Abstract
Context:
Aldosterone production in the adrenal zona glomerulosa is mainly regulated by angiotensin II, [K+], and ACTH. Genetic deletion of subunits of K+-selective leak (KCNK) channels TWIK-related acid sensitive K+-1 and/or TWIK-related acid sensitive K+-3 in mice results in primary hyperaldosteronism, whereas mutations in the KCNJ5 (potassium inwardly rectifying channel, subfamily J, member 5) gene are implicated in primary hyperaldosteronism and, in certain cases, in autonomous glomerulosa cell proliferation in humans.
Objective:
The objective of the study was to investigate the role of KCNK3, KCNK5, KCNK9, and KCNJ5 genes in a family with primary hyperaldosteronism and early-onset hypertension.
Patients and Methods:
Two patients, a mother and a daughter, presented with severe primary hyperaldosteronism, bilateral massive adrenal hyperplasia, and early-onset hypertension refractory to medical treatment. Genomic DNA was isolated and the exons of the entire coding regions of the above genes were amplified and sequenced. Electrophysiological studies were performed to determine the effect of identified mutation(s) on the membrane reversal potentials.
Results:
Sequencing of the KCNJ5 gene revealed a single, heterozygous guanine to thymine (G → T) substitution at nucleotide position 470 (n.G470T), resulting in isoleucine (I) to serine (S) substitution at amino acid 157 (p.I157S). This mutation results in loss of ion selectivity, cell membrane depolarization, increased Ca2+ entry in adrenal glomerulosa cells, and increased aldosterone synthesis. Sequencing of the KCNK3, KCNK5, and KCNK9 genes revealed no mutations in our patients.
Conclusions:
These findings explain the pathogenesis in a subset of patients with severe hypertension and implicate loss of K+ channel selectivity in constitutive aldosterone production.
Aldosterone, a steroid hormone synthesized and secreted by the zona glomerulosa of the adrenal cortex, is physiologically regulated by angiotensin II (AngII), [K+], and ACTH (1–3). AngII binds to its type 1 receptor (AT1R) and stimulates a variety of signaling cascades, leading to the entry of extracellular Ca2+ into the cytoplasm of cells and subsequent cell membrane depolarization with additional release of Ca2+ from the endoplasmic reticulum (4). The adrenal glomerulosa cells have a high resting outward K+ conductance, which produces a highly negative membrane potential (5). Membrane depolarization by either elevation of extracellular K+ or closure of K+ channels by AngII activates voltage-gated Ca2+ channels, thereby increasing intracellular Ca2+ concentrations. The latter provide signals for increased expression of proteins required for aldosterone biosynthesis, including steroidogenic acute regulatory protein and aldosterone synthase (CYP11B2) (1–3, 5).
The adrenal glomerulosa cells are uniquely sensitive to small increases in extracellular K+ due to the presence of low resting membrane potential maintained by the high background K+ conductance (6–8). This continuous outward K+ flow appears to be promoted by the K+-selective leak (KCNK)-type channels, including the TWIK-related acid sensitive K+ (TASK) 1 and 3, which are encoded by the KCNK3 and KCNK9 genes, respectively (9–11). These leak-type K+ channels are abundantly expressed in the adrenal glomerulosa cells in both humans and rodents (12). Small changes in extracellular K+ concentrations increase the sensitivity of adrenocortical cells to stimulation by AngII (6–8). AngII-induced activation of its AT1R results in closure of these K+ channels, depolarization of the membrane, opening of the voltage-gated Ca2+ channels, entry of extracellular Ca2+ into the cell, and increased intracellular Ca2+ concentrations. AngII may also increase intracellular Ca2+ concentrations by transiently stimulating the release of Ca2+ from the endoplasmic reticulum through activation of the inositol triphosphate-mediated signaling pathway. The physiological importance of these K+ channels has been verified in animals and humans: TASK1- and TASK1/TASK3-deficient mice develop primary hyperaldosteronism, whereas knockdown of TASK1 induces the expression of the steroidogenic acute regulatory protein and CYP11B2 and stimulates pregnenolone and aldosterone production (13).
