Abstract
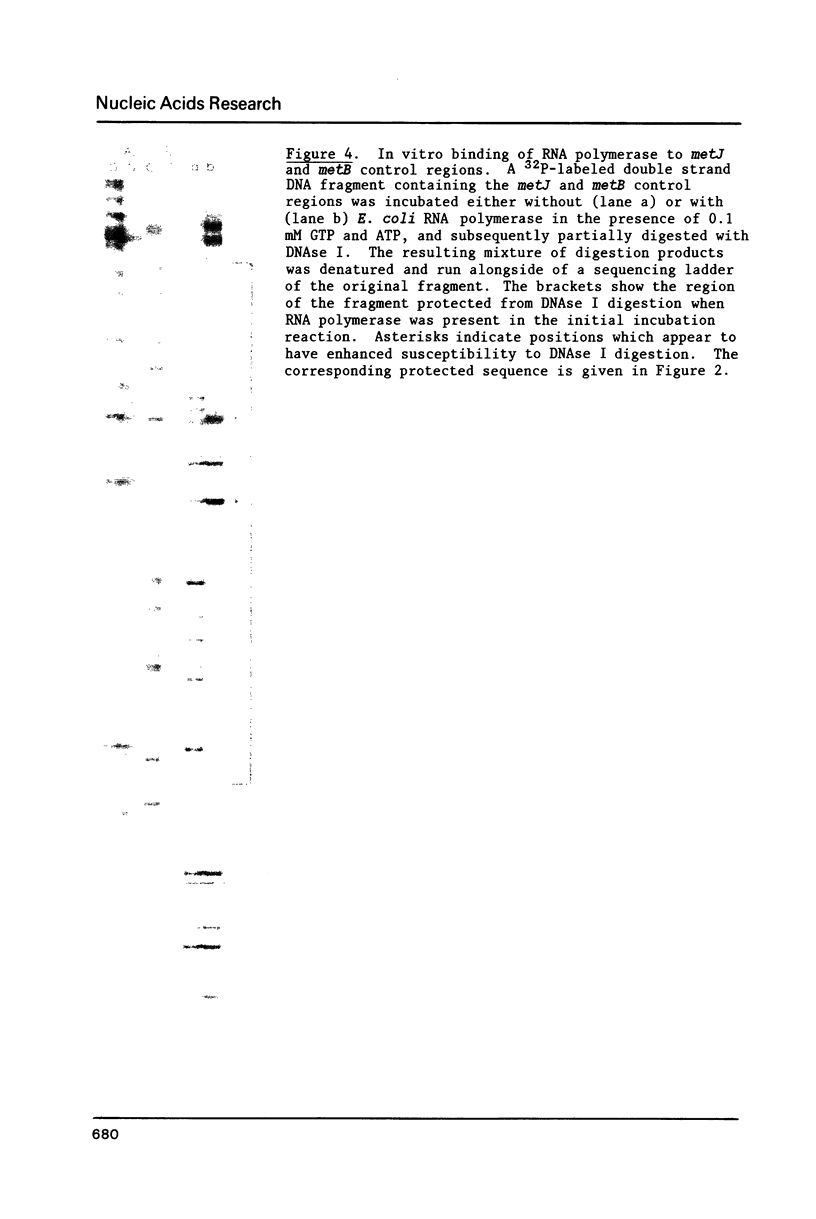
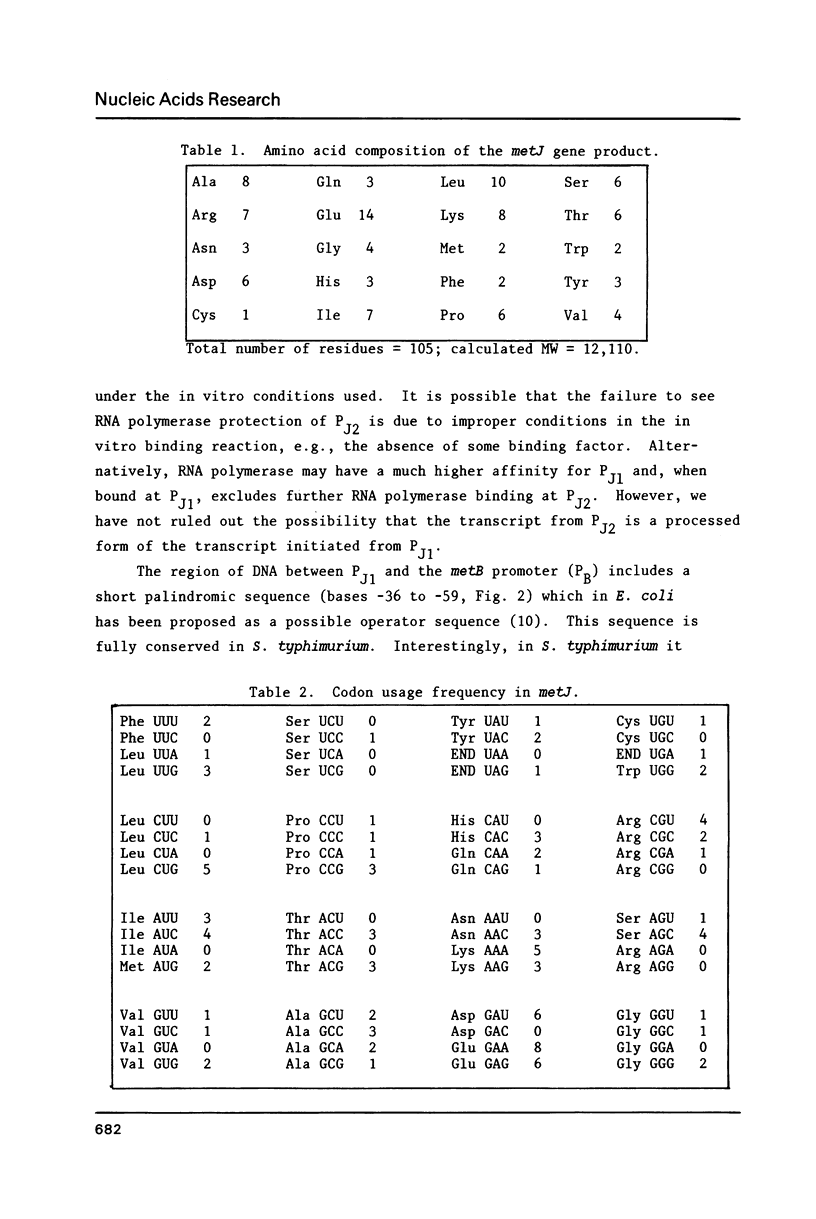
The nucleotide sequence of the Salmonella typhimurium metJ gene is presented along with the sequence of the promoter region for the closely linked metB gene. The two genes are transcribed in opposite directions, with transcription initiating from a single promoter for metB, and from two apparent promoters for metJ. RNA polymerase binding sites for metJ and metB, determined by in vitro protection studies, lie adjacent to each other and may overlap. The two metJ promoters, PJ1 and PJ2, are separated by approximately 65 base pairs. Binding of RNA polymerase in vitro could only be observed for PJ1, even though transcripts are initiated from both promoters in vivo. The metJ gene codes for a polypeptide of 105 amino acids with a calculated Mr of 12,110. The translation start site was determined by N-terminal amino acid sequence analysis of a metJ-lacZ fusion protein.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. Mechanism of repression of methionine biosynthesis in Escherichia coli. I. The role of methionine, s-adenosylmethionine, and methionyl-transfer ribonucleic acid in repression. Mol Gen Genet. 1973 Jul 16;123(4):299–324. doi: 10.1007/BF00433648. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchange N., Zakin M. M., Ferrara P., Saint-Girons I., Park I., Tran S. V., Py M. C., Cohen G. N. Structure of the metJBLF cluster in Escherichia coli K12. Sequence of the metB structural gene and of the 5'- and 3'-flanking regions of the metBL operon. J Biol