Abstract
Objective:
It is well documented that there is wide variation in the response of serum 25-hydroxyvitamin D [25(OH)D] to a given dose of vitamin D supplementation. Understanding factors affecting the response variation is important for identifying subjects who are susceptible to vitamin D deficiency or toxicity. This study aimed to evaluate potential predictors for vitamin D response variation.
Design and Participants:
A total of 1179 non-Hispanic white postmenopausal women were enrolled into a 4-yr calcium and vitamin D (1100 IU/d) clinical trial. Among them, serum 25(OH)D level of 1063 subjects were measured at both baseline and after 12 months treatment. Vitamin D response was computed for these 1063 subjects as the difference in levels of serum 25(OH)D concentration at the end of a 12-month vitamin D treatment compared with baseline. Stepwise linear regression was used to identify predictors of vitamin D response variation.
Results:
Increase in vitamin D intake, baseline serum 25(OH)D level, baseline blood collection season, baseline serum calcium level, and baseline body mass index were predictors of vitamin D response variation. These five factors explained 46.8% of the vitamin D response variation in the 1063 subjects. The first three factors [increase in vitamin D intake, baseline serum 25(OH)D level, baseline blood collection season] remained as predictors in the 392 subjects with trial vitamin D supplementation. For the first time, our study indicated that season is an important prediction factor for vitamin D response variation. Subjects who started vitamin D treatment in a cold season (autumn and winter) achieved a significantly higher serum 25(OH)D increase than those started in a hot season (summer) (P < 0.001).
Conclusion:
Our study suggests that the increase in vitamin D supplementation, baseline serum 25(OH)D level, and the season when initiating the vitamin D supplementation can partially predict vitamin D response variation in non-Hispanic postmenopausal women.
Serum 25-hydroxyvitamin D [25(OH)D] concentration is the best biomarker for assessing vitamin D status (1, 2). Although there is a debate regarding the cutoff line for an optimal serum 25(OH)D level for health outcomes, the importance of achieving and maintaining an optimal serum 25(OH)D level for overall health is well established.
Vitamin D supplementation is a simple, inexpensive, and effective strategy to achieve an optimal serum 25(OH)D level. However, the change in serum 25(OH)D levels in response to a given dose of vitamin D supplementation varies widely from person to person (3, 4). Because of the wide interindividual response variation, it is obvious that a one-size-fits-all approach will not work for vitamin D supplementation. Identifying factors accounting for the wide response variation is important for physicians to individualize vitamin D supplementation.
Few studies have been conducted to identify factors responsible for serum 25(OH)D variation in response to vitamin D supplementation. These studies suggest that body composition (5), genetic variants of the vitamin D binding protein (6), and the ratio of serum 24,25-dihydroxyvitamin D to 25(OH)D (7) may contribute to variation in serum 25(OH)D levels in response to vitamin D supplementation. However, these factors have not been confirmed in large-sample-size populations. In addition, most of the studies did not consider the season when subjects joined in a vitamin D clinical trial. This may confound the findings. This study aimed to identify environmental factors that would impact the efficacy of vitamin D supplementation in a large vitamin D intervention trial.
Materials and Methods
Subjects
Subjects come from a completed 4-yr, population-based, randomized, placebo-controlled, double-blinded vitamin D clinical trial (8). It was designed to investigate the effects of a calcium and vitamin D intervention on preventing fracture. The study enrolled 1179 community-dwelling postmenopausal women aged older than 55 yr from the rural area of east Nebraska centered at latitude 41.4° N. The study began in May 2000 and completed follow-up in 2005. The blinding of the study has been broken. Participants are non-Hispanic, white, postmenopausal women and generally healthy. Exclusion criteria included any chronic kidney diseases and Paget's or other metabolic bone diseases. Women with a history of cancer were included only if they had been cancer free for 10 yr or longer. All subjects provided written informed consent. The Institutional Review Board at Creighton University (Omaha, NE) approved the project.
Subjects were randomly assigned to receive 1400 mg/d supplemental calcium alone (Ca-only group), supplemental calcium (1400 mg/d) plus 1100 IU/d vitamin D3 (Ca+D group), or double placebos (placebo group). As reported (8), there was no significant difference in any of the baseline characteristics [age, weight, height, calcium supplement, body mass index (BMI), and baseline serum 25(OH)D levels] in the three groups. From the 1179 subjects, we selected 1063 subjects in whom were measured their serum 25(OH)D level at both baseline and the 12-month visit.