In addition to TASK1 and TASK3, which contribute to the negative resting potential of the adrenal glomerulosa cells, several K+ channels of other channel families are also expressed in adrenal glomerulosa cells. Although their exact function is still unclear, they may contribute to fine-tuning of the resting potential and/or the depolarization/repolarization of the cell membrane (12). One such channel, the rectifier potassium channels (Kir) 3.4, belongs to the inward rectifier K+ channel family, is encoded by the KCNJ5 (potassium inwardly rectifying channel, subfamily J, member 5) gene, and is expressed in the adrenal glomerulosa cells. Inherited and acquired mutations in the KCNJ5 gene were implicated in familial primary hyperaldosteronism and in patients with aldosteronomas (14–17). These mutations resulted in impaired ion selectivity and increased inflow Na+ conductance, which leads to cell depolarization and subsequent increased Ca2+ entry into the cells, the signal for aldosterone production in adrenal glomerulosa cells (14).
In the present study, we describe two patients, a mother and a daughter, with severe primary hyperaldosteronism and bilateral adrenal hyperplasia caused by a novel point mutation of the KCNJ5 gene.
Materials and Methods
Patients
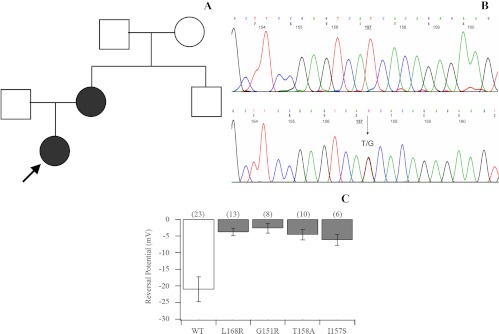
The index case (the daughter) presented at the age of 2 yr with severe hypertension (HTN) [arterial blood pressure (BP) 150/100 mm Hg]; hypokalemia (2.7 mEq/liter); hyperaldosteronism (3269 pmol/liter; normal range 194-1500 pmol/liter); and suppressed plasma renin activity (0.22 ng/dl·h; normal range 171-1115 ng/dl·h) levels (Fig. 1A). Her growth and development were normal. Dexamethasone suppression test (0.2 mg every 6 h for 4 d) showed no suppression of circulating aldosterone concentrations (3546 pmol/liter) or her BP measurements. She was treated with spironolactone at a dose of 12.5 mg daily, which had to be further increased gradually to 50 mg daily.
Fig. 1.
A, Kindred structure of the affected family. Affected members are shown as filled symbols. The arrow indicates the index case. B, Part of the sequencing chromatogram of exon 2 of the KCNJ5 gene in a normal subject (upper panel) and the patients carrying the heterozygous T-to-G transition n.G470T (lower panel). C, KCNJ5 mutations result in the depolarization of current reversal potential. Reversal potentials are plotted for wild-type (WT) Kir3.4 (KCNJ5)/Kir3.1 (KCNJ3) channels and each Kir3.4 (KCNJ5) mutant/Kir3.1 (KCNJ3). WT channels (white bar) show a negative reversal potential. Mutant channels (gray bars) show less negative reversal potentials (P < 0.05 in each case), suggesting a loss of ion selectivity. Numbers in parentheses are the number of cells recorded.
She presented again to us at the age of 15 yr. In the intervening time, she had undergone normal pubertal development, and there was no evidence of hirsutism or virilization. Her BP was 143/92 mm Hg. Her medication consisted of atenolol, amlodipine, spironolactone, and potassium chloride supplements. Magnetic resonance imaging of the adrenal glands confirmed massive bilateral adrenal hyperplasia. Because of her severe and refractory to treatment HTN, she underwent complete left adrenalectomy and right hemiadrenalectomy (her parents did not consent to bilateral adrenalectomy). Histopathological examination confirmed bilateral diffuse adrenocortical hyperplasia, predominantly on the left side. Over the subsequent years, further hyperplasia of the remaining right adrenal was documented and the symptoms and signs of primary hyperaldosteronism recurred. She is currently on amlodipine, spironolactone, and potassium chloride supplements and is awaiting surgery for removal of the remaining right adrenal gland.
