Abstract
Context:
Kisspeptin is the most powerful known stimulus of GnRH-induced LH secretion across mammalian species. However, the effects of kisspeptin are just being explored, and the dynamics of kisspeptin responsiveness across the menstrual cycle are incompletely understood.
Objective:
The objective of the study was to characterize the effects of kisspeptin on GnRH secretion in healthy women in different phases of the menstrual cycle.
Participants and Intervention:
Ten women in the early follicular phase, three women in the late follicular (preovulatory) phase, and 14 women in the midluteal phase received a bolus of kisspeptin 112–121 0.24 nmol/kg iv. An additional four women in the early to midfollicular phase received kisspeptin 112–121 0.72 nmol/kg iv.
Results:
The response to kisspeptin varied depending on the phase of the menstrual cycle. LH pulses were observed immediately after kisspeptin administration in all luteal and preovulatory women. However, only half the women in the early follicular phase had unambiguous kisspeptin responses. Increasing the kisspeptin dose did not increase the LH response in early to midfollicular phase women. Kisspeptin did not appear to reset the GnRH pulse generator in women as it does in men.
Conclusions:
Differences in responses to exogenous kisspeptin across the menstrual cycle suggest that kisspeptin tone is higher in the early follicular phase compared with other cycle phases. The mechanisms that determine the timing of GnRH pulse generation in men and women appear to be distinct.
Pushed into the spotlight in 2003 by the discovery of inactivating mutations of the kisspeptin receptor in patients with absent pubertal development (1, 2), kisspeptin is now accepted as a critical regulator of sexual maturation across mammalian species. Kisspeptin is a powerful stimulus for GnRH-induced gonadotropin release (3, 4), and just as exogenous GnRH has been used for decades to understand the control of pituitary gonadotropin secretion, the use of exogenous kisspeptin allows investigation of GnRH neuronal physiology. There is a growing literature regarding the administration of kisspeptin to humans (5–12) using different isoforms [kisspeptin 68–121 (54-mer), kisspeptin 112–121 (decapeptide)]; methods of administration (iv, sc); types of exposure (single bolus, continuous); chronicity of administration (single bolus, multiple doses); and study populations (healthy volunteers, patients with reproductive disorders).
This rapidly evolving repertoire of tools and techniques has contributed to an increasingly nuanced understanding of how kisspeptin stimulates the reproductive endocrine cascade. For example, by analyzing the morphology of kisspeptin-induced, GnRH-induced LH pulses in healthy men, our group deduced that a single bolus of kisspeptin 112–121 (0.24 nmol/kg, iv) induces 17 min of GnRH secretion (9), a duration that is strikingly concordant with that of kisspeptin-induced GnRH neuronal activation in rodent ex vivo experiments (13–17). In addition, by examining the timing of endogenous pulses after kisspeptin administration, we found that kisspeptin resets the GnRH pulse generator, such that the kisspeptin-induced pulse, rather than being an extra secretory event wedged between endogenous LH pulses, is somehow detected by the GnRH neuronal network and used to recalculate the timing of the subsequent endogenous pulse (9).
It is unknown whether similar responses occur in women and how these responses are influenced by the hormonal changes that occur across the menstrual cycle. Using the same protocol as our prior study in men, we have now examined the responses to kisspeptin 112–121 in healthy women in the early follicular phase, the preovulatory period, and the midluteal phase. These studies have allowed us to determine the effects of kisspeptin on endogenous GnRH pulse generation and to compare these effects between the different phases of the menstrual cycle and between women and men. Furthermore, because the same dose of kisspeptin was used in all groups, comparison of the size of endogenous pulses with that of pulses elicited by this fixed dose of kisspeptin could be used to infer the relative amounts of kisspeptin being secreted, i.e. the kisspeptin tone, across the menstrual cycle.
Materials and Methods
Subjects
Healthy adult women who received kisspeptin met the following inclusion criteria: 21–40 yr old; self-reported history of normal timing and pace of puberty; regular menstrual cycles of 25–35 d duration with no more than 5 d variability in cycle length; no use of prescription medications (including hormonal contraception) for at least 2 months before the study; body mass index 18.5–30 kg/m2; blood pressure less than 140/90 mm Hg; normal physical examination; normal white blood cell and platelet counts; hemoglobin 11 g/dl or greater in the follicular phase of the menstrual cycle or 12 g/dl or greater in other phases; no elevation of creatinine, blood urea nitrogen, or prolactin; aspartate aminotransferase and alanine aminotransferase no more than twice the upper limit of the reference range; normal TSH; and FSH, LH, and estradiol normal for the phase of the menstrual cycle. Exclusion criteria were the presence of a chronic medical condition, a history of a food or drug allergy, consumption of more than 10 alcoholic drinks per week, and self-reported use of illicit drugs.
Women in the early follicular phase who received the 0.24 nmol/kg dose of kisspeptin 112–121 were studied on d 2–5 of the menstrual cycle. Early to midfollicular-phase women who received the 0.72 nmol/kg dose were studied on d 4–7 of the cycle. Women in the preovulatory period were studied 11–16 d before the start of the next menstrual cycle, and women in the midluteal phase were studied 5–10 d before the start of the next menstrual cycle (estimated prospectively and confirmed retrospectively). Measurements of progesterone were consistent with the expected phase of the menstrual cycle for all subjects.
All protocols were approved by the Institutional Review Board of the Massachusetts General Hospital (MGH) and the U.S. Food and Drug Administration, and all subjects gave written informed consent before participation in these studies. The study was registered with www.ClinicalTrials.gov (NCT00914823).
Materials
Kisspeptin 112–121 and GnRH were synthesized using good manufacturing practices by NeoMPS (PolyPeptide Laboratories, San Diego, CA) under contract to the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Resuspended aliquots underwent additional tests for sterility, pyrogenicity, purity, and concentration as previously described (9).
Kisspeptin administration and frequent blood sampling
Subjects were admitted to the Harvard Catalyst Clinical Research Center of MGH. Blood sampling was performed every 10 min for 6 h before kisspeptin administration to establish baseline secretory patterns. Kisspeptin 112–121 was given as a single iv bolus at a dose of 0.24 nmol/kg (0.313 μg/kg) at the 6-h time point to 10 early follicular-phase women and all preovulatory and midluteal-phase women. Four women in the early to midfollicular-phase underwent the same protocol but received a higher dose of kisspeptin 0.72 nmol/kg (0.94 μg/kg). Blood sampling continued for 6 h after kisspeptin administration to chart the response to kisspeptin.
