Abstract
Context:
Variability in the pattern of change in estradiol (E2) and FSH levels over the menopause transition has not been well defined.
Objective:
The current study aimed to determine whether different trajectories of E2 and FSH could be identified and whether race/ethnicity and body mass index were related to the different trajectories.
Design:
The Study of Women's Health Across the Nation is a longitudinal observational study of the menopausal transition.
Setting:
Women aged 42–52 yr from seven participating sites were recruited and underwent up to 11 annual visits.
Participants:
Postmenopausal women with 12 or more months of amenorrhea that was not due to hysterectomy/oophorectomy and who were not using hormone therapy before the final menstrual period participated in the study.
Main Outcome Measures:
Annual serum E2 and FSH levels anchored to final menstrual period were measured.
Results:
Four distinct E2 trajectories and three distinct FSH trajectories were identified. The E2 trajectories were: slow decline (26.9%), flat (28.6%), rise/slow decline (13.1%), and rise/steep decline (31.5%). The FSH trajectories were: low (10.6%), medium (48.7%), and high (41.7%) rising patterns. Obesity increased the likelihood of a flat E2 and low FSH trajectory for all race/ethnic groups. Normal-weight Caucasian and African-American women tended to follow the rise/steep decline E2 and high FSH trajectories. Normal-weight Chinese/Japanese women tended to follow the slow decline E2 and the high/medium FSH trajectories.
Conclusions:
E2 and FSH trajectories over the menopausal transition are not uniform across the population of women. Race/ethnicity and body mass index affect the trajectory of both E2 and FSH change over the menopausal transition.
Irregularity of the menstrual cycle marks the start of the menopausal transition in most women (1). Usually around age mid-40s, cycle length may initially shorten and then progressively lengthen with the approach of the final menstrual period (FMP) (1, 2). This irregularity seems to correspond to changes in estrogen levels, which are predominantly the consequence of the decline of ovarian follicle number (3). In addition, age-related decreases in central nervous system function also drive changes in estrogen production (4). In early reproductive aging, FSH level increases, which initially maintains estradiol (E2) levels. As the menopause transition progresses, E2 levels eventually decline significantly and remain low, whereas FSH levels increase and remain high (3, 5). Although this general sequence of events has been assumed to be consistent for most women, several mostly very small studies have observed that E2 may increase immediately before the final menstrual period (4, 6–9). Moreover, the timing and magnitude of increase in FSH varies among individuals (7, 10); aggregation of FSH levels across individual women may be misleading and consequently overlooking factors related to FSH trajectory patterns. The literature so far is incomplete and does not conclusively define the variations of E2 and FSH trajectories or factors related to these variations.
The Study of Women's Health Across the Nation (SWAN) is a large multicenter, multiethnic longitudinal study of the menopausal transition. The availability of comprehensive and annually repeated measurements of biological and physical measures in SWAN allows a close examination of the long-term trajectory of E2 and FSH changes during the menopausal transition. The goal of the current study was to determine whether different trajectories of E2 and FSH could be identified and whether race/ethnicity and body mass index (BMI) were related to the different trajectories. Such information will help us to understand inconsistencies in the literature and differences in ovarian aging among different populations, which in turn may relate to clinical differences in menopausal-specific symptoms such as hot flashes and health outcomes such as changes in bone mineral density as well as initiation and progression of disease of aging such as osteoarthritis.
Materials and Methods
Study population
The current study included SWAN women who had up to 11 annual visits with serum FSH and E2 levels and an observable FMP without exogenous hormone therapy. The study protocol of SWAN has been described in detail previously (11). Briefly, eligibility criteria for study entry included the following: age 42–52 yr; no surgical removal of uterus and/or both ovaries; not currently using exogenous hormone medications that were known to affect ovarian function; at least one menstrual period within 3 months before study entry; and self-identification with a site's designated race/ethnic groups. All seven sites enrolled Caucasian women as well as one of the following five other racial/ethnic groups: Boston, MA, Detroit, MI, Chicago, IL, and Pittsburgh, PA, enrolled African-American (AA) women; Oakland, CA, Chinese; Los Angeles, CA, Japanese; and Newark, NJ, Hispanic women, respectively. A total of 3302 women were recruited: 1550 Caucasian, 935 AA, 250 Chinese, 281 Japanese, and 286 Hispanic. Eligible participants were then followed up annually. Institutional review boards approved the study protocol at each site; signed, written informed consent was obtained from all participants.
Menopausal status was assessed annually based on self-reported bleeding patterns. A woman was considered postmenopausal when she had no bleeding for at least 12 months. FMP was identified at the first visit when a woman became postmenopausal. Natural menopause was defined for women who did not have a hysterectomy and/or both ovaries removed and did not report any use of exogenous hormones one year before FMP.
Of the 3302 women in SWAN, 1366 SWAN participants experienced an observed natural menopause by follow-up visit 10, 1184 women reported FMP dates, and 150 women had missing FMP dates but the last visit before postmenopause was less than 2 yr earlier. These 150 missing FMP dates were estimated based on self-reported amenorrhea durations and visit dates. FMP dates were set to missing for 32 women who missed at least three consecutive visits before the first identified postmenopausal visit because imputation would be potentially unreliable, given the long gap in data collection. These 32 women were excluded. Finally, of the 1334 (1184 + 150) women with FMP dates, we excluded 18 women whose baseline BMI was missing. Thus, 1316 women with natural FMP were eligible for analysis.
Serum hormone assays
Annual fasting blood samples were collected. Two attempts were made to collect a follicular phase sample. When follicular phase samples were not available or when a woman stopped menstruating, a random fasting sample was collected within 90 d of the baseline recruitment date. All serum hormones were measured at the CLASS/RSP Central Laboratory at the University of Michigan (Ann Arbor, MI).