Clinical measurement
Blood for this analysis was drawn at baseline and again at 12 months. It was collected after a 3-h fast, and participants were asked not to take vitamin or mineral supplements that morning. The blood collection dates were transformed into four seasons (March through May, spring; June through August, summer, hot season; September through November, autumn; December through February, winter, cold season).
Serum 25(OH)D concentration was measured by RIA (Nichols/Quest Diagnostics, San Clemente, CA). It was the combination of 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3. All biochemical analyses were completed in a single laboratory that participates in the Quality Assurance Program for Vitamin D. The intraassay coefficient of variation (CV) was 5.1% and the interassay CV was 7.9%.
Other important variables that may influence variation in serum 25(OH)D such as age, weight, height, total body fat, total body lean, smoking habit, total calcium supplement, serum calcium levels, blood collection dates, adherence to the trial vitamin D supplementation, and self-selected vitamin D supplementation were collected at the baseline and at the end of the study. BMI was defined as weight (kilograms) divided by height squared (square meters). Compliance with trial vitamin D supplementation was assessed at 6-month intervals by bottle weight and mean adherence (defined as taking ≥ 80% of assigned doses) was 86% (8).
The primary phenotype for the study was vitamin D response variation. It was computed as:
where C12-month and Cbaseline were measurements of serum 25(OH)D concentration at the end of a 12-month vitamin D supplementation and at baseline, respectively.
The trial vitamin D supplement was calculated as per-protocol supplement dose adjusted for individual compliance rates.
In the study, in addition to the administrated trial vitamin D, we allowed subjects to take their self-selected vitamin D supplement. We named the self-selected vitamin D supplementation outside the trial as outside vitamin D supplement. We measured outside supplementation at baseline and at the end of the study. Some subjects changed their outside supplement dose during this time. We used the average amount of supplement (the outside vitamin D at baseline and the end of the study) as the outside vitamin D supplement intake at the end of 12-month treatment. The total vitamin D supplement intake is the sum of outside vitamin D supplement intake and the trial vitamin D supplement. The increase in vitamin D intake was the difference in total vitamin D supplement intake between baseline and the end of 12-months treatment.
Statistical analyses
Statistical analyses were performed with SPSS version 16.0 (SPSS, Chicago, IL). Unless otherwise stated, all tests were done two tailed, and P < 0.05 was considered statistically significant.
Descriptive analyses were conducted for serum 25(OH)D status as well as other major factors. One-way ANOVA was performed to compare the mean value of baseline serum 25(OH)D, the 12-month serum 25(OH)D, and the 12-month increase in serum 25(OH)D between subjects with different blood collection seasons. One-sample Kolmogorov-Smirnov tests were conducted for testing the normal distribution of traits.
Stepwise linear regressions were performed to detect potential predictors for vitamin D response variation in the 1063 subjects and the 392 subjects in the Ca+D subgroup. Potential predictors included age, weight variance inflation factor (VIF), height, BMI, total body fat, total body lean, blood collection season at baseline, smoking habit, trial vitamin D, total amount of calcium supplement, baseline serum 25(OH)D levels, and serum calcium levels. Multicollinearity of regressions was tested, and less than 5.0 was considered noncollinearity. Pearson correlations and partial correlations were used to confirm the association between the traits and vitamin D response variation.
Results
Among 1179 samples, 1063 subjects had their serum 25(OH)D level measured at both baseline and the 12-month visit. Descriptive information for the 1063 subjects is shown in Table 1. The range of baseline serum 25(OH)D level ranged from 23.6 to 144.6 nmol/liter, with an average of 72.0 nmol/liter. In the 392 subjects from the Ca+D group, the mean serum 25(OH)D level increased from 72.1 ± 19.7 to 96.0 ± 21.4 nmol/liter.
Table 1.