Laboratory assays
Measurements of LH for each sample, FSH and estradiol on 2-h pools, and progesterone on the first study sample were performed by the MGH Clinical Laboratory Research Core as previously described (9).
Pulse identification and calculated pulse characteristics
LH pulses were identified using a validated modification of the method of Santen and Bardin (18, 19) augmented by a deconvolution algorithm as previously described (9). Pulse amplitude was calculated as the difference between the nadir and peak of the pulse. An amplitude of zero was assigned for three subjects in whom LH decreased after kisspeptin. Results of statistical analysis did not differ if data from these three subjects were omitted. Area under the curve (AUC) for each pulse was calculated as previously described (20). For these calculations, the time of kisspeptin administration was always considered the start of a pulse, even if no pulse was present. This produced an AUC close to zero in subjects in whom kisspeptin did not elicit an immediate LH pulse. Subjects without an immediate pulse after kisspeptin were omitted from analysis of time from nadir to peak.
Statistical analysis
Data are presented in text and figures as mean ± sem. To examine the effects of kisspeptin on GnRH-induced LH secretion, one-way, repeated-measures ANOVA was used to compare LH at time points in the 1 h before vs. the 1 h after kisspeptin administration, and Dunnett's post hoc analysis was used to compare all LH values with the LH level just before kisspeptin administration; because not all subjects had data for all time points, analysis was performed either after removing data from that subject or after removing data from that time point, with similar results. To further examine the effects of kisspeptin on GnRH-induced LH secretion, paired t tests were used to compare mean LH in the hour before vs. the hour after kisspeptin administration. The binomial probability was used to calculate the likelihood of pulses occurring immediately after kisspeptin to determine whether kisspeptin administration may have coincided with endogenous pulses by chance. Individual paired t tests were also used for each phase of the menstrual cycle to compare LH pulse characteristics such as amplitude, AUC, and time from nadir to peak of kisspeptin-induced pulses to the mean of the values for each individual's endogenous pulses before kisspeptin. An unpaired t test was used to compare the mean time from nadir to peak in the early follicular vs. midluteal phase.
To examine effects of kisspeptin on FSH and estradiol, repeated-measures, one-way ANOVA was used to compare measurements for 2-h pools after kisspeptin administration (i.e. the 6–8, 8–10, and 10–12 h pools) to the mean of the three pools before kisspeptin (i.e. the 0–2, 2–4, and 4–6 h pools). To examine the effects of kisspeptin on the timing of endogenous pulses, paired t tests were used to compare pulse intervals. For women who did not exhibit an endogenous pulse in the 6 h before or the 6 h after kisspeptin administration, a pulse interval of 360 min was assigned; time-to-event analysis was also performed and did not change the conclusions of analysis. Because endogenous pulses could either be concealed by or be mistaken for kisspeptin-induced pulses, the likelihood of this occurring was calculated as described in Supplementary Materials, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org; correction for these potential issues did not change the results of analysis. All tests were two tailed, and P < 0.05 was considered significant (all P values < 0.05 are given in the text). Statistical calculations were performed using GraphPad Prism (La Jolla, CA).
Results
Subject characteristics
Ten healthy adult women in the early follicular phase, three women in the preovulatory period, and 14 women in the midluteal phase of the menstrual cycle received a single iv bolus of 0.24 nmol/kg of human kisspeptin 112–121 in the context of frequent blood sampling to establish baseline secretory patterns as well as the response to kisspeptin. Subject characteristics are summarized in Table 1.
Table 1.
Subject characteristics at screening
| Characteristic | Early follicular (n = 10) | Early/midfollicular (higher dose, n = 4) | Preovulatory (n = 3) | Midluteal (n = 14) |
|---|---|---|---|---|
| Age (yr) | 25.9 (22–35) | 25.3 (22–35) | 25.0 (24–26) | 27.8 (21–38) |
| Menstrual cycle length (d) | 28.1 (25–30) | 29.0 (27–31) | 28.7 (28–29) | 28.4 (26–31) |
| Gravidity | 0.4 (0–4) | 0.3 (0–4) | 0.3 (0–1) | 0.3 (0–2) |
| Body mass index (kg/m2) | 24.0 (19.5–27.4) | 20.7 (19.9–27) | 21.2 (20.5–21.6) | 24.9 (19.7–29.6) |
Data are shown as mean (range).
Effects of kisspeptin in healthy women across the menstrual cycle
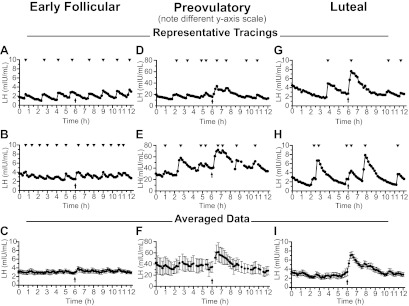
Figure 1 shows LH pulse profiles from representative women as well as data averaged across all women studied in each part of the menstrual cycle. Individual LH pulse profiles for all women are shown in Supplemental Figs. 1–4. Data from all groups are summarized in Table 2 along with previously published data in which the same dose of kisspeptin was administered to men (9).
Fig. 1.
Effects of kisspeptin on LH secretion. Representative patterns of LH secretion from two women each in the follicular (A and B), preovulatory (D and E), and luteal phases (G and H) of the menstrual cycle. Mean ± sem serum LH for all women in the early follicular (C), preovulatory (F), and luteal phases (I), aligned to the time of kisspeptin administration. Note the different y-axis in panels D–F. Arrows indicate time of kisspeptin administration; arrowheads indicate peaks of pulses.
Table 2.