FSH and SHBG assays were conducted in singlicate and E2 assays in duplicate using the automated Ciba Corning Diagnostics ACS:180 analyzer (Bayer Diagnostics Corp., Norwood, MA). Average inter- and intraassay coefficients of E2 variation was 10.6 and 6.4%, respectively, over the E2 assay range, and the lower limit of detection (LLD) was 1–7 pg/ml. FSH was measured with a two-site chemiluminometric immunoassay. Inter- and intraassay coefficients of FSH variation were 12.0 and 6.0%, respectively, and the LLD was 0.4–1.0 IU/liter. SHBG was measured with a two-site chemiluminescent assay. Inter- and intraassay coefficients of SHBG variation were 9.9 and 6.1%, respectively, and the LLD was 1.9–3.2 nm. A random number between zero and LLD (assume uniform distribution) was assigned to assays less than LLD for all 3302 SWAN women across 11 annual visits (n = 184 assays).
Measures
Time invariant baseline measures
BMI (kilograms per square meter) was calculated using measured weight (kilograms) and height2 (square meters) and categorized as normal/underweight (<25 kg/m2), overweight (25–29.9 kg/m2), or obese (≥30 kg/m2). Only 22 women had BMI less than 18.5 kg/m2; the normal/underweight group was thereafter called normal weight for simplicity. Potential baseline covariates included smoking, physical activity, education, health status, financial hardship, and enrollment site. Smoking was obtained based on seven questions adapted from the American Thoracic Society standard questions (12) and analyzed as current vs. past/never smoking. Physical activity was measured by a continuous score using an instrument adapted from the Kaiser Permanente Health Plan Activity Survey (13). Financial hardship was queried as how hard to pay for very basics and coded as very hard, somewhat hard, or not very hard. Distribution of baseline characteristics was shown in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.
Time-varying measures
Time-varying measures included annual visit age, SHBG levels, and cycle day of blood draw (within d 2–5 of the menstrual cycle vs. outside d 2–5/unknown). SHBG levels were adjusted in the modeling of E2 because of their importance in regulating the biological activity of E2.
Statistical analysis
Natural log transformation of E2 and FSH was performed for statistical modeling due to the lack of normality of the distributions and backtransformed for ease of interpretation. The time scale was anchored to the FMP, which facilitated the analysis of hormone levels at approximately similar time intervals relative to the FMP. The first and last time intervals were excluded from the statistical modeling to avoid small cell counts. This left 1316 women with 12586 observations available for modeling and a maximum time before or after the FMP of 8.3 yr.
Group-based trajectory modeling (GBTM) was used to detect the heterogeneity of E2 and FSH changes over the menopausal transition (14, 15). GBTM is an application of finite mixture modeling that approximates the unknown distribution of population developmental trajectories using trajectory groups or clusters. This approach takes uncertainty of classification into account. Within the same group, individuals follow approximately the same trajectory and different groups may vary in the same fashion or differently. A polynomial relationship was used to model the link between hormones and FMP. To allow for flexibility in estimating the shapes of the trajectories, linear (time), quadratic (time2), cubic (time3), quartic (time4), or quintic (time5) terms of time was tested. The maximum power term with a significant P value (<0.05) was selected along with all lower terms for each group separately. The number of groups was based on the model with the best fit statistically and reasonable scientific plausibility. Graphical visualization was checked for model fitting. Adequacy of the selected model was further evaluated using average posterior probabilities (AvePP) of group membership and odds of correct classification (OCC): the higher values indicate better adequacy. A selected model with an AvePP of 0.7 or greater and an OCC of 5 or greater was considered adequate (14).
Time-varying variables were considered as factors associated with the hormone levels but not with trajectory groups. Time-invariant variables were examined for their relation to latent trajectory group membership using multinomial logistic regressions. Coefficient estimates of time-invariant variables indicated their relations to the specific trajectory group. A positive/negative value indicated an increased/decreased likelihood of the trajectory group. Modeling details were presented in Supplemental Tables 2 and 3.
Each participant was assigned to a particular trajectory group based on her highest posterior (predicted) probability (PP). After the groups were identified, trajectory groups by race/ethnicity and BMI were compared using χ2 tests.
To illustrate the effects of baseline BMI and race/ethnicity on the probability of E2 and FSH trajectory group membership, we calculated the PP of E2 and FSH trajectory group membership given BMI and race/ethnicity. Using coefficient estimates from the trajectory models, PP for each trajectory group for BMI was calculated with adjustment for race/ethnicity, for race/ethnicity with adjustment for BMI, and for each race/ethnicity group within BMI categories. For all PP, 95% confidence intervals were estimated using the parametric bootstrap technique (16).
All analyses were performed with SAS Windows 9.2 (SAS Institute, Cary NC). The SAS macro PROC TRAJ program (17, 18) was used for the group-based trajectory modeling.
Results
The study sample included 1316 women who underwent natural menopause. The median age at FMP was 51.5 yr. Caucasians comprised 42.5% of the sample, AA 31.4%, Chinese 10.0%, Japanese 10.7%, and Hispanics 5.4%, respectively. At baseline, 25.3% of women were overweight, and 32.1% were obese. Current smokers comprised 17.6% of the sample, and 11.5% reported fair/poor health. Thirty-nine percent had some financial difficulties, and 25.0% had high school or less education. The study cohort was followed up an average of 9.5 ± 1.6 (mean ± sd) yr.