Baseline characteristics of 1063 subjects for the data analyses
| Total (n = 1063) | |
|---|---|
| Serum 25(OH)D (nmol/liter) | 72.0 ± 20.2 |
| Age (yr) | 67.6 ± 7.3 |
| Height (m) | 1.62 ± 0.06 |
| Weight (kg) | 76.7 ± 15.6 |
| BMI (kg/m2) | 29.1 ± 5.8 |
| Serum calcium (mg/dL) | 9.5 ± 0.4 |
| Amount of vitamin D intake (IU/d) | 327 ± 137 |
Wide variation in serum 25(OH)D levels in response to trial vitamin D supplementation
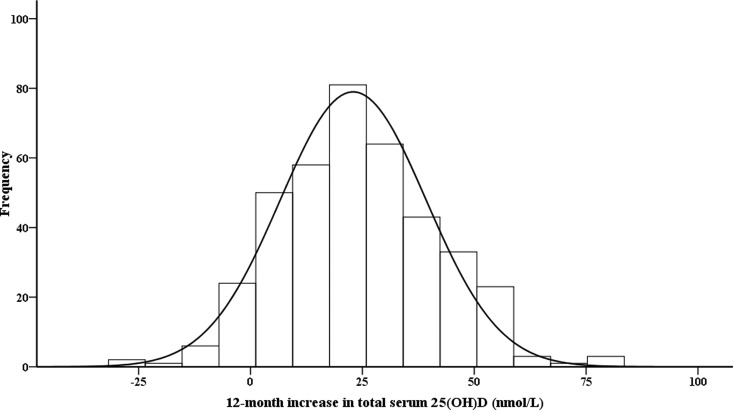
Consistent with other reported vitamin D supplementation studies (5, 9–13), a wide variation of serum 25(OH)D concentration in response to trial vitamin D supplementation was observed in the 392 subjects in the Ca+D group. The distribution of the 12-month increase of serum 25(OH)D was shown in Fig. 1. As was visually evident, the distribution is normal (Kolmogorov-Smirnov test, P = 0.366). Of greater interest was the great spread of response variance. The mean 12-month total serum 25(OH)D increase was 23.9 nmol/liter, the sd was 17.8 nmol/liter, the range was from −31.4 to 80.2 nmol/liter, and the CV was as high as 74.4%.
Fig. 1.
Frequency distribution of 12-month increase in serum 25(OH)D levels in 392 subjects in the calcium and vitamin D supplement (Ca+D) group. The 12-month increase in serum 25(OH)D level is the raw data, unadjusted by increase in vitamin D supplement.
Identifying predicators for vitamin D response variation
We used stepwise linear regression model to identify predictors for vitamin D response variation among the 1063 subjects. Table 2 shows the significant variables entered in the model and their contributions to the model. Increase in vitamin D intake entered first and accounted for the largest portion of the response variation (37.5%). Baseline serum 25(OH)D entered next, and it was negatively associated with the increase of 12-month serum 25(OH)D. Baseline blood collection season, serum calcium level, and BMI were entered later. In total, these five factors explained 46.8% to the overall model.
Table 2.
Stepwise multiple regression analysis for assessing predicators of vitamin response variation in 1063 subjects
| Unstandardized coefficients |
R2 change | P value | ||
|---|---|---|---|---|
| B | se | |||
| (Constant) | −14.676 | 11.260 | 0.193 | |
| Increase in vitamin D intake | 0.023 | 0.001 | 0.375 | <0.001 |
| Baseline serum 25(OH)D | −0.282 | 0.023 | 0.076 | <0.001 |
| Baseline blood collection season | 1.701 | 0.419 | 0.008 | <0.001 |
| Baseline serum calcium | 3.930 | 1.135 | 0.006 | 0.001 |
| Baseline BMI | −0.214 | 0.084 | 0.003 | 0.011 |
Multicollinearity diagnosis shows all traits have VIF less than 1.1, indicating no existence of significant collinearity. P value of regression is less than 0.001. The excluded variables are: baseline age, weight, height, total body fat mass, total body lean mass, percentage fat mass, smoking habit, and total calcium supplement.
We further conducted regression analysis in the 392 subjects with trial vitamin D supplementation. Baseline total serum 25(OH)D levels, trial vitamin D supplement, and baseline blood collection season entered in the regression model. The three variables explained 24.7% of the total variation. Baseline serum calcium level and BMI were not selected by the regression model (Table 3).
Table 3.
Regression analysis for investigating predicators for vitamin D response variation in 392 subjects in the calcium and vitamin D supplement (Ca+D) group
| Unstandardized coefficients |
R2 change | P value | ||
|---|---|---|---|---|
| B | se | |||
| (Constant) | 23.650 | 4.362 | <0.001 | |
| Baseline serum 25(OH)D | −0.341 | 0.041 | 0.125 | <0.001 |
| Trial vitamin D supplement | 0.021 | 0.003 | 0.106 | <0.001 |
| Baseline blood collection season | 2.172 | 0.749 | 0.016 | 0.004 |
Multicollinearity diagnosis shows all traits have VIF less than 1.1, indicating no existence of significant collinearity. Excluded variants are baseline age, weight, height, BMI, total body fat, total body lean, percentage fat mass, smoking habit, total calcium supplement, and serum calcium levels.