Summary of responses to kisspeptin 112–121 0.24 nmol/kg, iv, × 1
| Women |
Men (n = 13) (9) | |||
|---|---|---|---|---|
| Early follicular (n = 10) | Preovulatory (n = 3) | Midluteal (n = 14) | ||
| Percentage exhibiting an LH pulse immediately after kisspeptin | 50% | 100% | 100% | 100% |
| LH pulse amplitude (mIU/ml) | ||||
| Endogenous | 1.2 ± 0.2 | 10.4 ± 2.4 | 3.0 ± 0.4 | 2.1 ± 0.3 |
| Kisspeptin-induced | 1.1 ± 0.3 | 28.6 ± 5.1a | 4.0 ± 0.8 | 5.0 ± 1.0a |
| LH pulse AUC (min/mIU · ml) | ||||
| Endogenous | 163 ± 41 | 1723 ± 578 | 285 ± 46 | 312 ± 52 |
| Kisspeptin-induced | 161 ± 83 | 4528 ± 1143a | 593 ± 58a | 684 ± 118a |
| Time from nadir to peak (min) | ||||
| Endogenous | 15.8 ± 0.8 | 19.2 ± 2.2 | 22.1 ± 2.0 | 22.8 ± 1.4 |
| Kisspeptin-induced | 22.9 ± 3.6a | 36.7 ± 3.3a | 24.3 ± 2.0 | 27.7 ± 1.7a |
| FSH on 2-h pools (mIU/ml) | ||||
| Endogenous | 5.8 ± 0.3 | 9.8 ± 1.9 | 2.7 ± 0.3 | 2.9 ± 0.4 |
| Kisspeptin-induced | 5.9 ± 0.4 | 10.7 ± 1.4 | 2.9 ± 0.4a | 3.3 ± 0.5a |
| Estradiol on 2-h pools (pg/ml) | ||||
| Endogenous | 35 ± 3 | 145 ± 36 | 117 ± 9 | ND |
| Kisspeptin-induced | 34 ± 3 | 128 ± 34 | 123 ± 9a | ND |
| Endogenous interpulse interval, A (min) | 71 ± 7 | 73 ± 9 | 213 ± 33 | 130 ± 8 |
| Interval between kisspeptin-induced pulse and previous endogenous pulse, B1 (min) | 60 ± 12 | 40 ± 15 | 155 ± 37 | 62 ± 14 |
| Interval between kisspeptin-induced pulse and next endogenous pulse, B2 (min) | 78 ± 19 | 107 ± 23 | 130 ± 19a | 141 ± 21b |
Data are shown as mean ± sem. ND, Not determined.
P < 0.05 compared with endogenous.
P < 0.05 compared with interval B1.
Responses in the early follicular phase
In the early follicular phase, LH increased significantly after kisspeptin administration (P = 0.01; Fig. 1C), with significant elevations at 20 and 30 min after kisspeptin compared with the time just before kisspeptin (3.83 ± 0.54 and 3.60 ± 0.48 vs. 2.88 ± 0.40 mIU/ml, P < 0.05). Furthermore, mean LH was significantly higher across the 1 h after kisspeptin administration than across the 1 h before kisspeptin administration (3.41 ± 0.47 vs. 2.93 ± 0.34 mIU/ml, P = 0.04).
Despite the significant increase in mean LH, kisspeptin induced immediate LH pulses in only five of 10 early follicular-phase women (Supplemental Fig. 1). The lack of a consistent LH response to kisspeptin in the early follicular phase raised the possibility that these LH pulses could have coincided with kisspeptin administration simply by chance. However, when the endogenous pulse frequency was examined in the 6 h before kisspeptin administration, a mean of five pulses per 6 h was found (range three to seven pulses per 6 h). With this mean pulse frequency of five pulses per 6 h, the probability of having five or more LH pulses coincide with kisspeptin administration in 10 women is P = 0.007. Even when conservatively calculated using the fastest pulse frequency of seven pulses per 6 h, the probability is P = 0.03. Thus, it is highly unlikely that the LH pulses observed immediately after kisspeptin administration were unrelated to kisspeptin.
