph depicting glomerular WT-1 staining in glomeruli from WT-STZ vs. TG-STZ mice at 16 weeks. Original magnification × 400.
Kidney cortical expression of TGF-β1
As shown in Figure 13, protein expression of the profibrotic cytokine transforming growth factor-β1 (TGF-β1) was significantly increased in kidney cortices from WT-STZ mice at 8 weeks when compared with nondiabetic mice. In contrast, TG-STZ mice exhibited no increase in cortical TGF-β1 at 8 weeks. At 16 weeks, expression of TGF-β1 was increased in both WT-STZ and TG-STZ mice, with no significant difference between the two groups.
Figure 13.
Expression of transforming growth factor-β1 (TGF-β1) at 8 and 16 weeks. (a) Graph shows expression of TGF-β1 in kidney cortical lysates from wild-type (WT), transgenic (TG), wild-type streptozotocin-treated (WT-STZ), and transgenic streptozotocin-treated (TG-STZ) mice at 8 weeks, by immunoblot. Values are means±s.e., n=6 per group. *P<0.02 vs. all other groups (WT, TG, and TG-STZ). Above graph is a representative immunoblot for TGF-β1, depicting bands at 12.5 and 25 kDa, the latter representing the TGF-β1 dimer. Blot for β-actin, as loading control, is depicted below. (b) Graph shows expression of TGF-β1 from cortical lysates at 16 weeks, with representative immunoblot above graph. Values are means±s.e., n=6 per group. *P<0.05 vs. WT and TG. **P<0.01 vs. WT and TG. There was no significant difference between WT-STZ and TG-STZ mice.
DISCUSSION
The major finding of this study is that overexpression of human ACE2 in the glomerular podocyte attenuates the development of nephropathy in mouse STZ-diabetes. This was shown by (1) a delay in the development of albuminuria, independent of any effect on systolic BP; (2) histological evidence of renal protection, namely an early reduction in mesangial expansion, and attenuation of glomerular hypertrophy at 16 weeks; (3) partial preservation of expression of the podocyte proteins nephrin and synaptopodin; (4) prevention of podocyte loss by ACE2 overexpression; and (5) reduction in cortical TGF-β1 expression at 8 weeks. In addition to protection against diabetic nephropathy, this model of ACE2 overexpression should be useful to examine questions related to pathology and progression of CKD in other forms of glomerular injury.
Podocytes are highly specialized cells that form a critical component of the glomerular filtration barrier to proteins. Cultured podocytes express components of the renin–angiotensin system,16, 17, 18 and ACE2 has been localized to the podocyte in glomeruli from diabetic mice by immunohistochemistry and immunogold staining.15 Loss of podocytes via apoptosis contributes importantly to the pathogenesis of diabetic nephropathy. Multiple signaling pathways may mediate podocyte apoptosis in diabetes, including involvement of mammalian target of rapamycin signaling.19 However, Ang II is recognized as a key contributor to podocyte apoptosis, via interaction with AT1 (angiotensin II receptor, type 1) receptors, and production of reactive oxygen species.20, 21 In cultured podocytes exposed to high glucose, production of Ang II is enhanced, which may contribute to AT1 receptor–mediated apoptosis.16 In the db/db mouse model of type 2 diabetes, podocyte ACE2 expression is decreased,15 and renal biopsy specimens from humans with diabetic nephropathy similarly demonstrate decreased glomerular ACE2 mRNA and protein expression.22 As ACE2 degrades Ang II to Ang-(1–7), strategies to preserve or enhance podocyte ACE2 could prevent local increases in Ang II levels. Furthermore, ACE2 leads to enhanced production of Ang-(1–7), which has been associated with improvement of albuminuria in experimental diabetes.23