Trajectory of E2 relative to the FMP
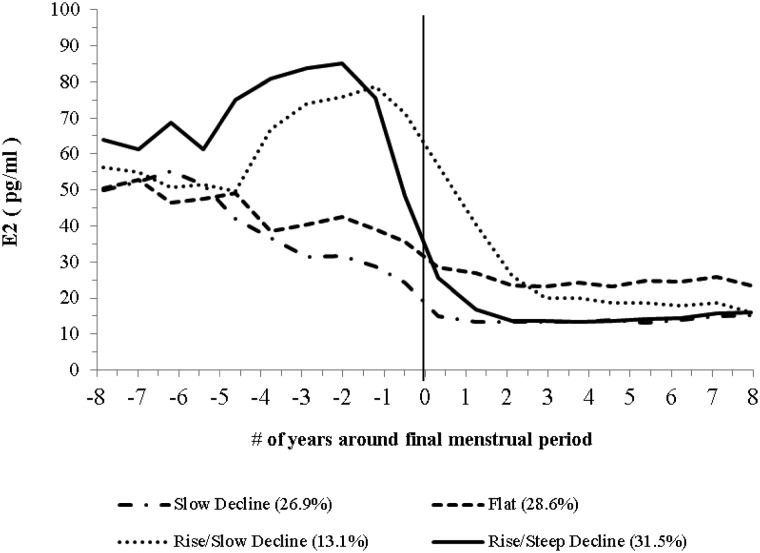
Using GBTM with adjustment for age, SHBG, and cycle day of blood draw, we found four distinct E2 trajectories (Fig. 1). The four E2 trajectories were summarized as slow gradual decline (slow decline, 26.9% of the sample); limited decline that appeared flat (flat, 28.6%); rise and then gradual decline (rise/slow decline, 13.1%); and rise and then steep decline (rise/steep decline, 31.5%).
Fig. 1.
Trajectory of E2 across the FMP. Significant predictors for trajectory separation were race/ethnicity and BMI (see Supplemental Table 2 for modeling details). E2 levels were the average observed serum E2 levels at each time point. Time-varying age, SHBG, and cycle day of blood draw were adjusted in the model. The model accuracy diagnostics showed that the capacity of the model to separate women among the groups was good. The AvePP ranged from 0.72 to 0.81 and the OCC ranged from 6.44 to 10.98.
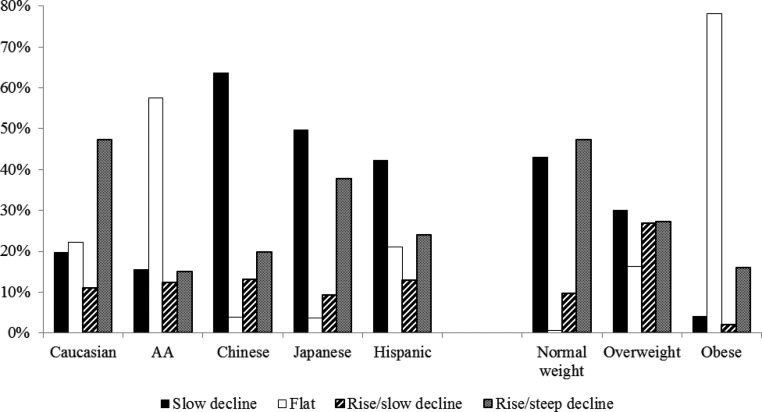
Race/ethnicity and BMI were significantly related to the E2 trajectory groups (P < 0.05) but not physical activity, smoking, health status, difficulty in paying basics, education, or site. Only race/ethnicity and BMI were included in the final model. Using the rise/steep decline trajectory as a reference, women exhibiting the slow decline trajectory were more likely to be Chinese or Japanese; women with the flat trajectory were more likely to be AA, overweight, or obese; and women with the rise/slow decline trajectory were more likely to be Chinese or overweight (Supplement Table 2 and Fig. 2).
Fig. 2.
Distribution of E2 trajectory subgroups by race/ethnicity and BMI. BMI was analyzed as normal weight (<25 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2). The percentages represented the percent of E2 trajectory subgroups within each race/ethnicity or BMI category. The total percentage within each race/ethnicity or BMI category was 100%. Overall P values based on χ2 tests for the distribution of E2 trajectory subgroups by race/ethnicity and BMI were both less than 0.0001.
The adjusted predicted probability of group membership for each of the trajectories was calculated (Table 1). For normal-weight women, rise/steep decline was the most likely E2 trajectory (PP: 0.46); for overweight women, rise/slow decline (PP: 0.29) or slow decline (PP: 0.28); and for obese women, flat (PP: 0.68). The most likely E2 trajectory for Caucasians was rise/steep decline (PP: 0.51); for AA, flat (PP: 0.31) or rise/steep decline (PP: 0.30); and for Chinese, Japanese, and Hispanics, slow decline. Flat trajectory was the most likely pattern for obese women among all race/ethnic groups. Normal/overweight Caucasian and normal weight AA were most likely to follow the rise/steep decline trajectory, whereas normal/overweight Chinese, Japanese, and Hispanics were most likely to follow the slow decline trajectory.
Table 1.