Seasonal effect on baseline serum 25(OH)D and vitamin D response variation
The regression results in the 1063 subjects and in the subgroup of 392 Ca+D subjects both showed that baseline blood collection season was an independent factor predicting vitamin D response variation. The results were also confirmed by partial correlation by controlling an increase in vitamin D supplement, baseline serum 25(OH)D, BMI, and baseline serum calcium levels in both the 1063 subjects (r = 0.124, P < 0.001) and in the 392 subgroup (r = 0.158, P = 0.002).
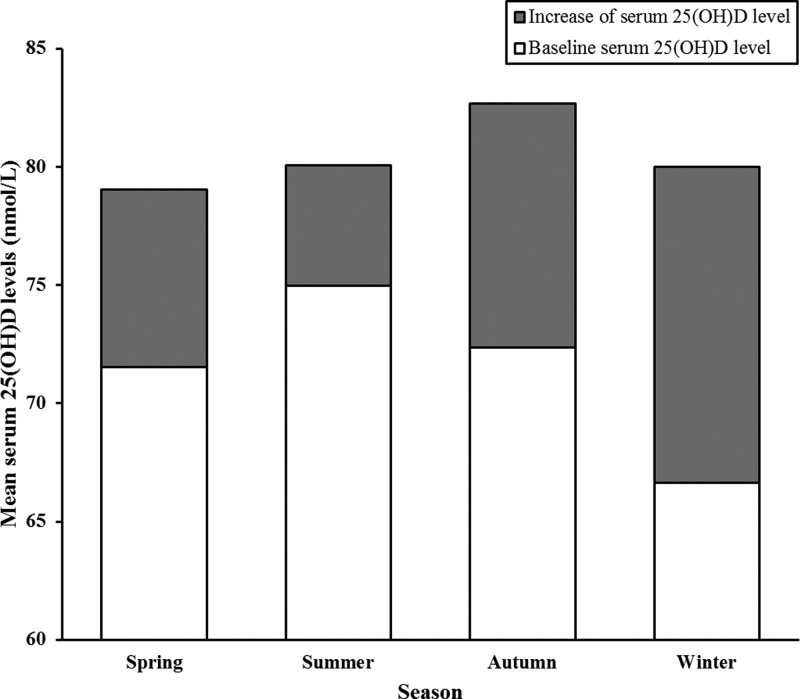
Figure 2 and Table 4 presented the average baseline serum 25(OH)D, the increase in serum 25(OH)D, and the serum 25(OH)D levels at the 12-month visit (baseline plus increase) among the 1063 subjects, distributed along with the four blood collection seasons. A significant difference of baseline serum 25(OH)D levels is found between summer and winter (P < 0.001) (Table 4). Consist with previous studies (14, 15), the baseline serum 25(OH)D levels were significantly higher in subjects enrolled in summer than in winter (P < 0.001). However, after a 12-month vitamin D supplementation, there was no significant difference in serum 25(OH)D levels among subjects enrolled in different seasons (P = 0.374) (Table 4). That is, the subjects who start vitamin D treatment in winter achieve a significantly greater serum 25(OH)D increase than those who start in the summer (P < 0.001).
Fig. 2.
Baseline serum 25(OH)D and 12-month increase in serum 25(OH)D in the four seasons among 1063 subjects. White histogram is the average baseline serum 25(OH)D level; gray histogram is the average 12-month increase in serum 25(OH)D level; white histogram plus gray histogram is the average serum 25(OH)D level at the 12-month visit. Baseline blood collection seasons were defined as follows: spring, March through May; summer, June through August; autumn, September through November; winter, December through February.
Table 4.