The glomerular ACE2-specific TG mice that we describe in this study did not exhibit major phenotypic differences compared with WT counterparts. For unclear reasons, TG mice had lower body weights compared with WT mice matched for age. However, both WT-STZ and TG-STZ mice developed similar elevations of plasma glucose, associated with loss of body weight, kidney hypertrophy, glomerular hyperfiltration, and parallel increases in systolic BP. The human ACE2 cDNA was linked to the mouse nephrin promoter in these studies, as nephrin gene expression is relatively specific to podocytes.24 Several lines of evidence support the conclusion that human ACE2 was selectively overexpressed in podocytes from TG mice. First, robust mRNA expression of the transgene occurred in glomeruli from TG mice, at a level ∼50-fold higher than that in kidney cortex, whereas endogenous mouse ACE2 expression was lower in glomeruli than cortex. Other tissues from TG mice, including brain (which expresses nephrin24) did not significantly express human ACE2 mRNA. Second, by immunoblot, the human ACE2 protein was detected in glomerular isolates, but not kidney cortex, as a protein of ∼120 kDa. Third, the ACE2 protein colocalized with synaptopodin by immunofluorescence, indicating its presence in the podocyte. Fourth, ACE2 activity was significantly enhanced in glomeruli from TG mice, and elevated activity (and glomerular expression by immunoblot and immunofluorescence) persisted after 16 weeks of STZ-diabetes. In this regard, nephrin expression decreases in diabetic nephropathy,25, 26 and nephrin deficiency is associated with activation of nuclear factor-κB–mediated pathways that enhance glomerular injury.27 Our data suggest that the nephrin promoter remained active and stimulated transcription of the human ACE2 gene in TG-STZ mice, despite the presence of severe diabetes. Finally, selective overexpression of the human ACE2 gene in podocytes was not associated with changes in systolic BP. Plasma ACE2 activity did not increase above the level of WT-STZ mice, in keeping with the localized increase in ACE2 at the podocyte level.
In WT-STZ mice, a significant increase in albuminuria occurred at 4 weeks as compared with nondiabetic mice. In contrast, urinary albumin excretion in TG-STZ mice at 4 weeks did not differ from levels in non-diabetic mice. The 24 h albuminuria levels in our diabetic mouse model were significantly higher than those reported by Qi et al.28 in FVB mice with STZ-diabetes. There is considerable variability in urinary albumin measures in mouse models of diabetes, and assay procedures differ. Studies using the same assay procedure we used in this study have reported high measures of urinary albumin in other mouse strains with diabetes.29, 30 Despite the initial prevention of albuminuria in TG-STZ mice, albuminuria increased to the level of WT-STZ mice at later time points. In diabetes, increased Ang II is thought to promote podocyte dysfunction and albuminuria. Ang II reduces expression of the antioxidant protein peroxiredoxin 2 in podocytes in vitro and in vivo, associated with increased production of reactive oxygen species, inhibition of the Akt pathway, and apoptosis.20 As TG-STZ mice were only protected against albuminuria at 4 weeks, podocyte ACE2 activity may not have been sufficiently enhanced at later time points to reduce this Ang II–mediated adverse signaling, or other non-Ang II–dependent pathways affecting podocyte function may have dominated and contributed to albuminuria.