Chem. 1983 Dec 25;258(24):14868–14871. [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A., Smith D. A., Rowbury R. J. Regulation of methionine synthesis in Salmonella typhimurium: mutants resistant to inhibition by analogues of methionine. Genetics. 1968 Apr;58(4):473–492. doi: 10.1093/genetics/58.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Plamann M. D., Stauffer G. V. Characterization of the Escherichia coli gene for serine hydroxymethyltransferase. Gene. 1983 Apr;22(1):9–18. doi: 10.1016/0378-1119(83)90059-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Schmitz A., Galas D. J. The interaction of RNA polymerase and lac repressor with the lac control region. Nucleic Acids Res. 1979 Jan;6(1):111–137. doi: 10.1093/nar/6.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Steers E., Jr, Cuatrecasas P., Pollard H. B. The purification of beta-galactosidase from Escherichia coli by affinity chromatography. J Biol Chem. 1971 Jan 10;246(1):196–200. [PubMed] [Google Scholar]
- Su C. H., Greene R. C. Regulation of methionine biosynthesis in Escherichia coli: mapping of the metJ locus and properties of a metJ plus-metJ minus diploid. Proc Natl Acad Sci U S A. 1971 Feb;68(2):367–371. doi: 10.1073/pnas.68.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran S. V., Schaeffer E., Bertrand O., Mariuzza R., Ferrara P. Appendix. Purification, molecular weight, and NH2-terminal sequence of cystathionine gamma-synthase of Escherichia coli. J Biol Chem. 1983 Dec 25;258(24):14872–14873. [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]