Seasonal effects on serum 25(OH)D and vitamin D response
| Season when vitamin D supplementation starts | Spring (1) | Summer (2) | Autumn (3) | Winter (4) | P value | Significant difference |
|---|---|---|---|---|---|---|
| Baseline serum 25(OH)D (nmol/liter) | 71.53 ± 20.34 | 74.98 ± 19.93 | 72.37 ± 19.08 | 66.62 ± 20.97 | <0.001 | 2 > 4 |
| Serum 25(OH)D at 12-month visit (nmol/liter) | 79.03 ± 23.40 | 80.05 ± 23.54 | 82.66 ± 24.64 | 79.99 ± 24.27 | 0.374 | |
| Increase in serum 25(OH)D level (nmol/liter) | 7.49 ± 18.37 | 5.07 ± 19.25 | 10.29 ± 19.73 | 13.37 ± 20.34 | <0.001 | 1 < 4, 2 < 3, 2 < 4 |
Discussion
This large vitamin D clinical trial identified that an increase in vitamin D, baseline serum 25(OH)D level, and baseline blood collection season were the major predictors of vitamin D response variation. The baseline blood collection season was the season that an individual started her vitamin D clinical trial. To the best of our knowledge, this is the first study reporting that the season when vitamin D supplementation starts, an important factor in predicting vitamin D response variation. A start in the cold season is more sensitive to vitamin D supplementation than a start in the hot season.
Seasonal impact on baseline serum 25(OH)D is well documented. Consistent with other studies (14, 15), our study confirmed that serum 25(OH)D levels were lower in the winter than in summer because synthesis of vitamin D was affected by the amount of sun exposure and time of year.
The seasonal impact on vitamin D response variation has not been reported. Our study indicates that subjects starting vitamin D treatment during a cold season (autumn and winter) would gain a greater 12-month increase in 25(OH)D compared with those who started during a summer season, although treatment time for each one was a whole year. After 1100 IU/d vitamin D supplementation, the seasonal effect was removed. There is no seasonal difference of serum 25(OH)D levels at the 12-month visit (Fig. 2 and Table 4), which indicate that adequate vitamin D supplementation can remove the seasonal differences. The feedback mechanism may explain part of the seasonal effect. Low serum 25(OH)D levels in a cold season would give a subject more room to increase serum 25(OH)D levels than those who started in summer. However, the association of blood collection season and vitamin D response variation remained significant, even after adjusting for baseline serum 25(OH)D, BMI, and other significant factors. The mechanism underlying the association between blood collection season and vitamin D response variation is unknown but hints of an endogenous mechanism that protects against vitamin D intoxication during long-term sun exposure.
Many studies have documented a negative association between BMI and total serum 25(OH)D levels due to an expanded pool for storage of 25(OH)D in adipose tissue (16–19). However, the impact of BMI on dose response to vitamin D supplementation is not well studied. In the study by Blum et al. (5), 257 healthy men and women older than 65 yr were randomly assigned to treatment with either supplemental vitamin D and calcium or placebo. After adjusting for baseline 25(OH)D, season, and gender, baseline BMI was inversely correlated with a 12-month increase in 25(OH)D. However, some studies found no correlation between BMI and change in 25(OH)D levels (18, 20). We also found inconsistent results in this study. After adjusting for trial vitamin D supplement, baseline serum 25(OH)D, and blood collection season, BMI was significantly inversely associated with 12-month increase in serum 25(OH)D in subjects with a wide range of increases in vitamin D supplement (n = 1063). However, the association was not identified among subjects with vitamin D supplement subgroups (n = 392). Given the tiny contribution of BMI to vitamin D response in large samples, the contribution of BMI to vitamin D response variation may be undetectable in small samples.
Our study showed an inverse relationship between baseline serum 25(OH)D and 12-month increase in serum 25(OH)D levels. This finding is consistent with previous studies. Nelson et al. (18) studied 86 subjects by giving them placebo or 800 IU vitamin D supplement per day and observed a strong response to vitamin D supplements in participants with lower baseline serum 25(OH)D levels. In the study by Trang et al. (21), 72 subjects who received 4000 IU/d vitamin D2 or vitamin D3 showed an increase in serum 25(OH)D, which was also negatively associated with baseline serum 25(OH)D. Interestingly, such a phenomenon was observed in not only vitamin D supplement trials but also UV irradiation treatment. By giving UV irradiation, both serum 25(OH)D and 1,25-dihydroxyvitamin D levels were significantly increased and were also negatively correlated with baseline concentrations of 25(OH)D (22, 23). Feedback inhibition of 25(OH)D production may account for reversal in the correlation.