The mother of the index case was noted to have HTN at the age of 7 yr, when her arterial blood pressure was found to be elevated at 170/100 mm Hg upon routine measurement before tonsillectomy (Fig. 1A). She had hypokalemia, markedly increased plasma aldosterone concentrations, and low plasma renin activity levels. A computed tomography scan confirmed massive bilateral adrenal hyperplasia. Because of severe and refractory to treatment HTN, the patient underwent bilateral adrenalectomy at the age of 13 yr, which resulted in complete normalization of her blood pressure and serum potassium concentrations. She has since remained normotensive, with normal electrolytes and plasma renin concentrations, on replacement therapy with hydrocortisone and 9α-fludrocortisone. No other members of the immediate family reported any similar manifestations; however, they have not provided consent for formal endocrinological and genetic evaluation (Fig. 1A).
Given that the features resembled those of familial hyperaldosteronism (FH) type I or glucocorticoid-remediable aldosteronism, both the mother and the daughter underwent genetic testing for glucocorticoid-remediable aldosteronism, which was negative. In addition, sequencing of the AT1R was undertaken, which was also negative.
The above clinical manifestations, in association with the findings of the endocrinological evaluation, suggested the possibility of familial hypertension secondary to a defect in the genes encoding K+ channels. Written informed consent was obtained by both patients and further molecular studies were undertaken.
Amplification and sequencing of the KCNK3, KCNK5, KCNK9, and KCNJ5 genes
Genomic DNA was isolated from peripheral blood lymphocytes using the Maxwell 16 instrument for automated DNA extraction (Promega Corp., Madison, WI). The coding sequences and the intron-exon junctions of the genes KCNK3 (TASK1), KCNK5 (TASK2), KCNK9 (TASK3), and KCNJ5 were amplified by PCR and bidirectionally sequenced using the Big Dye Terminator cycle sequencing kit (Applied Biosystems Inc., Foster City, CA) on an ABI 3100 sequencer (ABI 3100; Applied Biosystems). The primers used for the PCR amplification of the genomic DNA were designed using the exon/intron junction sequences and are listed in Table 1. In silico analysis of the mutation was carried out using the programs PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/dokuwiki/start) and SIFT (http://sift.jcvi.org/).
Table 1.
Primers used to amplify each exon of the KCNK3, KCNK5, KCNK9, and KCNJ5 genes
| Gene | Exon | Primer sequence (5′–3′) |
|---|---|---|
| KCNK3 | 1F | GGG TGG TGC TGA AGG GAC A |
| 1R | CAC ACA CGC CAG GCT GAA C | |
| 2F | AGC CAG GGC CTA GCA CAG | |
| 2R | AGT GGT GAG GGA TGT TGT GC | |
| 2F | TAC ATC CTT ACG GGC CTC AC | |
| 2R | ACC ACG AGG TTG AGG AAG G | |
| KCNK5 | 1F | AGC TGT TTT CAG AGG CTG GA |
| 1R | GAC AGA TCC TCG AAG CCT GA | |
| 2F | CTG CCA CCT TCT TCT TGT GA | |
| 2R | CTG ATT GTA CCC CCA ACA GG | |
| 3F | TAG AGA ACG GGC TGT GTG TG | |
| 3R | TCC TCT TGC CAT CTG TCC TT | |
| 4F | ACC CAG CTA GCT TCT CCT CT | |
| 4R | AAG TGC CCA GAA CAT GGA AC | |
| 5F | ATA GTC CAG GGT CTA GAA CAG | |
| 5R | TGA GGA TGA TGG AAG GTT AGG | |
| 5F | CAA AGG CCA CGT ATC AAG GT | |
| 5R | TGG AAG ATG AGT GGG TGG T | |
| KCNK9 | 1F | CGC CGC TTA CAA CTT GGA G |
| 1R | GCT GCG TTT AAC CCT CGA C | |
| 2F | GTA CCA GGT CCC TCT GCA TT | |
| 2R | CGA GTT GGA CCA ATG GAA ATT | |
| 2F | AGA AGA AGC CGC TCT ACG TG | |
| 2R | GGT CAA GAA CCT GAG GAC GA | |
| KCNJ5 | 2F | GTG GCC TTC CAT CTT GTG TT |
| 2R | AGG CAC AGA CCT GCA TCT TC | |
| 2AF | CGA CCA AGA GTG GAT TCC TT | |
| 2AR | AGG GTC TCC GCT CTC TTC TT | |
| 3F | TTT CCA TAT CTG GAT GGA TGG | |
| 3R | GGC TCT GCA GTG TCT GTG TT |
The above primers were designed using the primer3 (version 0.0.4.0) design program. The PCR amplification products of each exon were directly sequenced using the same primers as for the PCR and two additional internal primers for exon 2. Sequencing was performed using the Big Dye Terminator cycle sequencing kit (Applied Biosystems) on an ABI 3100 sequencer (ABI 3100 Avant; Applied Biosystems). F, Forward; R, reverse; AF, additional internal forward; AR, additional internal reverse.