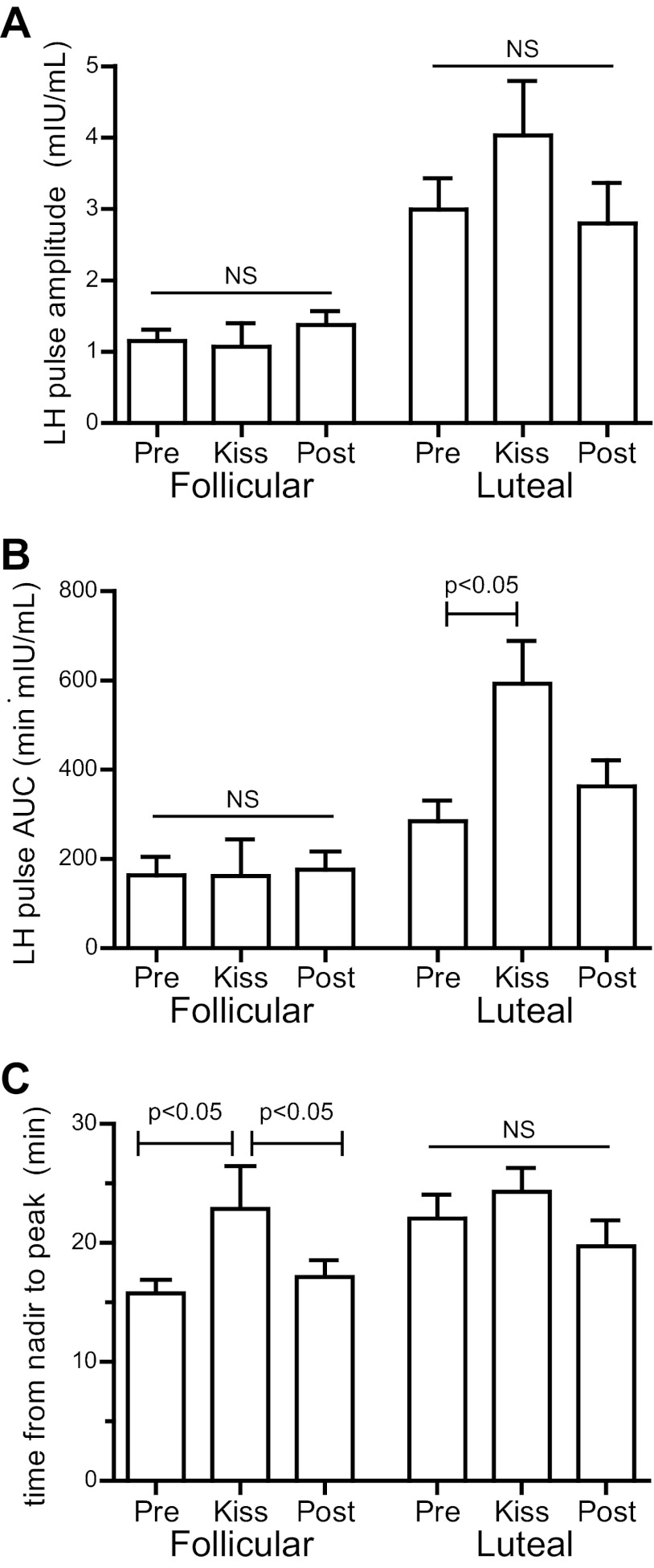
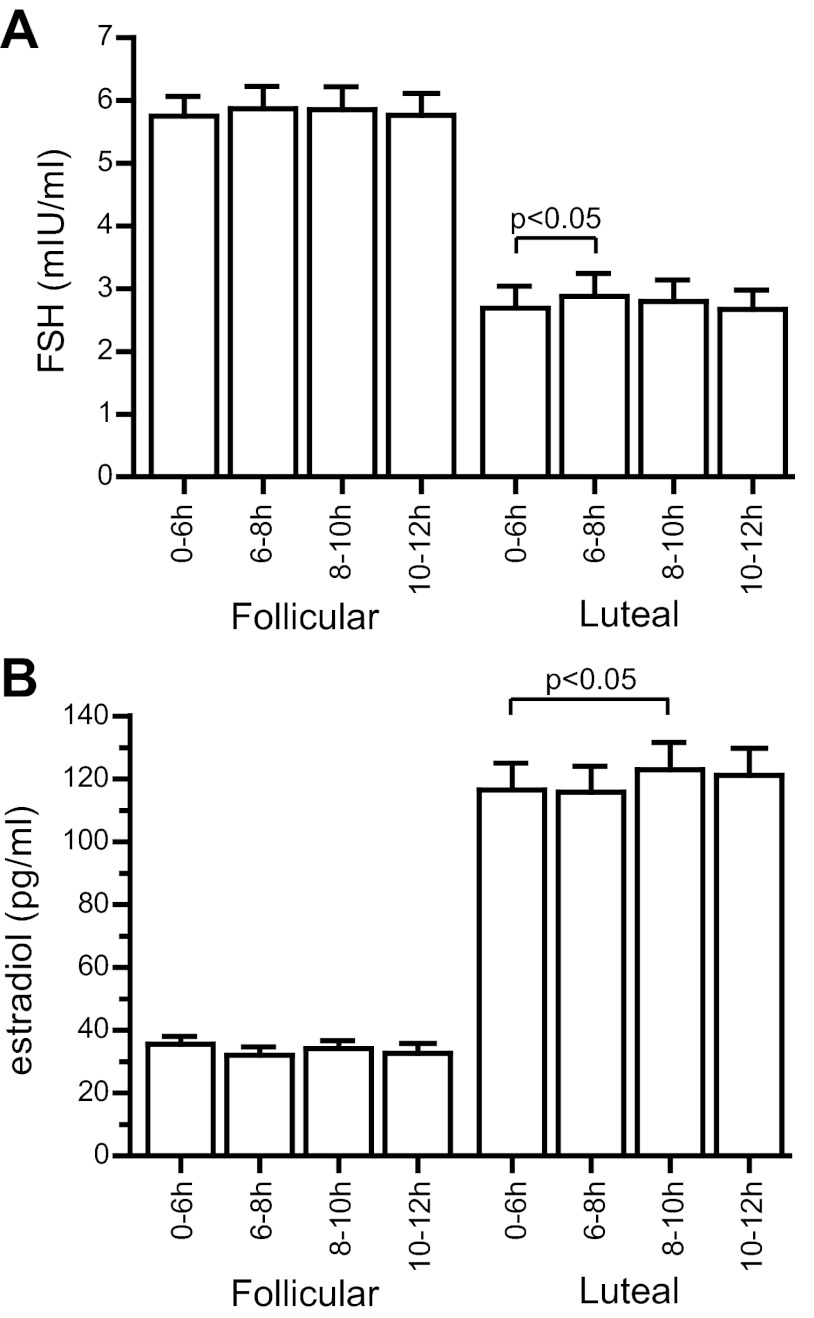
The amplitude and AUC of kisspeptin-induced GnRH-induced LH pulses in early follicular-phase women were not significantly different from those of endogenous pulses (amplitude 1.1 ± 0.3 vs. 1.2 ± 0.2 mIU/ml, P = 0.8; AUC 161 ± 83 vs.163 ± 41 min/mIU · ml, P = 1; Fig. 2, A and B). However, kisspeptin-induced pulses were more prolonged than endogenous pulses (time from nadir to peak 22.9 ± 3.6 vs. 15.8 ± 0.8 min, P = 0.048; Fig. 2C). No significant changes in FSH or estradiol were observed after kisspeptin in the early follicular phase (Fig. 3).
Fig. 2.
Characteristics of LH pulses. A, Amplitude. B, AUC. C, Time from nadir to peak. NS, Not significant; Pre, before kisspeptin administration; Post, after kisspeptin administration.
Fig. 3.
Effects of kisspeptin on hormone concentrations. FSH (A) and estradiol (B) were measured in 2-h study pools.
To determine whether a higher dose of kisspeptin would elicit a more robust LH response, four women in the early to midfollicular phase underwent the same protocol using a 3-fold higher dose of kisspeptin (0.72 nmol/kg, iv). Even with this higher dose, kisspeptin elicited an immediate LH pulse in only half of the women (Supplemental Figure 2).
Responses in the preovulatory period
Kisspeptin consistently elicited a robust LH pulse in preovulatory women (n = 3; Fig. 1, D and E, Supplemental Fig. 3), with LH peaking at 61.3 ± 16.7 mIU/ml 40 min after kisspeptin, compared with the prekisspeptin baseline of 34.7 ± 9.7 mIU/ml (Fig. 1F, P < 0.01). The AUC of kisspeptin-induced pulses was more than twice that of endogenous pulses (4528 ± 1143 vs. 1723 ± 578 min/mIU · ml, P = 0.04). No significant changes in FSH or estradiol were seen after kisspeptin (Table 2).
Responses in the midluteal phase
As in preovulatory women, kisspeptin consistently elicited an immediate LH pulse in all 14 luteal-phase women (Fig. 1, G and H, and Supplemental Fig. 4). LH peaked at 7.13 ± 0.73 mIU/mL 30 min after kisspeptin, compared with the prekisspeptin baseline of 3.39 ± 0.87 (P < 0.01; Fig. 1I). The AUC of kisspeptin-induced pulses was twice as large as that of endogenous pulses (593 ± 58 vs. 285 ± 46 min/mIU · ml, P = 0.04; Fig. 2B). In contrast to the early follicular phase, kisspeptin-induced LH pulses in the luteal phase were not significantly more prolonged than endogenous pulses (time from nadir to peak 24.3 ± 2.0 vs. 22.1 ± 2.0 min, P = 0.7; Fig. 2C). The duration of kisspeptin-induced pulses did not differ between the two phases (follicular 22.9 ± 3.6 min, luteal 24.3 ± 2.0 min, P = 0.7), whereas the duration of endogenous pulses was longer in the luteal phase than in the early follicular phase (22.1 ± 2.0 vs. 15.8 ± 0.8 min, P = 0.01). In the luteal phase, kisspeptin administration resulted in significant increases in FSH (Fig. 3A, baseline, 2.70 ± 0.35 mIU/ml; 0–2 h after kisspeptin, 2.88 ± 0.36 mIU/ml; P < 0.05) and estradiol (Fig. 3B, baseline, 117 ± 9 pg/ml; 2–4 h after kisspeptin, 123 ± 9 pg/ml; P < 0.05).