Nonetheless, the early reduction in albuminuria in TG-STZ mice was associated with improvement in features of diabetic nephropathy. First, a significant decrease in mesangial area occurred at 8 weeks, with decreased glomerular hypertrophy at 16 weeks. Second, expression of the podocyte proteins nephrin and synaptopodin, reported to be decreased in diabetic models,31, 32 was partly preserved in TG-STZ mice. In TG mice, baseline nephrin expression was decreased compared with WT mice, and treatment with STZ did not induce a further decrease. As our model used the mouse nephrin promoter to induce podocyte-specific ACE2 overexpression, the decrease in basal nephrin expression in TG mice could be because of competition for transcription factors between the nephrin gene and the transgene. Importantly, partial protection against loss of podocyte number was evident at 16 weeks, but not at 8 weeks. Finally, the expression of TGF-β1 in kidney cortex was significantly attenuated in TG-STZ mice at 8 weeks. We postulate that this partial protection is because of enhanced ACE2-dependent degradation of Ang II in the diabetic glomerulus. Our data support the hypothesis that in the diabetic kidney, glomerular Ang II initiates a reduction in nephrin and synaptopodin expression, and stimulation of TGF-β1, which induces podocyte loss (diabetic podocytopathy), leading to proteinuria and subsequent tubulointerstitial inflammation and fibrosis, which in turn is associated with increased elaboration of TGF-β1.33 Consistent with this view, in rats with STZ-diabetes, intravenous adenoviral gene transfer of ACE2 reduces kidney cortical levels of Ang II, and increases Ang-(1–7), associated with protection from glomerular injury.11 In rat mesangial cells, transfection of ACE2 inhibits Ang II–stimulated cell proliferation, oxidative stress, and collagen IV synthesis.11
Although the beneficial effects of ACE2 may have derived largely from a reduction in Ang II levels in our study, the role of potentially enhanced podocyte generation of Ang-(1–7) in TG mice remains unclear. Although Ang-(1–7) inhibits Ang II–mediated proinflammatory signaling in proximal tubular cells and the vasculature,3, 4, 5 its effects on podocyte function are unknown. In mesangial cells, Ang-(1–7) has been reported to either stimulate profibrotic pathways34 or inhibit Ang II signaling.35 Thus, although overexpression of ACE2 in the podocyte has a renoprotective effect, the relative contribution of diminished levels of Ang II versus increased Ang-(1–7) requires further study. Finally, this study focused on early diabetic nephropathy, and the long-term effects of overexpression of ACE2 on disease progression remain unclear.
In summary, after STZ-induced diabetes, TG mice with podocyte-specific overexpression of the human ACE2 protein are protected against the early development of albuminuria and show partial preservation of podocyte proteins and podocyte number, reduced glomerular histological injury, and decreased kidney cortical TGF-β1 expression. ACE2 may represent a therapeutic target in the prevention and treatment of diabetic nephropathy.
MATERIALS AND METHODS
TG mice
The cDNA encoding the open reading frame of the human ACE2 gene, which contained a double hemagglutinin epitope tag at the 5′ end, was inserted immediately downstream of the murine nephrin promoter (provided by Dr C Kennedy, University of Ottawa, Ottawa, ON, Canada), and cloned into the XhoI restriction site of the expression vector pcDNA3 (Invitrogen, Carlsbad, CA). The correct orientation of the insert was confirmed by DNA sequencing (StemCore Laboratories, Ottawa Hospital Research Institute, Ottawa, ON, Canada). Transfection of the expression plasmid containing the cytomegalovirus promoter but lacking the nephrin promoter into cultured COS-7 cells induced robust expression of the human ACE2 protein, as determined by western blot analysis (not shown). The linearized expression plasmid containing the nephrin promoter was microinjected into FVB mouse embryos (Charles River Laboratories, Wilmington, MA) and founder mice were identified by DNA genotyping. Three mouse founder lines were characterized for podocyte ACE2 expression, and one line was selected for further studies.
Diabetes model
All mice used in these studies were on a congenic FVB/n background. At 8 weeks of age, male WT and TG mice were injected over 5 consecutive days with either STZ (50 μg/g per day intraperitoneally; Sigma-Aldrich, St Louis, MO) or sodium-citrate vehicle. Hindlimb blood glucose levels were monitored weekly by glucometer readings. Urine was analyzed for ketones using Ketostix (Bayer HealthCare LLC, Elkhart, IN). All mice were housed and cared for in the Animal Care Facility at the University of Ottawa with free access to food and water. All protocols were approved by the University of Ottawa Animal Care Committee and conducted according to the guidelines of the Canadian Council on Animal Care.
Systolic BP
Systolic BP was measured using tail-cuff plethysmography (BP-2000; Visitech Systems, Apex, NC). Mice were trained at 6 weeks of age for 5 consecutive days and a baseline measurement was taken at 7 weeks of age. Systolic BP was measured weekly from 1 week after STZ or vehicle injection until 16 weeks.