Plasmids
The plasmids expressing the wild-type Kir3.4 (KCNJ5) as well as those with the G151R, T158A, and L168R mutations were kindly provided by Dr. Richard P. Lifton (Departments of Genetics and Internal Medicine, Howard Hughes Medical Institute, Yale University School of Medicine, New Haven, CT) (14). Specifically, for the wild-type construct, the KCNJ5 gene was cloned into the BamH1 site of the PIRES2-EGFP plasmid (CLONTECH Laboratories, Mountain View, CA). The plasmid expressing the KCNJ5I157S was constructed by introducing the indicated mutation into the wild-type Kir3.4 plasmid using PCR-assisted, site-directed mutagenesis (Stratagene, La Jolla, CA). The successful introduction of the I157S mutation into the wild-type construct was confirmed by sequencing.
Electrophysiology
Human embryonic kidney (HEK) 293T cells were cotransfected with either wild-type Kir3.4 (KCNJ5) (0.5 μg) or its mutants and Kir3.1 (KCNJ3) (0.5 μg) in 35-mm dishes using Fugene 6 (Roche Applied Science, Indianapolis, IN). An empty vector was used as a control. Recordings were made 24 h after transfection. Coverslips containing transfected HEK 293T cells were submerged in the recording chamber and exposed to a continuous flow of artificial cerebrospinal fluid consisting of the following: 140 mm NaCl, 5 mm KCl, 1.8 mm CaCl2, 1.8 mm MgCl2, and 10 mm HEPES. The artificial cerebrospinal fluid was perfused with 95% O2-5% CO2. An infrared differential interference contrast videomicroscopy system (Zeiss Instruments, Oberkochen, Germany; Diagnostic Instruments, Sterling Heights, MI) was used to visualize cells. Patch pipettes were pulled from thick-walled borosilicate glass (Warner Instruments, Hamden, CT) to achieve a tip resistance of 2–5 mΩ. Pipettes were filled with an internal solution containing the following: 140 mm KCl, 5 mm HEPES, 1 mm EGTA, 1 mm CaCl2, and 4 mm MgCl2. Whole-cell voltage clamp recordings were made using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA) and Clampex 10.1 software (Molecular Devices). Signals were digitized at 10 kHz with a Digidata 1440A (Molecular Devices) and filtered at 4 kHz. Currents were elicited by delivering a second long pulse to −100 to +60 mV in 20-mV increments from a 0-mV holding. This protocol was repeated three times for each cell. Leak currents were recorded from empty vector controls and subtracted from each recording. A linear fit between averaged least negative and positive currents from each cell was used to estimate the reversal potential. All recordings were performed at room temperature. Recordings were analyzed using Clampfit 10.1 (Molecular Devices) and Microsoft Excel (Redmond, WA). Statistical significance was evaluated using a Student's t test (two sided, unpaired).
Structural biology
A model of the monomer of human Kir3.4 (KCNJ5) was created by submitting its sequence to the I-TASSER web service and specifying the structure of chicken Kir2.2 (KCNJ12 ortholog) as a template (18, 19). The chicken Kir2.2 (KCNJ12), whose crystal structure was reported recently, has an 89% amino acid homology to the human Kir3.4 (KCNJ5) (19). The physiological tetramer was reassembled by structure superposition in PyMOL (The PyMOL molecular graphics system, version 1.2r3pre; Schrödinger, LLC, Portland, OR) using the resulting model. The TM score of the alignment was 0.94, suggesting an excellent agreement between the model and template (20). LigPlot was used to analyze the molecular contacts of the native and I157S mutant KCNJ5 at that position (21). For the alignment of KCNJ5 encoded by several species and other inward Kir, the coding sequences of indicated channels were obtained from GenBank, and amino acid alignment was performed with the AlignX software (Invitrogen, Carlsbad, CA).