Effects of kisspeptin on the timing of endogenous pulses
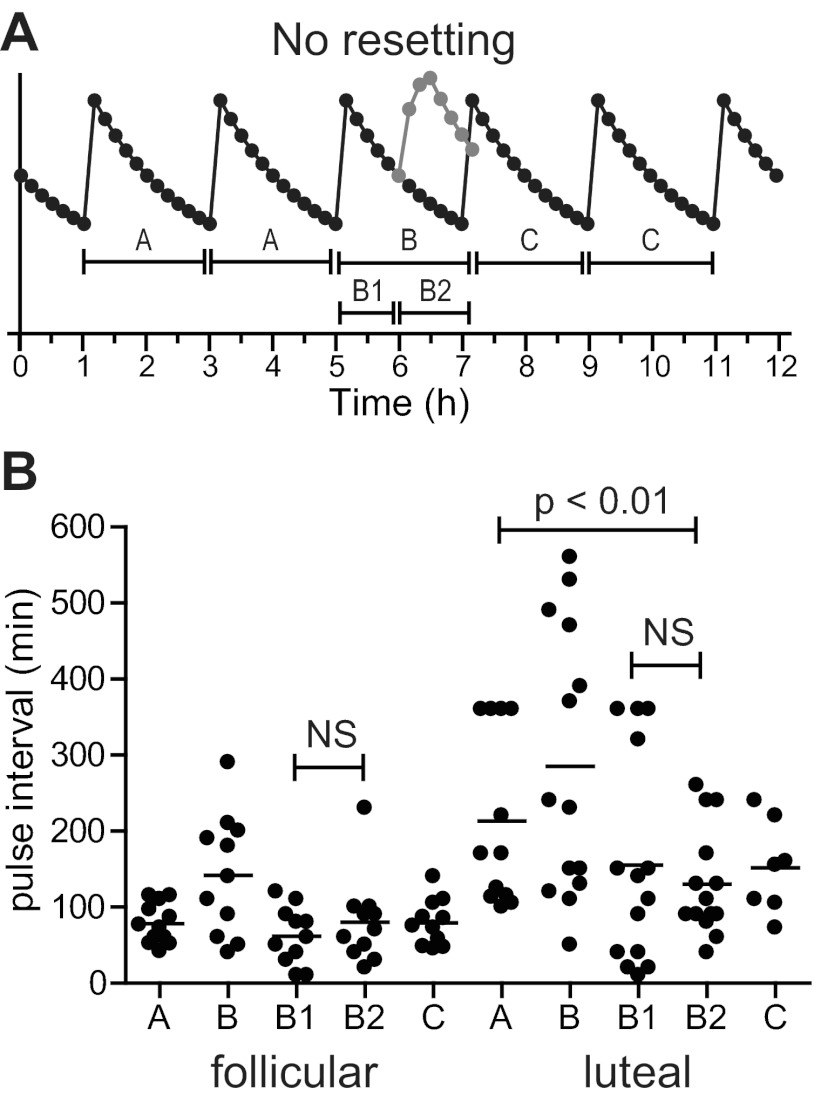
To determine whether kisspeptin delays the appearance of the next endogenous pulse in women, as it does in men, two intervals were compared: the interval from the pulse immediately preceding the kisspeptin bolus to the time of kisspeptin administration (the Pre-to-Kiss interval = interval B1 in Fig. 4), and the interval from the time of kisspeptin administration to the pulse immediately after kisspeptin (the Kiss-to-Post interval = interval B2). In men, these two intervals were significantly different, demonstrating that kisspeptin delayed the appearance of the next endogenous pulse (9). However, no significant differences were seen between these intervals in either the early follicular phase or the midluteal phase (early follicular B1, 60 ± 12 min; B2, 78 ± 19 min, P = 0.4; midluteal B1, 155 ± 37 min; B2, 130 ± 19 min, P = 0.5; Fig. 4); there were not enough data from preovulatory women for this analysis. Thus, the observed intervals do not support resetting by kisspeptin in women.
Fig. 4.
Effects of kisspeptin on pulse intervals. A, Schematic of the predicted result if kisspeptin were to have no effect on the timing of endogenous pulses. Note that this is an idealized schematic and that actual pulse profiles show more variability in pulse intervals. A, Interval between endogenous pulses before kisspeptin; B, interval between the endogenous pulses immediately preceding and following the kisspeptin-induced pulse; B1, interval between the kisspeptin-induced pulse and the immediately preceding endogenous pulse; B2, interval between the kisspeptin-induced pulse and the immediately following endogenous pulse; C, interval between endogenous pulses after kisspeptin. B, Observed pulse intervals. Bars, Means. NS, Not significant.
Further evidence of resetting in men came from the observation that the time from kisspeptin administration to the next pulse (“Kiss-to-Post” = B2) was no different from the normal resting interpulse interval (A) (9). However, in the midluteal phase, the time from kisspeptin administration to the next LH pulse was significantly shorter than the endogenous pulse interval (130 ± 19 vs. 213 ± 33 min, P = 0.008), a result again inconsistent with resetting. In the early follicular phase, these intervals appeared similar (78 ± 19 vs. 71 ± 7 min, P = 0.2).
Discussion
Although the ability of kisspeptin to potently and selectively stimulate GnRH-induced LH secretion is well known, detailed quantification of the neuroendocrine responses of healthy women to exogenous kisspeptin in this study has led to novel insights regarding kisspeptin-GnRH secretory dynamics. Understanding how kisspeptin stimulates GnRH pulses in varying sex-steroid milieus is critical to understanding how kisspeptin or its analogs might be used to modify GnRH pulses in reproductive disorders.