Heaney et al. (1) demonstrated that the rising of serum 25(OH)D level was biphasic. From values close to zero to about 100 nmol/liter, serum 25(OH)D levels increased rapidly. The increment began to slow down when serum 25(OH)D level achieved at 80–100 nmol/liter. These findings indicated that less than 100 nmol/liter, serum 25(OH)D level would respond more sensitively to supplementation. This might explain our results regarding the relationship of vitamin D response, baseline serum 25(OH)D, and blood collection season. For subjects with low baseline serum 25(OH)D or in cold season [subjects who generally had low baseline serum 25(OH)D], there would be more space to increase their serum 25(OH)D to approximately 100 nmol/liter. They showed significant higher response to vitamin D supplementation. It is a different case for subjects with higher baseline serum 25(OH)D or start vitamin D supplementation in hot season. These may explain why subjects who started a vitamin D supplementation trial during a cold season and with low baseline serum 25(OH)D would be more sensitive to vitamin D supplementation.
A recent study shows that genetic variants of vitamin D binding protein and the ratio of serum 24,25-dihydroxyvitamin D to 25(OH)D are associated with vitamin D response variation (6, 7). In addition, large genome-wide association studies (24, 25) indicated that genetic variants of vitamin D receptor (VDR), 7-dehydrocholesterol reductase (DHCR7), and cytochrome P450 2R1 (CYP2R1) genes are associated with baseline serum 25(OH)D variation. Due to the lack of data, it is unknown whether these genes are important for vitamin D response variation in our sample.
The advantage of this study lies in its large sample size compared with previous studies. In addition, using 12-month vitamin D supplementation data reduced the confounding effect of season. Several limitations existed in the study. First, we did not have data for dietary vitamin D intake and sun exposure. Second, the quality of self-reported vitamin D supplementation data is not as high as the trial vitamin D supplementation. Third, our research was limited to non-Hispanic postmenopausal women. The results from the study may not be generalized to other populations.
In summary, our study suggests that increase in vitamin D intake, baseline serum 25(OH)D level, and baseline blood collection season are the three most important predictors for serum 25(OH)D levels in response to vitamin D supplementation in non-Hispanic white postmenopausal women. For a given dose of vitamin D supplement, subjects who started a vitamin D supplementation trial during a cold season and with low baseline serum 25(OH)D would be more sensitive to vitamin D supplementation, compared with subjects who started during a hot season and with high baseline serum 25(OH)D levels.
Acknowledgments
This work was partially supported by grants from the Cancer and Smoking Disease Research Bone Biology Program; the Nebraska Tobacco Settlement Biomedical Research Development Award; Grant 3R01CA129488-01A2S2 from the National Institutes of Health); and Grant LB595 from the State of Nebraska Cancer and Smoking Disease Research Program. Y.Z., X.X., A.Y., and L.-J.Z. were partially supported by a start-up fund from Tulane University (to L.-J.Z.). H.-W.D. is partially supported by Grants R01AG026564, R01AR050496, R01AR057049, and R03TW008221 from the National Institutes of Health; Grant P50 AR055081 from the Specialized Center of Research supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and from the Office of Research on Women's Health; and an Edward G. Schlieder Endowment from Tulane University.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- Body mass index
- CV
- coefficient of variation
- 25(OH)D
- 25-hydroxyvitamin D
- VIF
- variance inflation factor.