Predicted E2 trajectory group membership probabilities (95% confidence intervals)
| n (%) | Slow decline | Flat | Rise/slow decline | Rise/steep decline | |
|---|---|---|---|---|---|
| BMI (kg/m2) | |||||
| Normal (<25) | 561 (42.6) | 0.39 (0.30, 0.47) | 0.03 (0.01, 0.12) | 0.12 (0.08, 0.19) | 0.46 (0.38, 0.53) |
| Overweight (25–29.9) | 333 (25.3) | 0.28 (0.20, 0.37) | 0.16 (0.08, 0.29) | 0.29 (0.19, 0.40) | 0.27 (0.18, 0.37) |
| Obese (≥30) | 422 (32.1) | 0.09 (0.04, 0.19) | 0.68 (0.55, 0.77) | 0.04 (0.01, 0.14) | 0.19 (0.12, 0.26) |
| Race/ethnicity | |||||
| Caucasian | 568 (43.2) | 0.22 (0.15, 0.31) | 0.13 (0.06, 0.25) | 0.13 (0.07, 0.23) | 0.51 (0.42, 0.59) |
| African-Americans | 401 (30.5) | 0.25 (0.14, 0.37) | 0.31 (0.17, 0.50) | 0.14 (0.07, 0.27) | 0.30 (0.20, 0.40) |
| Chinese | 133 (10.1) | 0.50 (0.24, 0.65) | 0.11 (0.01, 0.53) | 0.17 (0.06, 0.32) | 0.22 (0.10, 0.35) |
| Japanese | 142 (10.8) | 0.43 (0.25, 0.57) | 0.09 (0.01, 0.36) | 0.12 (0.04, 0.27) | 0.36 (0.22, 0.50) |
| Hispanic | 72 (5.5) | 0.44 (0.25, 0.62) | 0.06 (0.02, 0.20) | 0.13 (0.04, 0.32) | 0.36 (0.17, 0.57) |
| BMI by race/ethnicity (kg/m2) | |||||
| Caucasian | |||||
| Normal (<25) | 247 (44.2) | 0.29 (0.21, 0.38) | 0.02 (0.00, 0.10) | 0.11 (0.06, 0.19) | 0.58 (0.48, 0.65) |
| Overweight (25–29.9) | 143 (25.6) | 0.22 (0.14, 0.32) | 0.14 (0.06, 0.26) | 0.28 (0.17, 0.40) | 0.36 (0.25, 0.49) |
| Obese (≥30) | 169 (30.2) | 0.07 (0.03, 0.17) | 0.61 (0.48, 0.71) | 0.04 (0.01, 0.15) | 0.27 (0.19, 0.36) |
| African-Americans | |||||
| Normal (<25) | 80 (19.4) | 0.38 (0.24, 0.52) | 0.07 (0.01, 0.25) | 0.14 (0.07, 0.26) | 0.41 (0.28, 0.53) |
| Overweight (25–29.9) | 124 (30.0) | 0.23 (0.14, 0.33) | 0.30 (0.18, 0.45) | 0.27 (0.16, 0.41) | 0.20 (0.12, 0.30) |
| Obese (≥0) | 209 (50.6) | 0.05 (0.02, 0.11) | 0.84 (0.72, 0.90) | 0.03 (0.01, 0.11) | 0.09 (0.05, 0.15) |
| Chinese | |||||
| Normal (<25) | 105 (79.6) | 0.61 (0.34, 0.79) | 0.02 (0.01, 0.24) | 0.13 (0.02, 0.31) | 0.24 (0.11, 0.44) |
| Overweight (25–29.9) | 21 (15.9) | 0.44 (0.21, 0.61) | 0.11 (0.01, 0.54) | 0.31 (0.11, 0.52) | 0.14 (0.02, 0.26) |
| Obese (≥30) | 6 (4.6) | 0.19 (0.03, 0.53) | 0.61 (0.14, 0.93) | 0.06 (0.01, 0.27) | 0.13 (0.02, 0.34) |
| Japanese | |||||
| Normal (<25) | 114 (80.9) | 0.51 (0.38, 0.63) | 0.01 (0.00, 0.09) | 0.09 (0.04, 0.20) | 0.38 (0.25, 0.51) |
| Overweight (25–29.9) | 19 (13.5) | 0.42 (0.22, 0.57) | 0.09 (0.01, 0.43) | 0.24 (0.08, 0.48) | 0.25 (0.12, 0.41) |
| Obese (≥30) | 8 (5.7) | 0.19 (0.03, 0.46) | 0.52 (0.12, 0.88) | 0.05 (0.01, 0.23) | 0.25 (0.06, 0.48) |
| Hispanic | |||||
| Normal (<25) | 15 (21.1) | 0.52 (0.28, 0.72) | 0.01 (0.00, 0.06) | 0.10 (0.03, 0.26) | 0.37 (0.16, 0.61) |
| Overweight (25–29.9) | 26 (36.6) | 0.43 (0.23, 0.62) | 0.06 (0.02, 0.20) | 0.27 (0.10, 0.50) | 0.24 (0.10, 0.45) |
| Obese (≥30) | 30 (42.3) | 0.22 (0.07, 0.44) | 0.44 (0.18, 0.71) | 0.07 (0.01, 0.28) | 0.28 (0.12, 0.48) |
The highest probability is in bold.
Trajectory of FSH relative to the FMP
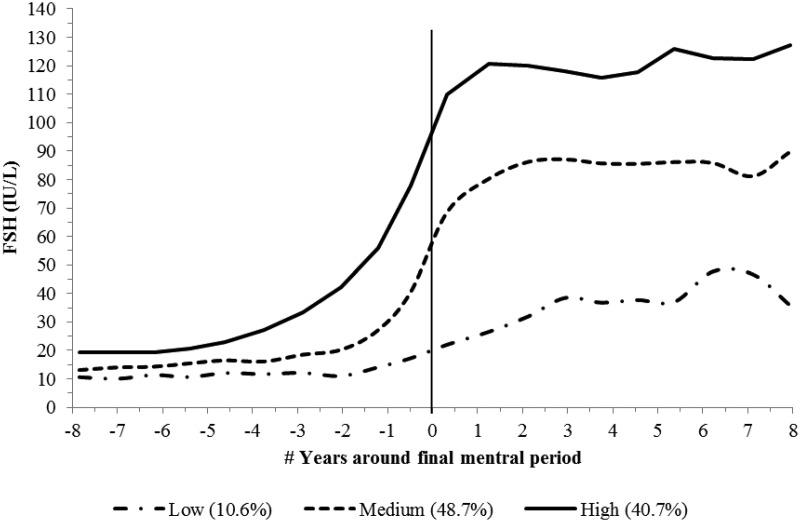
Using GBTM with adjustment for age and cycle day of blood draw, we found three distinct FSH trajectories (Fig. 3). The variation in trajectories was most evident after the FMP. These trajectories can be described as low- (10.6% of the population), medium- (48.7%), and high (40.7%)-rising trajectories.
Fig. 3.
Trajectory of FSH across the FMP. Significant predictors for trajectory separation were race/ethnicity and BMI (see Supplemental Table 3 for modeling details). FSH levels were the average observed serum FSH levels at each time point. Time-varying age and cycle day of blood draw were adjusted in the model. The model accuracy diagnostics showed that the capacity of the model to separate women among the groups was very good. The AvePP ranged from 0.87 to 0.92 and the OCC ranged from 7.00 to 105.51.