Compared across women in various phases of the menstrual cycle and men, the response to kisspeptin 112–121 (as measured by amplitude and AUC of the kisspeptin induced, GnRH induced LH pulse) was largest in preovulatory women, intermediate in men and luteal-phase women, and smallest in follicular-phase women. Dhillo et al. (6) similarly observed the largest LH responses in preovulatory women after sc administration of kisspeptin 68–121 compared with the follicular and luteal phases, and more recently Jayasena et al. (11) found that preovulatory women had a large LH response to iv kisspeptin 112–121 but early to midfollicular-phase women did not. These findings are also concordant with studies in rats (21) and sheep (22), in which the largest LH response to kisspeptin was seen just before ovulation.
The size of the LH pulses induced by a fixed dose of exogenous kisspeptin was also compared with that of endogenously generated LH pulses, a comparison enabled by the 6-h interval of frequent blood sampling before kisspeptin administration. If each pulse of GnRH secretion is driven by a pulse of kisspeptin, as has been suggested (23), and if the size of endogenous LH pulses is determined primarily by the amount of kisspeptin secreted, comparison of kisspeptin-induces pulses with endogenous pulses allows an estimate of the relative amounts of kisspeptin secreted in different phases of the cycle. In early follicular-phase women, endogenous LH pulses were comparable in size with those induced by kisspeptin 0.24 nmol/kg, iv, suggesting that endogenous kisspeptin secretion is approximately equivalent to that achieved by this dose of exogenous kisspeptin. In contrast, in preovulatory women and luteal-phase women and men (9), endogenous pulses were smaller than those induced by the same dose of kisspeptin. This suggests that more kisspeptin is secreted in the early follicular phase than in other phases of the menstrual cycle and in men.
Not only were kisspeptin-induced pulses smallest in the early follicular phase, only half of early follicular-phase women exhibited an LH pulse in response to kisspeptin. These findings mirror those of a recent study in female rhesus monkeys by Guerriero et al. (24), who performed hypothalamic administration of kisspeptin and direct measurement of GnRH to demonstrate that kisspeptin stimulates GnRH secretion in intact animals. In contrast, they found that kisspeptin administration did not enhance GnRH secretion in ovariectomized animals, much as our study observed dampened responses to kisspeptin in the early follicular phase, when estradiol levels are relatively low. Guerriero et al. further found that the GnRH response to kisspeptin in ovariectomized monkeys was restored by estradiol replacement, pinpointing estradiol as a key modulator of the GnRH neuronal response to kisspeptin and paralleling our observation that kisspeptin robustly stimulated LH secretion in women in the late follicular phase just before ovulation, when estradiol is high. Consistent with these collective findings, estradiol has been shown to enhance GnRH secretion in response to kisspeptin in GnRH neuronal cell lines (25, 26).
There are a number of potential explanations for the lack of a consistent response to kisspeptin in low-estradiol states such as the early follicular phase. One possibility is that the dose of kisspeptin used in this study (0.24 nmol/kg) was close to the threshold required to induce LH pulses. However, even with a 3-fold higher dose of kisspeptin, a consistent LH response to kisspeptin was not observed in early follicular-phase women. Furthermore, Jayasena et al. (11) reported no evidence of an augmented LH response to kisspeptin at doses up to 10 nmol/kg, iv. A second possibility is that there is a refractory period to kisspeptin after endogenous pulses, an effect that would be particularly relevant in the follicular phase when the pulse frequency is high. However, consistent responses to kisspeptin were seen in the preovulatory period, when the pulse frequency is higher than in the early follicular phase. In addition, even when kisspeptin was given shortly after an endogenous pulse in luteal-phase women and in men (9), there was unequivocal evidence of an LH response, arguing against a significant refractory period. A third possibility for the inconsistent response to kisspeptin in the early follicular phase is that GnRH secretion is largely kisspeptin-independent in the early follicular phase. However, there were more LH responses to kisspeptin in early follicular-phase women than would be expected by chance alone. Thus, it is unlikely that GnRH neurons are universally unresponsive to kisspeptin in the early follicular phase.
This leaves a fourth possibility: that stimulation of GnRH neurons by kisspeptin in the early follicular phase is close to saturation, such that administration of additional kisspeptin has little or no effect (and could even cause desensitization of the kisspeptin receptor). This possibility offers an alternative explanation for the observation that kisspeptin-induced pulses are larger than endogenous pulses in the preovulatory and luteal phases but not in the early follicular phase: the endogenous response to kisspeptin may already be maximal in the early follicular phase, and higher doses would therefore have little additional effect. This possibility could be tested by blocking kisspeptin signaling either through continuous administration of kisspeptin (27–29) or through the use of kisspeptin receptor antagonists (30).
The LH response to exogenous kisspeptin was observed to be smallest in the early follicular phase (both in absolute measurements and relative to endogenous pulses), yet we postulate that GnRH neurons are close to maximally stimulated by kisspeptin in the early follicular phase. This suggests that there is a limit on the ability of GnRH neurons to be stimulated by kisspeptin, and potentially a limit on GnRH secretion more generally, in the follicular phase. Why would such a limit exist? Any answer to this question is speculative, but one possibility is that this may be a physiological mechanism to ensure singleton pregnancies. Studies in GnRH-deficient women have demonstrated a very precise requirement for GnRH stimulation in the follicular phase, such that inadequate doses fail to achieve ovulation reliably, whereas supraphysiological doses result in overproduction of FSH, driving multifollicular development and increasing the risk for multiple gestation (31). The results of the current study raise the possibility that such precise regulation of GnRH secretion may be achieved through maximal kisspeptin drive to GnRH neuronal secretion combined with a limit on the amount of GnRH that can be secreted in response. Such a mechanism, in conjunction with other modulators of FSH secretion (e.g. estradiol and inhibin) (32), would ensure that only single follicle is selected for ovulation.
Because kisspeptin stimulates LH secretion indirectly through GnRH (3, 33–35), the differences in the LH response to kisspeptin across the menstrual cycle could be due to changes in GnRH neuronal sensitivity, changes in pituitary sensitivity to GnRH, or both. These possibilities can potentially be resolved by comparing the LH responses to kisspeptin observed in this study to the LH responses to GnRH observed in prior studies. The average size (amplitude and AUC) of kisspeptin-induced LH pulses is 3- to 4-fold greater in the midluteal phase than in the early follicular phase. Similarly, the LH response to GnRH has been shown to be 3- to 4-fold greater in the midluteal phase than in the early follicular phase (36–41). These differences in LH responsiveness are attributable to higher estrogen concentrations in the luteal phase because they can be mimicked by exogenous estrogen (42) and abolished by the estrogen antagonist clomiphene (41). Thus, the LH response to kisspeptin mirrors the LH response to GnRH. This comparison is complicated by the fact that only half of early follicular-phase women had a measurable LH pulse after kisspeptin, and it assumes that kisspeptin acts by simply inducing a single burst of GnRH secretion. With these caveats in mind, our results suggest that the difference in the size of the LH response to kisspeptin in the early follicular vs. midluteal phase can be attributed mainly to changes in pituitary sensitivity. Stated alternatively, the amount of GnRH secreted in response to a fixed dose of kisspeptin does not appear to differ between these phases of the cycle, but the response of the pituitary to GnRH does. This conclusion is concordant with prior findings that changes in pituitary responsiveness to GnRH, not the amount of GnRH secreted, primarily account for the changes in gonadotropin secretion across the human menstrual cycle (43).