Results
Sequencing of the KCNK3, KCNK5, KCNK9, and KCNJ5 genes
The exons of the entire coding region of the KCNK3, KCNK5, KCNK9, and KCNJ5 genes, including the intron/exon junctions, were amplified by PCR and sequenced. In both our patients, a single, heterozygous guanine to thymine (G → T) substitution was identified at nucleotide position 470 (n.G470T) of the KCNJ5 gene, resulting in isoleucine (I) to serine (S) substitution at amino acid 157 (p.I157S) (Fig. 1B). The in silico analysis of the amino acid change detected, using the PolyPhen program, predicted that the mutation is probably damaging. When the SIFT program was used, the I-to-S substitution at position 157 was predicted to affect the protein function with a score of 0.00. Sequencing of the KCNK3, KCNK5, and KCNK9 genes revealed no mutations in our patients.
Electrophysiology
We examined the properties of normal and mutant Kir3.4 channels by coexpressing them with Kir3.1 in HEK 293T cells. In whole-cell voltage-clamp recordings, we measured currents at voltages ranging between −100 and +60 mV. We observed an inwardly rectifying current with a reversal potential of −21.0 ± 3.7 mV in the wild-type channels (Fig. 1C). This current was eliminated in the presence of barium. Similar to the previously reported mutations T158A, G151R, and L168R, the I157S mutation showed loss-of-channel selectivity and less negative reversal potentials. Although the substitution of choline for sodium in the bath solution did not affect the wild-type current, it significantly reduced the current in mutant recordings (current at −100 mV: WT control = −115 ± 26 pA, n = 23; WT + barium = −14 ± 8 pA, n = 9; and WT + barium and choline = 5 ± 7 pA, n = 6; I157S control = −156 ± 70 pA, n = 6; I157S + barium = −100 ± 50 pA, n = 7; I157S + barium and choline = −16 ± 12 pA, n = 6). These findings indicate that the mutations near the channel pore lead to loss of K+ ion selectivity and increased Na+ conductance.
Structural biology
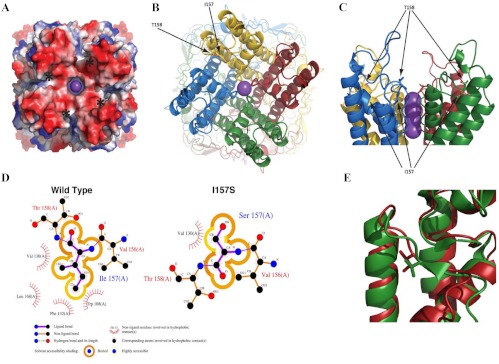
The residue corresponding to Kir3.4 (KCNJ5) I157 in the chicken KCNJ12 structure is a V151. Both residues are found at the C-terminal end of the loop leading away from the selectivity filter, in which they pack into a hydrophobic pocket formed by three nearby α-helices. Substitution of a polar residue in that region would be energetically unfavorable; a conformational change in the loop would be required to satisfy the hydrogen bond of the serine hydroxyl group and thereby disrupt the selectivity filter, which is sensitive to small perturbations (Fig. 2). The amino acid alignment of KCNJ5 among different species revealed that the isoleucine mutated in our cases was conserved among all species examined (Fig. 3A). The inward rectifier potassium channel family consists of seven subgroups (22). In the analysis on representative channels of this family (Kir1.1, 2.1, 2.2, 3.1, 3.2, 3.3, 3.4, 4.1, 5.1, 6.1, and 7.1), all subtypes of the human Kir3 subgroup had isoluecine in this amino acid position, whereas most of the channels in the other subgroups had the hydrophobic amino acids (valine or methionine) at this position (Fig. 3B). These results suggest that hydrophobicity provided by these residues at this position is important for proper function of inward rectifier potassium channels. Additional modeling and simulation may reveal the mechanism of disruption.
Fig. 2.