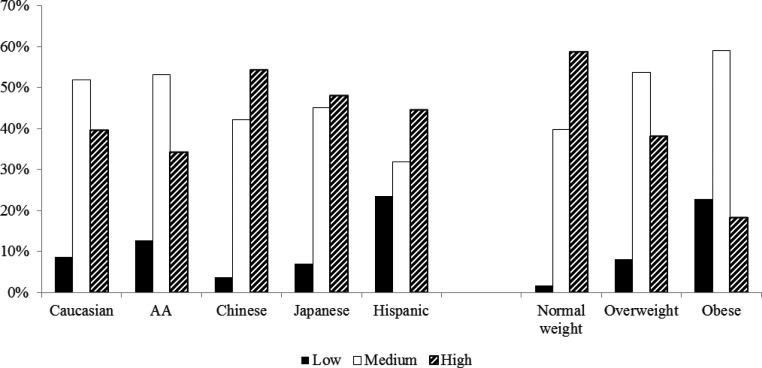
Race/ethnicity and BMI were significantly related to FSH trajectory groups but not physical activity, smoking, health status, difficulty in paying basics, education, or site; only race/ethnicity and BMI were included in the final model. Compared with women following the high FSH trajectory, women following the low trajectory were more likely to be AA, Hispanic, overweight, or obese; and women following the medium trajectory were more likely to be Japanese and overweight/obese (Supplemental Table 3 and Fig. 4).
Fig. 4.
Distribution of FSH trajectory subgroups by race/ethnicity and BMI. BMI was analyzed as normal weight (<25 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2). Percentages represented the percent of FSH trajectory subgroups within each race/ethnicity or BMI category. The total percentage within each race/ethnicity or BMI category was 100%. Overall P values based on χ2 tests for the distribution of FSH trajectory subgroups by race/ethnicity and BMI were both less than 0.0001.
The adjusted predictive effects of race/ethnicity and BMI were estimated based on the predicted probability of FSH trajectory groups (Table 2). For normal-weight women, the high trajectory was the most likely trajectory (PP: 0.61), whereas for overweight (PP: 0.55) and obese (PP: 0.62) women, the medium trajectory was the most likely trajectory. For Caucasians (PP: 0.53) and Hispanics (PP: 0.51), the most likely trajectory was the high trajectory, whereas for AA and Chinese/Japanese, the most likely trajectory was the medium trajectory. For Caucasians, AA, and Chinese, normal-weight women were most likely to follow the high trajectory but overweight/obese women were most likely to follow the medium trajectory. Japanese women were most likely to follow the medium trajectory, regardless of BMI (Table 2).
Table 2.
Predicted FSH group membership probabilities (95% confidence intervals)
| n, % | Low | Medium | High | |
|---|---|---|---|---|
| BMI (kg/m2) | ||||
| Normal (<25) | 561 (42.6) | 0.02 (0.01, 0.04) | 0.37 (0.31, 0.44) | 0.61 (0.54, 0.67) |
| Overweight (25–29.9) | 333 (25.3) | 0.08 (0.04, 0.13) | 0.55 (0.47, 0.62) | 0.38 (0.31, 0.45) |
| Obese (≥30) | 422 (32.1) | 0.20 (0.14, 0.27) | 0.62 (0.54, 0.68) | 0.19 (0.14, 0.25) |
| Race/ethnicity | ||||
| Caucasian | 568 (43.2) | 0.05 (0.03, 0.08) | 0.42 (0.35, 0.49) | 0.53 (0.46, 0.59) |
| African-Americans | 401 (30.5) | 0.09 (0.06, 0.14) | 0.50 (0.43, 0.58) | 0.40 (0.33, 0.47) |
| Chinese | 133 (10.1) | 0.05 (0.01, 0.16) | 0.56 (0.44, 0.66) | 0.39 (0.28, 0.50) |
| Japanese | 142 (10.8) | 0.04 (0.01, 0.15) | 0.67 (0.55, 0.76) | 0.29 (0.20, 0.39) |
| Hispanic | 72 (5.5) | 0.18 (0.10, 0.30) | 0.31 (0.17, 0.48) | 0.51 (0.34, 0.67) |
| BMI by race/ethnicity (kg/m2) | ||||
| Caucasian | ||||
| Normal (<25) | 247 (44.2) | 0.02 (0.01, 0.04) | 0.36 (0.29, 0.45) | 0.62 (0.53, 0.70) |
| Overweight (25–29.9) | 143 (25.6) | 0.06 (0.03, .11) | 0.55 (0.46, 0.63) | 0.39 (0.31, 0.48) |
| Obese (≥30) | 169 (30.2) | 0.16 (0.11, 0.24) | 0.63 (0.55, 0.71) | 0.20 (0.15, 0.27) |
| African-Americans | ||||
| Normal (<25) | 80 (19.4) | 0.03 (0.01, 0.07) | 0.36 (0.27, 0.45) | 0.61 (0.51, 0.70) |
| Overweight (25–29.9) | 124 (30.0) | 0.11 (0.07, 0.18) | 0.52 (0.43, 0.60) | 0.37 (0.29, 0.46) |
| Obese (≥30) | 209 (50.6) | 0.27 (0.21, 0.34) | 0.56 (0.48, 0.63) | 0.18 (0.13, 0.24) |
| Chinese | ||||
| Normal (<25) | 105 (79.6) | 0.01 (0.00, 0.06) | 0.40 (0.30, 0.50) | 0.59 (0.48, 0.69) |
| Overweight (25–29.9) | 21 (15.9) | 0.05 (0.01, 0.20) | 0.58 (0.43, 0.70) | 0.36 (0.25, 0.49) |
| Obese (≥30) | 6 (4.6) | 0.14 (0.04, 0.41) | 0.67 (0.45, 0.80) | 0.19 (0.10, 0.29) |
| Japanese | ||||
| Normal (<25) | 114 (80.9) | 0.01 (0.00, 0.06) | 0.52 (0.40, 0.62) | 0.47 (0.36, 0.58) |
| Overweight (25–29.9) | 19 (13.5) | 0.05 (0.01, 0.20) | 0.69 (0.54, 0.79) | 0.26 (0.17, 0.38) |
| Obese (≥30) | 8 (5.7) | 0.12 (0.03, 0.38) | 0.75 (0.52, 0.86) | 0.13 (0.07, 0.21) |
| Hispanic | ||||
| Normal (<25) | 15 (21.1) | 0.05 (0.02, 0.13) | 0.21 (0.10, 0.38) | 0.74 (0.55, 0.85) |
| Overweight (25–29.9) | 26 (36.6) | 0.21 (0.11, 0.34) | 0.32 (0.18, 0.50) | 0.47 (0.31, 0.64) |
| Obese (≥30) | 30 (42.3) | 0.47 (0.31, 0.63) | 0.32 (0.18, 0.49) | 0.21 (0.12, 0.34) |
The highest probability is in bold.