Plasma analysis
At 16 weeks after injections, mice were anesthetized using isoflurane and killed by cardiac puncture. Blood was collected with a heparinized needle and plasma was separated by centrifugation at 3000 g for 10 min at 4 °C. Plasma electrolytes, glucose, albumin, carbon dioxide, triglycerides, and cholesterol were assayed using the Synchron CX5 Delta analyzer (Beckman Coulter, Fullerton, CA).
Glomerular isolation
Mice were anesthetized and cardiac perfusion was initiated with 4 × 107 Dynabeads (Invitrogen Dynal AS, Oslo, Norway), diluted in 20 ml of phosphate-buffered saline. Kidney cortices were digested in collagenase Type 1 (1.0 mg/ml; Worthington Biochemical, Lakewood, NJ) and DNase 1 (0.1 mg/ml; Roche Diagnostics, Indianapolis, IN) at 37 °C for 30 min, and tissue was passed through a 100 μm sieve. Glomeruli were isolated using a magnetic particle concentrator.36
Reverse transcriptase–PCR for human and mouse ACE2
RNA was isolated from glomeruli and other tissues for real-time PCR for ACE2. Details of the protocol can be found in Supplementary Information online.
Immunoblotting
Kidney cortical tissue and glomeruli were prepared for immunoblot analysis with antibodies to ACE2, nephrin, and TGF-β1. Details of the protocol can be found in Supplementary Information online.
Measurement of glomerular filtration rate
The glomerular filtration rate was measured in mice at 16 weeks of study, using plasma fluorescein isothiocyanate–inulin clearance kinetics, as we have described.37
Urinary albumin excretion
At 4, 8, and 16 weeks after injections, urine was collected over 24 h in metabolic cages. Urinary albumin excretion was measured using a murine albumin ELISA (Bethyl Laboratories, Montgomery, TX).
Kidney histology
Dissected kidneys were incubated with 4% paraformaldehyde for 48 h at 4 °C, and were subsequently dehydrated, paraffin-embedded, and cut into 4 μm sections. Sections were stained with periodic acid–Schiff for measurement of mesangial and glomerular cross-sectional areas. Podocyte counts were determined by staining for WT-1,38 and immunofluorescent staining was performed for ACE2, hemagglutinin, nephrin, and synaptopodin using standard procedures. All analyses were performed in a blinded fashion. Details on the histological protocols can be found in Supplementary Information online.
ACE2 enzymatic activity assay
ACE2 activity in glomeruli and plasma was determined using a fluorogenic ACE2 synthetic substrate, essentially as described.37, 39 Details of the assay can be found in Supplementary Information online.
Statistics
Data are presented as mean±s.e. Data were analyzed using GraphPad Prism (Software version 4.02, San Diego, CA). For multiple comparisons, analysis was by one-way analysis of variance followed by Bonferroni or Newman–Keuls post-comparison test. For comparisons involving two groups, Student's t-test was used. A P-value of <0.05 was considered statistically significant.
Acknowledgments
We thank Mr A Carter for management of the mouse colonies. We thank Dr R Kothary (OHRI) and Mr Yves de Repentigny (OHRI) for performance of the mouse embryo injections. This study was supported by grants from the Canadian Institutes of Health Research (CIHR), and the Kidney Foundation of Canada (KFOC) to KDB and by NIDDK (grant 1R01DK080089-01A2) and JDRF grants to DB.
All the authors declared no competing interests.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary Information. RT-PCR for human and mouse ACE2.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
Parts of this work have been presented in an abstract form at the World Congress of Nephrology in Vancouver, BC, Canada in April 2011.