A, Electrostatic potential of the Kir3.4 (KCNJ5) tetramer. The mutation site is on the edge of the electronegative bowl (red) leading to the channel and is indicated by asterisks. B and C, Mutations that affect channel selectivity. The solvent-exposed T158A mutation reduces selectivity (14). The adjacent I157S mutation was buried in a hydrophobic pocket and also affected selectivity. D, The hydrophobic pocket in which I157 is buried would not easily accommodate the hydroxyl group of serine mutation, inducing conformational change to the loop, which may propagate a perturbation down the loop to the selectivity filter a few residues away. E, Structure superposition of the template (3JYC; green) and the model of Kir3.4 (KCNJ5) (red). Note that the residues are both hydrophobic (valine and isoleucine) and bury into a pocket at the intersection of the three α-helices.
Fig. 3.
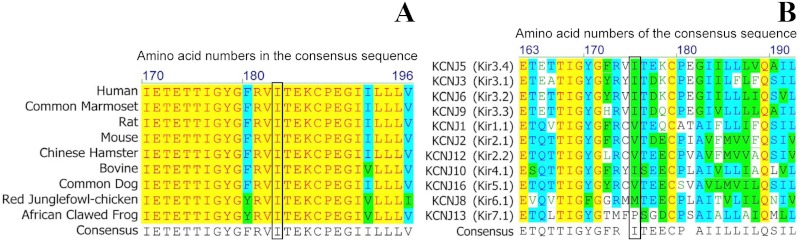
Multiple alignment of KCNJ5 protein sequence in different species (A) and different members of the KCNJ potassium channel family (B), indicating conservation of the residue affected by this mutation. Isoleucine at amino acid position 157 in the human KCNJ5 (Kir3.4) is conserved among several species, whereas most of the inward rectifier potassium channels have hydrophobic amino acids at this position. The yellow color indicates the amino acids conserved in all species compared, the blue color indicates those conserved in most of the species, whereas the green color indicates the amino acids in the same group.
Discussion
In this study, we describe a family with hereditary hypertension and massive bilateral adrenal hyperplasia caused by a novel mutation of the KCNJ5 gene. Both the index case and her mother presented in childhood with markedly elevated aldosterone concentrations, suppressed plasma renin activity, and early onset severe hypertension refractory to treatment, which required bilateral adrenalectomy. Sequencing of the KCNJ5 gene revealed a single, heterozygous guanine to thymine (G → T) substitution at nucleotide position 470, resulting in I-to-S substitution at amino acid position 157.
Primary hyperaldosteronism (PA) is the most common cause of secondary hypertension. Its prevalence is estimated to be approximately 10% within the hypertensive population (23, 24) and higher among patients with hypertension refractory to medical treatment (25). The elevated aldosterone concentrations in PA are not suppressed by dietary sodium loading, volume expansion, or antagonism of the renin-angiotensin system (26). Early diagnosis and treatment of patients with PA is of fundamental importance because they have significantly higher incidence of stroke, myocardial infarction, and atrial fibrillation compared with age-, sex-, and BP-matched patients with essential HTN (27); however, they can be cured in many cases and substantially improved in the remainder by removal of the affected adrenal gland.
Three familial forms of PA have been described to date, referred to as FH-I, FH-II, and FH-III. FH-I, also known as glucocorticoid remediable aldosteronism, is an autosomal dominant condition characterized by early onset hypertension, elevated ACTH-dependent aldosterone concentrations, suppressed plasma renin concentrations, and increased urinary excretion of the hybrid steroids, 18-hydroxycortisol and 18-oxocortisol. Despite the elevated aldosterone concentrations, hypokalemia is uncommon (28, 29). The molecular basis of the condition is attributed to a chimeric CYP11B1/CYP11B2 gene encoding a hybrid enzyme that synthesizes aldosterone under the control of ACTH (28). Therefore, in patients with FH-I, aldosterone concentrations are persistently suppressed by glucocorticoid administration. Most affected individuals develop severe hypertension in early life and are prone to cerebrovascular events, although patients with mild hypertension or blood pressure within the normal range have been described in many families (30, 31).
FH-II is characterized by nonglucocorticoid remediable hyperaldosteronism and bilateral adrenocortical hyperplasia often combined with adenomas. FH-II is phenotypically indistinguishable from sporadic PA and can be transmitted in an autosomal dominant fashion (32–37). The molecular basis of FH-II is still unknown; however, a linkage with the chromosomal region 7p22 has been shown in some, but not all, families with the condition (38).