Combinations of E2 and FSH trajectory groups
When looking at the cooccurrence of E2 and FSH trajectory groups, certain pairs tended to be more likely than others. The low FSH trajectories were most likely to occur in conjunction with the flat E2 trajectories. The high FSH trajectory was most likely to be paired with the slow decline E2 trajectory. Most of those who followed the combination of the low FSH and the flat E2 trajectories were obese women (97.3%). Women following the high FSH/slow decline E2 combinations were predominantly normal weight (72.2%).
Discussion
These data show clearly that, during the menopausal transition, the trajectories of change in the levels of both E2 and FSH vary between women but fall into several distinct patterns. We were able to distinguish four unique E2 and three unique FSH trajectory groups or clusters. These differing hormone trajectories were strongly related to BMI and race/ethnicity but not site, smoking, physical activity, or demographic variables.
We found that a large group of women (44.6%) experienced a significant elevation of E2 before the FMP. This group was predominantly non-obese women and was observed among all race/ethnicity groups. This finding was not observed in some previous menopausal transition studies (3, 5, 19–21) and was considered atypical when observed (6), although studies reported the elevation of E2 before the FMP was limited by a small sample size, short study durations, and mostly limited to Caucasian women (6, 8, 9, 22). Hale et al. (6) found that among 78 Caucasian women, serum E2 levels were erratic and often elevated (37%). They observed that this E2 secretion pattern appeared to be triggered by prolonged high FSH levels during the follicular phase and was associated with abnormally short or long cycle lengths (6). The Fertility Recognition Enabling Early Detection of Menopause study also reported an elevation of estrogen during the menopausal transition among Caucasian women (study sample = 37) (8) and that increasing FSH was related to increased urinary estrone 3-glucuronide.
The SWAN data presented here put these prior findings in context by providing a comprehensive picture of how both race/ethnicity and BMI are related to changes in E2 levels. The high E2 levels with the onset of the menopausal transition seen with the rise/decline trajectories could be related to the decrease in the number of ovarian follicles and the resultant drop in negative feedback enabling a marked elevation in FSH (6). FSH may eventually be high enough to cause normally silent preantral follicles to become FSH responsive and these may in turn be responsible for the increase in E2 production (6). Another possibility is that the higher FSH may have a hyperstimulating effect on ovarian production of E2. This may lead to a disturbance of the normal follicle development and consequent faster as well as multicycstic follicular growth with higher levels of estrogens earlier in the follicular phase, when our E2 assays were obtained (23). A final explanation may be related to the finding that the hypothalamus-pituitary becomes less sensitive to estrogen during the perimenopause and this may stimulate elevation in E2 levels before the FMP (4).
Clinically the increase in E2 levels in the perimenopause may manifest as excessive volume of bleeding, prolonged menstrual periods, increased breast discomfort, more frequent headaches, and an altered response to hormonal therapies. Indeed, it is the likeliest explanation for the frequently reported transient occurrence of very heavy menses by many perimenopausal women. Future studies of clinical phenotypes of this subgroup of women may help to understand these menopause related symptomatic presentations.
We found that obese women followed a flat E2 decline and a low FSH increase trajectory, regardless of their race/ethnicity. In other words, the change in hormone levels from pre- to postmenopause was less pronounced for obese women than nonobese women. These attenuation effects of obesity on E2 and FSH change confirm previous cross-sectional and longitudinal reports (5, 19–21). Randolph et al. (19) reported that obesity limited the rise in FSH levels after the FMP and obese women had lower pre-FMP but higher post-FMP E2 levels). They did not find a significant effect of obesity on the timing of E2/FSH change (19), which is similar to our results that the trajectory patterns differ by BMI largely due to hormone levels rather than the timing of changes. The failure of E2 to increase pre-FMP among obese women may be related to the adverse effects of obesity on ovarian function. Increased BMI may activate lipotoxicity in obese women (24). This in turn may contribute to perturbation of the hormone balance that regulates follicular development and ovulation and the inability to respond adequately to menopausal transition related changes (24). In postmenopausal women when ovaries ceased to be the principal sources of estrogens, estrogens are produced in a number of extragonadal sites including mesenchymal cells of adipose tissue (25). The aromatization process of converting androgens to estrogens in extragonadal tissues is accelerated with higher BMI (26). The higher post-FMP E2 levels among the flat trajectory group potentially reflect an increased adipose tissue mass with an accelerated aromatization among obese women. Additionally, increased BMI has an inhibitory effect on gonadotropin secretion, and overweight and obese women have lower FSH levels compared with normal-weight women (5, 19, 27, 28). This is consistent with our observation of a slower/less steep FSH increase among overweight and obese women.
We found that AA women had a significantly increased probability of the flat E2 trajectory independent of obesity status (Supplemental Tables 2 and 3). This may be related to differences in adipose tissue distribution and the functional status of adipose tissues between AA and other racial/ethnic groups (29, 30). AA women have less abdominal visceral fat than Caucasian women (31), and Caucasian women have less abdominal visceral fat than Japanese women (32). Studies have shown that a reverse relationship exists between visceral fat and estrogen levels (33, 34). For the AA women in our study, their low levels of visceral fat and their high prevalence of obesity (50.6%) may contribute to their less dramatic decrease of E2 after the FMP.