Supplementary Material
References
- Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:e1–e9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Tipnis S, Hooper N, Hyde R, et al. A human homolog of angiotensin-converting enzyme. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- Freeman EJ, Chisolm GM, Ferrario CM, et al. Angiotensin-(1-7) inhibits vascular smooth muscle cell growth. Hypertension. 1996;28:104–108. doi: 10.1161/01.hyp.28.1.104. [DOI] [PubMed] [Google Scholar]
- Sampaio WO, Henrique de Castro C, Santos RA, et al. Angiotensin-(1-7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension. 2007;50:1093–1098. doi: 10.1161/HYPERTENSIONAHA.106.084848. [DOI] [PubMed] [Google Scholar]
- Su Z, Zimpelmann J, Burns KD. Angiotensin-(1-7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int. 2006;69:2212–2218. doi: 10.1038/sj.ki.5001509. [DOI] [PubMed] [Google Scholar]
- Rice G, Thomas D, Grant P, et al. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Wysocki J, Naaz P, et al. Increased ACE 2 and decreased ACE protein in renal tubules from diabetic mice: a renoprotective combination. Hypertension. 2004;43:1120–1125. doi: 10.1161/01.HYP.0000126192.27644.76. [DOI] [PubMed] [Google Scholar]
- Soler M, Wysocki J, Ye M, et al. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int. 2007;72:614–623. doi: 10.1038/sj.ki.5002373. [DOI] [PubMed] [Google Scholar]
- Wong D, Oudit G, Reich H, et al. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol. 2007;171:438–451. doi: 10.2353/ajpath.2007.060977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit G, Liu G, Zhong J, et al. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes. 2010;59:529–538. doi: 10.2337/db09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CX, Hu Q, Wang Y, et al. Angiotensin-converting enzyme (ACE) 2 overexpression ameliorates glomerular injury in a rat model of diabetic nephropathy: a comparison with ACE inhibition. Mol Med. 2011;17:59–69. doi: 10.2119/molmed.2010.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle D, Wysocki J, Soler MJ, et al. Angiotensin-converting enzyme 2: enhancing the degradation of angiotensin II as a potential therapy for diabetic nephropathy. Kidney Int. 2011;81:520–528. doi: 10.1038/ki.2011.381. [DOI] [PubMed] [Google Scholar]
- Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- Toyoda M, Najafian B, Kim Y, et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56:2155–2160. doi: 10.2337/db07-0019. [DOI] [PubMed] [Google Scholar]
- Ye M, Wysocki J, Soler M, et al. Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17:3067–3075. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- Durvasula RV, Shankland SJ. Activation of a local renin angiotensin system in podocytes by glucose. Am J Physiol Renal Physiol. 2008;294:F830–F839. doi: 10.1152/ajprenal.00266.2007. [DOI] [PubMed] [Google Scholar]
- Velez JC, Bland AM, Arthur JM, et al. Characterization of renin-angiotensin system enzyme activities in cultured mouse podocytes. Am J Physiol Renal Physiol. 2007;293:F398–F407. doi: 10.1152/ajprenal.00050.2007. [DOI] [PubMed] [Google Scholar]
- Yoo TH, Li JJ, Kim JJ, et al. Activation of the renin-angiotensin system within podocytes in diabetes. Kidney Int. 2007;71:1019–1027. doi: 10.1038/sj.ki.5002195. [DOI] [PubMed] [Google Scholar]
- Inoki K, Mori H, Wang J, et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011;121:2191–2196. doi: 10.1172/JCI44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HH, Hoffmann S, Di Marco GS, et al. Downregulation of the antioxidant protein peroxiredoxin 2 contributes to angiotensin II-mediated podocyte apoptosis. Kidney Int. 2011;80:959–969. doi: 10.1038/ki.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Ding G, Zhu J, et al. Angiotensin II infusion induces nephrin expression changes and podocyte apoptosis. Am J Nephrol. 2008;28:500–507. doi: 10.1159/000113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich HN, Oudit GY, Penninger JM, et al. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 2008;74:1610–1616. doi: 10.1038/ki.2008.497. [DOI] [PubMed] [Google Scholar]