FH-III is an autosomal dominant condition characterized by early-onset hypertension, nonglucocorticoid-remediable hyperaldosteronism and hypokalemia. The genetic cause of FH-III is attributed to mutations in the KCNJ5 gene encoding the potassium channel Kir 3.4 (14). The clinical spectrum of the condition in the limited number of families reported to date appears to be broad, ranging from mild or moderate hypertension, which is responsive to medical therapy, to more severe and refractory to medical treatment hypertension that requires bilateral adrenalectomy (14–16, 39, 40). Furthermore, bilateral adrenocortical hyperplasia has been described in some (14) but not all subjects (15) with the condition. The absence of adrenal hyperplasia in subjects with genetically confirmed FH-III is thought to arise as a result of mutations in the KCNJ5 gene that produce a much larger Na+ conductance, leading to rapid Na+-dependent cell lethality (15). Interestingly, somatic mutations in KCNJ5 have also been detected in aldosterone-producing adenomas from patients with severe hypertension and hypokalemia (14, 16, 17).
Similarly to the previously reported mutations G151R, T158A, and L168R (14), in our electrophysiological studies, the I157S mutation showed loss of K+ ion selectivity and less negative reversal potentials. Hydrophobicity carried by isoleucine at amino acid position 157 together with a hydrophobic pocket formed by three nearby α-helices appears to be essential to confer K+ selectivity by maintaining proper alignment/structure of the selectivity filter, whereas I to S (nucleophilic amino acid) replacement at this position could have damaged this protein tertiary structure, eventually losing the ability of the mutated channel to exclude Na+ (Fig. 3). Loss of ion selectivity caused by this amino acid replacement in Kir3.4 channel increased cell membrane depolarization, increased Ca2+ entry in the adrenal glomerulosa cells and increased aldosterone synthesis. These findings implicate inherited and acquired mutations in the KCNJ5 gene in primary hyperaldosteronism, which in certain cases might be associated with autonomous cell proliferation.
In summary, we report a new family with FH-III, in which two affected members, a mother and a daughter, presented with severe primary hyperaldosteronism and bilateral adrenal hyperplasia caused by a novel point mutation of the KCNJ5 gene. The findings of this study explain the pathogenesis in a subset of patients with severe early onset familial hypertension and implicate loss of K+ channel selectivity in constitutive aldosterone production and, in some cases, glomerulosa cell proliferation. In addition, these findings may help develop rational pharmacological therapies for the management of primary hyperaldosteronism caused by mutations in the KCNJ5 gene.
Acknowledgments
This work was supported in part by the University of Athens Medical School (Athens, Greece), the intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases (National Institutes of Health, Bethesda, MD).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AngII
- Angiotensin II
- AT1R
- AngII type 1 receptor
- BP
- blood pressure
- CYP11B2
- aldosterone synthase
- FH
- familial hyperaldosteronism
- HEK
- human embryonic kidney
- HTN
- hypertension
- I
- isoleucine
- KCNK
- K+-selective leak
- Kir
- rectifier potassium channel
- PA
- primary hyperaldosteronism
- S
- serine
- TASK
- TWIK-related acid sensitive K+.