We observed an increased probability of Japanese, Chinese, and Hispanic women following the low decline E2 trajectory. This is consistent with previous SWAN reports (35, 36) of lower E2 serum levels in Chinese and Japanese women. Genetic variations and environmental differences, such as Asian people's greater soy intake (37), may contribute to this difference. Studies with larger samples of Hispanic women are needed for a more conclusive understanding of the hormonal changes within this racial/ethnic group.
Our results show that the three FSH trajectories all had rising patterns despite the relatively greater variation in E2 trajectory patterns. Consistent with our findings, a recent study showed similar FSH levels between regular cycling AA and Caucasian women, even though they had different E2 levels during the late follicle phase (38). It is possible that estrogen's negative feedback, which occurs during the early and midfollicular phase, was similar, although at different levels, among the various E2 trajectory groups.
The unique strengths of our study included the large sample size, inclusion of multiple racial/ethnic groups, repeated annual longitudinal measures symmetrically around the FMP, and the implementation of a novel sophisticated statistical methodology that takes classification uncertainty into account. The annual measures of serum hormones limit the representativeness and comparability of hormones within a specific phase of the menstrual cycle over time because samples were obtained in the early follicular phase for women with a regular menstrual cycle, but this was less restrictive or random when women's menstrual cycles became irregular. This variation in blood sampling may have caused the misclassification of some women, and the higher levels of E2 closer to the FMP may partially reflect collection of E2 measurements in the later follicular phase or midcycle in some women. However, the inconsistency in the menstrual phase timing of the blood draw would affect all women and would not be expected to be associated with either obesity or race/ethnicity. SWAN recruited different racial/ethnic groups at different sites, which could result in the apparent race/ethnicity effect in fact caused by factors shared by women in the same community. We did not find site to influence the hormone trajectories independently, but a potential effect cannot be excluded.
In conclusion, our findings show that the trajectories of E2 and FSH change over the menopausal transition are not uniform across women. Appropriate methodological techniques to separate women into subgroups are important in future studies of menopause and the endocrinology of midlife women.
Supplementary Material
Acknowledgments
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Institute of Nursing Research, the Office of Research on Women's Health, or the National Institutes of Health. Clinical centers included the following: University of Michigan, Ann Arbor, Siobán Harlow, principal investigator (PI) 2011, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA, Joel Finkelstein, PI 1999 to present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL, Howard Kravitz, PI 2009 to present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser, Davis, CA, Ellen Gold, PI; University of California, Los Angeles, Los Angeles, CA, Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY, Carol Derby, PI 2011, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry, New Jersey Medical School, Newark, NJ, Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA, Karen Matthews, PI. The National Institutes of Health Program Office: National Institute on Aging, Bethesda, MD, Sherry Sherman 1994 to present; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD, Program Officers. The Central laboratory was the University of Michigan, Ann Arbor, Daniel McConnell (Central Ligand Assay Satellite Services). The Coordinating centers were the following: University of Pittsburgh, Pittsburgh, PA, Kim Sutton-Tyrrell, PI 2001 to present; New England Research Institutes, Watertown, MA, Sonja McKinlay, PI 1995–2001. The Steering Committee was Susan Johnson, current chair, Chris Gallagher, former chair. We thank the study staff at each site and all the women who participated in SWAN. Special thanks go to Dr. Bobby Jones for his assistance in implementing SAS PROC TRAJ.
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health, Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research, and the National Institutes of Health Office of Research on Women's Health (Grants NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, and AG012495).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AA
- African-American
- AvePP
- average posterior probabilities
- BMI
- body mass index
- E2
- estradiol
- FMP
- final menstrual period
- GBTM
- group-based trajectory modeling
- LLD
- lower limit of detection
- OCC
- odds of correct classification
- PP
- posterior (predicted) probability
- SWAN
- Study of Women's Health across the Nation.
References
- 1. O'Connor KA, Holman DJ, Wood JW. 2001. Menstrual cycle variability and the perimenopause. Am J Hum Biol 13:465–478 [DOI] [PubMed] [Google Scholar]
- 2. Taffe JR, Dennerstein L. 2002. Menstrual patterns leading to the final menstrual period. Menopause 9:32–40 [DOI] [PubMed] [Google Scholar]
- 3. Burger HG, Hale GE, Robertson DM, Dennerstein L. 2007. A review of hormonal changes during menopausal transition: focus on findings from the Melbourne Women's Midlife Health Project. Hum Reprod Update 13:559–565 [DOI] [PubMed] [Google Scholar]
- 4. Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. 2004. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA 292:2991–2996 [DOI] [PubMed] [Google Scholar]
- 5. Randolph JF, Jr, Zheng H, Sowers MR, Crandall C, Crawford S, Gold EB, Vuga M. 2011. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab 96:746–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hale GE, Huges CL, Burger HG, Robertson DM, Fraser IS. 2009. Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase events underlying irregular ovulatory menstrual cycles in the menopausal transition. Menopause 16:50–59 [DOI] [PubMed] [Google Scholar]