In addition to stimulating GnRH secretion, kisspeptin was found in our studies of men to reset the GnRH pulse generator (9), with the interval from the kisspeptin-induced pulse to the next endogenous pulse being indistinguishable from the endogenous interpulse interval. In the current study, kisspeptin did not appear to reset the GnRH pulse generator in the luteal phase because these two intervals were significantly different. (In the early follicular phase, these two intervals appeared similar, but this may have been due to difficulty in distinguishing kisspeptin induced pulses from endogenous pulses; see Supplemental Materials.) By suggesting differences in the mechanisms of GnRH pulse generation between men and women, this conclusion is consistent with previous work that demonstrated that GnRH pulse generation is a renewal process in men but may not be in luteal-phase women (44, 45). Our results further suggest that the two effects of kisspeptin on GnRH secretion, induction of an immediate pulse (seen in both men and luteal phase women) and resetting of the pulse generator (seen in men but not in luteal phase women), are not obligately linked and thus are likely to involve separate mechanisms.
By characterizing the effects of kisspeptin in women in different phases of the menstrual cycle and by comparing these effects to endogenous patterns of reproductive endocrine activity, we are beginning to garner insight into the fundamental physiology of kisspeptin across the human menstrual cycle. Our results suggest that endogenous kisspeptin secretion, GnRH neuronal sensitivity to kisspeptin, and the maximal GnRH response to kisspeptin all vary across the menstrual cycle. Specifically the early follicular phase appears to have the highest kisspeptin tone yet also appears to have a maximal limit on how much GnRH can be secreted in response to kisspeptin. Furthermore, our results demonstrate differences in the effects of kisspeptin in women vs. men, with kisspeptin resetting the GnRH pulse generator in men but not women. The physiological basis for these differences may be determined by future studies administering kisspeptin under conditions where the sex-steroid milieu and other factors are directly controlled and manipulated.
Supplementary Material
Acknowledgments
We thank members of the Massachusetts General Hospital Reproductive Endocrine Unit for critical discussions and reading of the manuscript, the staff of the Massachusetts General Hospital Clinical Research Center for assistance with the studies, D. Hayden and H. Feldman for statistical advice, and the volunteers who participated in the studies. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
This work was supported by Grant R01 HD43341 from the Eunice Kennedy Shriver National Institute for Child Health and Human Development and Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 RR 025758, and financial contributions from Harvard University and its affiliated academic health care centers). Y.-M.C. was supported by a Charles A. King Trust postdoctoral fellowship and a Career Development Award from Children's Hospital Boston.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- Area under the curve
- MGH
- Massachusetts General Hospital.
References
- 1. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 3. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. 2004. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 4. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. 2005. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. 2005. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab 90:6609–6615 [DOI] [PubMed] [Google Scholar]
- 6. Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V, Kokkinos A, Donaldson M, Ghatei MA, Bloom SR. 2007. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab 92:3958–3966 [DOI] [PubMed] [Google Scholar]
- 7. Jayasena CN, Nijher GM, Chaudhri OB, Murphy KG, Ranger A, Lim A, Patel D, Mehta A, Todd C, Ramachandran R, Salem V, Stamp GW, Donaldson M, Ghatei MA, Bloom SR, Dhillo WS. 2009. Subcutaneous injection of kisspeptin-54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea, but chronic administration causes tachyphylaxis. J Clin Endocrinol Metab 94:4315–4323 [DOI] [PubMed] [Google Scholar]
- 8. Jayasena CN, Nijher GM, Abbara A, Murphy KG, Lim A, Patel D, Mehta A, Todd C, Donaldson M, Trew GH, Ghatei MA, Bloom SR, Dhillo WS. 2010. Twice-weekly administration of kisspeptin-54 for 8 weeks stimulates release of reproductive hormones in women with hypothalamic amenorrhea. Clin Pharmacol Ther 88:840–847 [DOI] [PubMed] [Google Scholar]
- 9. Chan YM, Butler JP, Pinnell NE, Pralong FP, Crowley WF, Jr, Ren C, Chan KK, Seminara SB. 2011. Kisspeptin resets the hypothalamic GnRH clock in men. J Clin Endocrinol Metab 96:E908–E915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. George JT, Veldhuis JD, Roseweir AK, Newton CL, Faccenda E, Millar RP, Anderson RA. 2011. Kisspeptin-10 is a potent stimulator of LH and increases pulse frequency in men. J Clin Endocrinol Metab 96:E1228–E1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jayasena CN, Nijher GM, Comninos AN, Abbara A, Januszewki A, Vaal ML, Sriskandarajah L, Murphy KG, Farzad Z, Ghatei MA, Bloom SR, Dhillo WS. 2011. The effects of kisspeptin-10 on reproductive hormone release show sexual dimorphism in humans. J Clin Endocrinol Metab 96:E1963–E1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Young J, George JT, Tello JA, Francou B, Bouligand J, Guiochon-Mantel A, Brailly-Tabard S, Anderson RA, Millar RP. 24 February 2012. Kisspeptin restores pulsatile LH secretion in patients with neurokinin B signaling deficiencies: physiological, pathophysiological and therapeutic implications. Neuroendocrinology 10.1159/000336376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. 2005. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pielecka-Fortuna J, Chu Z, Moenter SM. 2008. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 149:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. 2008. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 28:4423–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dumalska I, Wu M, Morozova E, Liu R, van den Pol A, Alreja M. 2008. Excitatory effects of the puberty-initiating peptide kisspeptin and group I metabotropic glutamate receptor agonists differentiate two distinct subpopulations of gonadotropin-releasing hormone neurons. J Neurosci 28:8003–8013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Constantin S, Caligioni CS, Stojilkovic S, Wray S. 2009. Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in gonadotropin-releasing hormone-1 neurons. Endocrinology 150:1400–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Santen RJ, Bardin CW. 1973. Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest 52:2617–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spratt DI, Carr DB, Merriam GR, Scully RE, Rao PN, Crowley WF., Jr 1987. The spectrum of abnormal patterns of gonadotropin-releasing hormone secretion in men with idiopathic hypogonadotropic hypogonadism: clinical and laboratory correlations. J Clin Endocrinol Metab 64:283–291 [DOI] [PubMed] [Google Scholar]