- Benter IF, Yousif MH, Cojocel C, et al. Angiotensin-(1-7) prevents diabetes-induced cardiovascular dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H666–H672. doi: 10.1152/ajpheart.00372.2006. [DOI] [PubMed] [Google Scholar]
- Putaala H, Soininen R, Kilpeläinen P, et al. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet. 2001;10:1–8. doi: 10.1093/hmg/10.1.1. [DOI] [PubMed] [Google Scholar]
- Benigni A, Gagliardini E, Tomasoni S, et al. Selective impairment of gene expression and assembly of nephrin in human diabetic nephropathy. Kidney Int. 2004;65:2193–2200. doi: 10.1111/j.1523-1755.2004.00636.x. [DOI] [PubMed] [Google Scholar]
- Doublier S, Salvidio G, Lupia E, et al. Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II. Diabetes. 2003;52:1023–1030. doi: 10.2337/diabetes.52.4.1023. [DOI] [PubMed] [Google Scholar]
- Hussain S, Romio L, Saleem M, et al. Nephrin deficiency activates NF-kappaB and promotes glomerular injury. J Am Soc Nephrol. 2009;20:1733–1740. doi: 10.1681/ASN.2008111219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Fujita H, Jin J, et al. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes. 2005;54:2628–2637. doi: 10.2337/diabetes.54.9.2628. [DOI] [PubMed] [Google Scholar]
- Gil N, Goldberg R, Neuman T, et al. Heparanase is essential for the development of diabetic nephropathy in mice. Diabetes. 2012;61:208–216. doi: 10.2337/db11-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Huang Y, Yang L, et al. Uninephrectomy of diabetic OVE26 mice greatly accelerates albuminuria, fibrosis, inflammatory cell infiltration and changes in gene expression. Nephron Exp Nephrol. 2011;119:e21–e32. doi: 10.1159/000327586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi J, Hayden MR, Sowers JR, et al. Nebivolol attenuates redox-sensitive glomerular and tubular mediated proteinuria in obese rats. Endocrinology. 2011;152:659–668. doi: 10.1210/en.2010-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menini S, Iacobini C, Oddi G, et al. Increased glomerular cell (podocyte) apoptosis in rats with streptozotocin-induced diabetes mellitus: role in the development of diabetic glomerular disease. Diabetologia. 2007;50:2591–2599. doi: 10.1007/s00125-007-0821-y. [DOI] [PubMed] [Google Scholar]
- Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev. 2008;4:39–45. doi: 10.2174/157339908783502370. [DOI] [PubMed] [Google Scholar]
- Zimpelmann J, Burns KD. Angiotensin-(1-7) activates growth-stimulatory pathways in human mesangial cells. Am J Physiol Renal Physiol. 2009;296:F337–F346. doi: 10.1152/ajprenal.90437.2008. [DOI] [PubMed] [Google Scholar]
- Moon JY, Tanimoto M, Gohda T, et al. Attenuating effect of angiotensin-(1-7) on angiotensin II-mediated NAD(P)H oxidase activation in type 2 diabetic nephropathy of KK-A(y)/Ta mice. Am J Physiol Renal Physiol. 2011;300:F1271–F1282. doi: 10.1152/ajprenal.00065.2010. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Asker N, Gerhardt H, et al. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilauro M, Zimpelmann J, Robertson SJ, et al. Effect of ACE2 and angiotensin-(1-7) in a mouse model of early chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1523–F1532. doi: 10.1152/ajprenal.00426.2009. [DOI] [PubMed] [Google Scholar]
- Yu D, Petermann A, Kunter U, et al. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol. 2005;16:1733–1741. doi: 10.1681/ASN.2005020159. [DOI] [PubMed] [Google Scholar]
- Joyner J, Neves LAA, Granger JP, et al. Temporal-spatial expression of ANG-(1-7) and angiotensin-converting enzyme 2 in the kidney of normal and hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:169–177. doi: 10.1152/ajpregu.00387.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.