- 7. Prior JC. 1998. Perimenopause: the complex endocrinology of the menopausal transition. Endocr Rev 19:397–428 [DOI] [PubMed] [Google Scholar]
- 8. Miro F, Parker SW, Aspinall LJ, Coley J, Perry PW, Ellis JE. 2004. Origins and consequences of the elongation of the human menstrual cycle during the menopausal transition: the FREEDOM study. J Clin Endocrinol Metab 89:4910–4915 [DOI] [PubMed] [Google Scholar]
- 9. Santoro N, Crawford SL, Lasley WL, Luborsky JL, Matthews KA, McConnell D, Randolph JF, Jr, Gold EB, Greendale GA, Korenman SG, Powell L, Sowers MF, Weiss G. 2008. Factors related to declining luteal function in women during the menopausal transition. J Clin Endocrinol Metab 93:1711–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrell RJ, O'Connor KA, Holman DJ, Brindle E, Miller RC, Rodriguez G, Simon JA, Mansfield PK, Wood JW, Weinstein M. 2007. Monitoring reproductive aging in a 5-year prospective study: aggregate and individual changes in luteinizing hormone and follicle-stimulating hormone with age. Menopause 14:29–37 [DOI] [PubMed] [Google Scholar]
- 11. Sowers MF, Crawford SL, Morgenstein D. 2000. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, Kelsey J, Marcus R, eds. Menopause: biology and pathobiology. San Diego: Academic Press; 175–188 [Google Scholar]
- 12. Ferris BG. 1978. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis 118:1–120 [PubMed] [Google Scholar]
- 13. Sternfeld B, Ainsworth BE, Quesenberry CP. 1999. Physical activity patterns in a diverse population of women. Prev Med 28:313–323 [DOI] [PubMed] [Google Scholar]
- 14. Nagin 2005. Group-based modeling of development. Cambridge, MA: Harvard University Press [Google Scholar]
- 15. Nagin DS, Odgers CL. 2010. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 6:109–138 [DOI] [PubMed] [Google Scholar]
- 16. Efron B. 1979. Bootstrap methods: another look at the jackknife. Ann Stat 7:1–26 [Google Scholar]
- 17. Jones BL, Nagin D, Roeder K. 2001. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res 29:374–393 [Google Scholar]
- 18. Jones BL, Nagin DS. 2007. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Socio Methods Res 35:542–571 [Google Scholar]
- 19. Randolph JF, Jr, Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, McConnell DS, Finkelstein JS, Korenman SG, Matthews KA, Sternfeld B, Lasley BL. 2003. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab 88:1516–1522 [DOI] [PubMed] [Google Scholar]
- 20. Sowers MR, Zheng H, McConnell D, Nan B, Harlow SD, Randolph JF., Jr 2008. Estradiol rates of change in relation to the final menstrual period in a population-based cohort of women. J Clin Endocrinol Metab 93:3847–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Freeman EW, Sammel MD, Lin H, Gracia CR. 2010. Obesity and reproductive hormone levels in the transition to menopause. Menopause 17:718–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. 2007. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab 92:3060–3067 [DOI] [PubMed] [Google Scholar]
- 23. Fraser IS, Baird DT. 1974. Blood production and ovarian secretion rates of estradiol-17β and estrone in women with dysfunctional uterine bleeding. J Clin Endocrinol Metab 39:564–570 [DOI] [PubMed] [Google Scholar]
- 24. Robker RL, Wu LL, Yang X. 2011. Inflammatory pathways linking obesity and ovarian dysfunction. J Reprod Immunol 88:142–148 [DOI] [PubMed] [Google Scholar]
- 25. Simpson ER. 2003. Sources of estrogen and their importance. J Steroid Biochem Mol Biol 86:225–230 [DOI] [PubMed] [Google Scholar]
- 26. Siiteri PK. 1987. Adipose tissue as a source of hormones. Am J Clin Nutr 45:277–282 [DOI] [PubMed] [Google Scholar]
- 27. De Pergola G, Maldera S, Tartagni M, Pannacciulli N, Loverro G, Giorgino R. 2006. Inhibitory effect of obesity on gonadotropin, estradiol, and inhibin B levels in fertile women. Obesity 14:1954–1960 [DOI] [PubMed] [Google Scholar]
- 28. Grenman S, Rönnemaa T, Irjala K, Kaihola HL, Grönroos M. 1986. Sex steroid, gonadotropin, cortisol, and prolactin levels in healthy, massively obese women: correlation with abdominal fat cell size and effect of weight reduction. J Clin Endocrinol Metab 63:1257–1261 [DOI] [PubMed] [Google Scholar]
- 29. Hamdy O, Porramatikul S, Al-Ozairi E. 2006. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev 2:367–373 [DOI] [PubMed] [Google Scholar]
- 30. Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, Ravussin E, Ryan DH, Smith SR, Katzmarzyk PT. 2011. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity 19:402–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Conway JM, Yanovski SZ, Avila NA, Hubbard VS. 1995. Visceral adipose tissue differences in black and white women. Am J Clin Nutr 61:765–771 [DOI] [PubMed] [Google Scholar]
- 32. Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. 2007. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr 86:353–359 [DOI] [PubMed] [Google Scholar]
- 33. Brown LM, Clegg DJ. 2010. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol 122:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bouchard C, Després JP, Mauriège P. 1993. Genetic and nongenetic determinants of regional fat distribution. Endocr Rev 14:72–93 [DOI] [PubMed] [Google Scholar]
- 35. Randolph JF, Jr, Sowers M, Bondarenko IV, Harlow SD, Luborsky JL, Little RJ. 2004. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab 89:1555–1561 [DOI] [PubMed] [Google Scholar]
- 36. Sowers MR, Wilson AL, Kardia SR, Chu J, Ferrell R. 2006. Aromatase gene (CYP 19) polymorphisms and endogenous androgen concentrations in a multiracial/multiethnic, multisite study of women at midlife. Am J Med 119(9 Suppl 1):S23–S30 [DOI] [PubMed] [Google Scholar]
- 37. North American Menopause Society 2011. The role of soy isoflavones in menopausal health: report of The North American Menopause Society/Wulf H. Utian Translational Science Symposium in Chicago, IL (October 2010). Menopause 18:732–753 [DOI] [PubMed] [Google Scholar]
- 38. Marsh EE, Shaw ND, Klingman KM, Tiamfook-Morgan TO, Yialamas MA, Sluss PM, Hall JE. 2011. Estrogen levels are higher across the menstrual cycle in African-American women compared with Caucasian women. J Clin Endocrinol Metab 96:3199–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.