References
- 1. Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW. 2008. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr 87:1738–1742 [DOI] [PubMed] [Google Scholar]
- 2. Hollis BW. 2005. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr 135:317–322 [DOI] [PubMed] [Google Scholar]
- 3. Armas LA, Ilahi M, Heaney RP. 2007. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab 92:2130–2135 (Letter to the Editor) [DOI] [PubMed] [Google Scholar]
- 4. Aloia JF, Patel M, Dimaano R, Li-Ng M, Talwar SA, Mikhail M, Pollack S, Yeh JK. 2008. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr 87:1952–1958 [DOI] [PubMed] [Google Scholar]
- 5. Blum M, Dallal GE, Dawson-Hughes B. 2008. Body size and serum 25 hydroxy vitamin D response to oral supplements in healthy older adults. J Am Coll Nutr 27:274–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fu L, Yun F, Oczak M, Wong BY, Vieth R, Cole DE. 2009. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin Biochem 42:1174–1177 [DOI] [PubMed] [Google Scholar]
- 7. Wagner D, Hanwell HE, Schnabl K, Yazdanpanah M, Kimball S, Fu L, Sidhom G, Rousseau D, Cole DE, Vieth R. 2011. The ratio of serum 24,25-dihydroxyvitamin D(3) to 25-hydroxyvitamin D(3) is predictive of 25-hydroxyvitamin D(3) response to vitamin D(3) supplementation. J Steroid Biochem Mol Biol 126:72–77 [DOI] [PubMed] [Google Scholar]
- 8. Lappe JM, Davies KM, Travers-Gustafson D, Heaney RP. 2006. Vitamin D status in a rural postmenopausal female population. J Am Coll Nutr 25:395–402 [DOI] [PubMed] [Google Scholar]
- 9. Cashman KD, Hill TR, Lucey AJ, Taylor N, Seamans KM, Muldowney S, Fitzgerald AP, Flynn A, Barnes MS, Horigan G, Bonham MP, Duffy EM, Strain JJ, Wallace JM, Kiely M. 2008. Estimation of the dietary requirement for vitamin D in healthy adults. Am J Clin Nutr 88:1535–1542 [DOI] [PubMed] [Google Scholar]
- 10. Cashman KD, Wallace JM, Horigan G, Hill TR, Barnes MS, Lucey AJ, Bonham MP, Taylor N, Duffy EM, Seamans K, Muldowney S, Fitzgerald AP, Flynn A, Strain JJ, Kiely M. 2009. Estimation of the dietary requirement for vitamin D in free-living adults >=64 y of age. Am J Clin Nutr 89:1366–1374 [DOI] [PubMed] [Google Scholar]
- 11. Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O'Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, et al. 2010. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 376:180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Genaro PS, Pereira GA, Pinheiro MM, Szejnfeld VL, Martini LA. 2007. Relationship between nutrient intake and vitamin D status in osteoporotic women. Int J Vitam Nutr Res 77:376–381 [DOI] [PubMed] [Google Scholar]
- 13. Ilahi M, Armas LA, Heaney RP. 2008. Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr 87:688–691 [DOI] [PubMed] [Google Scholar]
- 14. Rajakumar K, de las Heras J, Chen TC, Lee S, Holick MF, Arslanian SA. 2011. Vitamin D status, adiposity, and lipids in black American and Caucasian children. J Clin Endocrinol Metab 96:1560–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, Kim KJ, Rhee Y, Lim SK. 2011. Vitamin D insufficiency in Korea—a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES), 2008. J Clin Endocrinol Metab 96:643–651 [DOI] [PubMed] [Google Scholar]
- 16. Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, Seidell JC, Lips P. 2005. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab 90:4119–4123 [DOI] [PubMed] [Google Scholar]
- 17. Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. 2002. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: Third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 76:187–192 [DOI] [PubMed] [Google Scholar]
- 18. Nelson ML, Blum JM, Hollis BW, Rosen C, Sullivan SS. 2009. Supplements of 20 microg/d cholecalciferol optimized serum 25-hydroxyvitamin D concentrations in 80% of premenopausal women in winter. J Nutr 139:540–546 [DOI] [PubMed] [Google Scholar]
- 19. Jorde R, Sneve M, Emaus N, Figenschau Y, Grimnes G. 2010. Cross-sectional and longitudinal relation between serum 25-hydroxyvitamin D and body mass index: the Tromso study. Eur J Nutr 49:401–407 [DOI] [PubMed] [Google Scholar]
- 20. Canto-Costa MH, Kunii I, Hauache OM. 2006. Body fat and cholecalciferol supplementation in elderly homebound individuals. Braz J Med Biol Res 39:91–98 [DOI] [PubMed] [Google Scholar]
- 21. Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. 1998. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr 68:854–858 [DOI] [PubMed] [Google Scholar]
- 22. Mawer EB, Berry JL, Sommer-Tsilenis E, Beykirch W, Kuhlwein A, Rohde BT. 1984. Ultraviolet irradiation increases serum 1,25-dihydroxyvitamin D in vitamin-D-replete adults. Miner Electrolyte Metab 10:117–121 [PubMed] [Google Scholar]
- 23. Snell AP, MacLennan WJ, Hamilton JC. 1978. Ultra-violet irradiation and 25-hydroxy-vitamin D levels in sick old people. Age Ageing 7:225–228 [DOI] [PubMed] [Google Scholar]
- 24. Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB, Li Q, Weinstein SJ, Purdue M, Virtamo J, Horst R, Wheeler W, Chanock S, Hunter DJ, Hayes RB, Kraft P, Albanes D. 2010. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet 19:2739–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]