- 20. Pralong FP, Boepple PA, Conn PM, Whitcomb RW, Butler JP, Schoenfeld D, Crowley WF., Jr 1996. Contour of the GnRH pulse independently modulates gonadotropin secretion in the human male. Neuroendocrinology 64:247–256 [DOI] [PubMed] [Google Scholar]
- 21. Roa J, Vigo E, Castellano JM, Navarro VM, Fernández-Fernández R, Casanueva FF, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. 2006. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female rat. Endocrinology 147:2864–2878 [DOI] [PubMed] [Google Scholar]
- 22. Smith JT, Saleh SN, Clarke IJ. 2009. Seasonal and cyclical change in the luteinizing hormone response to kisspeptin in the ewe. Neuroendocrinology 90:283–291 [DOI] [PubMed] [Google Scholar]
- 23. Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. 2010. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 30:3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guerriero KA, Keen KL, Millar RP, Terasawa E. 2012. Developmental changes in GnRH release in response to kisspeptin agonist and antagonist in female rhesus monkeys (Macaca mulatta): implication for the mechanism of puberty. Endocrinology 153:825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Novaira HJ, Ng Y, Wolfe A, Radovick S. 2009. Kisspeptin increases GnRH mRNA expression and secretion in GnRH secreting neuronal cell lines. Mol Cell Endocrinol 311:126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tonsfeldt KJ, Goodall CP, Latham KL, Chappell PE. 2011. Oestrogen induces rhythmic expression of the Kisspeptin-1 receptor GPR54 in hypothalamic gonadotrophin-releasing hormone-secreting GT1-7 cells. J Neuroendocrinol 23:823–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. 2006. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology 147:2122–2126 [DOI] [PubMed] [Google Scholar]
- 28. Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley WF, Jr, Plant TM. 2007. Effect of continuous intravenous administration of human metastin 45-54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta). Endocrinology 148:3364–3370 [DOI] [PubMed] [Google Scholar]
- 29. Roa J, Vigo E, García-Galiano D, Castellano JM, Navarro VM, Pineda R, Diéguez C, Aguilar E, Pinilla L, Tena-Sempere M. 2008. Desensitization of gonadotropin responses to kisspeptin in the female rat: analyses of LH and FSH secretion at different developmental and metabolic states. Am J Physiol Endocrinol Metab 294:E1088–E1096 [DOI] [PubMed] [Google Scholar]
- 30. Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. 2009. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 29:3920–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santoro N, Wierman ME, Filicori M, Waldstreicher J, Crowley WF., Jr 1986. Intravenous administration of pulsatile gonadotropin-releasing hormone in hypothalamic amenorrhea: effects of dosage. J Clin Endocrinol Metab 62:109–116 [DOI] [PubMed] [Google Scholar]
- 32. Hall JE. 2009. Neuroendocrine control of the menstrual cycle. In: Strauss JF, III, Barbieri RL, ed. Yen and Jaffe's reproductive endocrinology. 6th ed Philadelphia, PA: Elsevier Saunders; 139–154 [Google Scholar]
- 33. Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. 2004. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol 16:850–858 [DOI] [PubMed] [Google Scholar]
- 34. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. 2005. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Caraty A, Smith JT, Lomet D, Ben Saïd S, Morrissey A, Cognie J, Doughton B, Baril G, Briant C, Clarke IJ. 2007. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology 148:5258–5267 [DOI] [PubMed] [Google Scholar]
- 36. Nillius SJ, Wide L. 1972. Variation in LH and FSH response to LH-releasing hormone during the menstrual cycle. J Obstet Gynaecol Br Commonw 79:865–873 [DOI] [PubMed] [Google Scholar]
- 37. Thomas K, Donnez J, Ferin J. 1972. LH and FSH releasing potency of the synthetic decapeptide p-Glu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH 2 in human beings. Contraception 6:55–64 [DOI] [PubMed] [Google Scholar]
- 38. Yen SS, VandenBerg G, Rebar R, Ehara Y. 1972. Variation of pituitary responsiveness to synthetic LRF during different phases of the menstrual cycle. J Clin Endocrinol Metab 35:931–934 [DOI] [PubMed] [Google Scholar]
- 39. Aono T, Minagawa J, Kinugasa T, Tanizawa O, Kurachi K. 1973. Response of pituitary LH and FSH to synthetic LH-releasing hormone in normal subjects and patients with Sheehan's syndrome. Am J Obstet Gynecol 117:1046–1052 [DOI] [PubMed] [Google Scholar]
- 40. Franchimont P, Becker H, Ernould C, Thys C, Demoulin A, Bourguignon JP, Legros JJ, Valcke JC. 1974. The effect of hypothalamic luteinizing hormone releasing hormone (LH-RH) on plasma gonadotrophin levels in normal subjects. Clin Endocrinol (Oxf) 3:27–39 [DOI] [PubMed] [Google Scholar]
- 41. Wang CF, Yen SS. 1975. Direct evidence of estrogen modulation of pituitary sensitivity to luteinizing hormone-releasing factor during the menstrual cycle. J Clin Invest 55:201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thompson IE, Arfania J, Taymor ML. 1973. Effects of estrogen and progesterone on pituitary response to stimulation by luteinizing hormone-releasing factor. J Clin Endocrinol Metab 37:152–155 [DOI] [PubMed] [Google Scholar]
- 43. Hall JE, Taylor AE, Martin KA, Rivier J, Schoenfeld DA, Crowley WF., Jr 1994. Decreased release of gonadotropin-releasing hormone during the preovulatory midcycle luteinizing hormone surge in normal women. Proc Natl Acad Sci USA 91:6894–6898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Butler JP, Spratt DI, O'Dea LS, Crowley WF., Jr 1986. Interpulse interval sequence of LH in normal men essentially constitutes a renewal process. Am J Physiol 250:E338–E340 [DOI] [PubMed] [Google Scholar]
- 45. Santoro N, Butler JP, Filicori M, Crowley WF., Jr 1988. Alterations of the hypothalamic GnRH interpulse interval sequence over the normal menstrual cycle. Am J Physiol 255:E696–E701 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.