References
- 1. Quinn SJ, Williams GH. 1988. Regulation of aldosterone secretion. Annu Rev Physiol 50:409–426 [DOI] [PubMed] [Google Scholar]
- 2. Müller J. 1995. Aldosterone: the minority hormone of the adrenal cortex. Steroids 60:2–9 [DOI] [PubMed] [Google Scholar]
- 3. Spät A, Hunyady L. 2004. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev 84:489–539 [DOI] [PubMed] [Google Scholar]
- 4. Hunyady L, Catt KJ. 2006. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol 20:953–970 [DOI] [PubMed] [Google Scholar]
- 5. Spät A. 2004. Glomerulosa cell—a unique sensor of extracellular K+ concentration. Mol Cell Endocrinol 217:23–26 [DOI] [PubMed] [Google Scholar]
- 6. Hollenberg NK, Williams G, Burger B, Hooshmand I. 1975. The influence of potassium on the renal vasculature and the adrenal gland, and their responsiveness to angiotensin II in normal man. Clin Sci Mol Med 49:527–534 [DOI] [PubMed] [Google Scholar]
- 7. Vallotton MB, Rossier MF, Capponi AM. 1995. Potassium-angiotensin interplay in the regulation of aldosterone biosynthesis. Clin Endocrinol (Oxf) 42:111–119 [DOI] [PubMed] [Google Scholar]
- 8. Chen XL, Bayliss DA, Fern RJ, Barrett PQ. 1999. A role for T-type Ca2+ channels in the synergistic control of aldosterone production by ANG II and K+. Am J Physiol 276:F674–F683 [DOI] [PubMed] [Google Scholar]
- 9. Lotshaw DP. 1997. Effects of K+ channel blockers on K+ channels, membrane potential, and aldosterone secretion in rat adrenal zona glomerulosa cells. Endocrinology 138:4167–4175 [DOI] [PubMed] [Google Scholar]
- 10. Czirják G, Fischer T, Spät A, Lesage F, Enyedi P. 2000. TASK (TWIK-related acid-sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Mol Endocrinol 14:863–874 [DOI] [PubMed] [Google Scholar]
- 11. Czirják G, Enyedi P. 2002. TASK-3 dominates the background potassium conductance in rat adrenal glomerulosa cells. Mol Endocrinol 16:621–629 [DOI] [PubMed] [Google Scholar]
- 12. Bandulik S, Penton D, Barhanin J, Warth R. 2010. TASK1 and TASK3 potassium channels: determinants of aldosterone secretion and adrenocortical zonation. Horm Metab Res 42:450–457 [DOI] [PubMed] [Google Scholar]
- 13. Nogueira EF, Gerry D, Mantero F, Mariniello B, Rainey WE. 2010. The role of TASK1 in aldosterone production and its expression in normal adrenal and aldosterone-producing adenomas. Clin Endocrinol (Oxf) 73:22–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choi M, Scholl UI, Yue P, Björklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Åkerström G, Wang W, Carling T, Lifton RP. 2011. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 331:768–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scholl UI, Nelson-Williams C, Yue P, Grekin R, Wyatt RJ, Dillon MJ, Couch R, Hammer LK, Harley FL, Farhi A, Wang WH, Lifton RP. 2012. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc Natl Acad Sci USA 109:2533–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mulatero P, Tauber P, Zennaro MC, Monticone S, Lang K, Beuschlein F, Fischer E, Tizzani D, Pallauf A, Viola A, Amar L, Williams TA, Strom TM, Graf E, Bandulik S, Penton D, Plouin PF, Warth R, Allolio B, Jeunemaitre X, Veglio F, Reincke M. 2012. KCNJ5 mutations in European families with nonglucocorticoid remediable familial hyperaldosteronism. Hypertension 59:235–240 [DOI] [PubMed] [Google Scholar]
- 17. Xekouki P, Hatch MM, Lin L, Rodrigo DA, Azevedo M, de la Luz Sierra M, Levy I, Saloustros E, Moraitis A, Horvath A, Kebebew E, Hoffman DA, Stratakis CA. 2012. KCNJ5 mutations in the National Institutes of Health cohort of patients with primary hyperaldosteronism: an infrequent genetic cause of Conn's syndrome. Endocr Relat Cancer 19:255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roy A, Kucukural A, Zhang Y. 2010. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5:725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tao X, Avalos JL, Chen J, MacKinnon R. 2009. Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 A resolution. Science 326:1668–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu J, Zhang Y. 2010. How significant is a protein structure similarity with TM-score = 0.5? Bioinformatics 26:889–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wallace AC, Laskowski RA, Thornton JM. 1995. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng 8:127–134 [DOI] [PubMed] [Google Scholar]
- 22. Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. 2010. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90:291–366 [DOI] [PubMed] [Google Scholar]
- 23. Rossi GP, Pessina AC, Heagerty AM. 2008. Primary aldosteronism: an update on screening, diagnosis and treatment. J Hypertens 26:613–621 [DOI] [PubMed] [Google Scholar]
- 24. Rossi GP, Seccia TM, Pessina AC. 2011. Adrenal gland: a diagnostic algorithm-the holy grail of primary aldosteronism. Nat Rev Endocrinol 7:697–699 [DOI] [PubMed] [Google Scholar]
- 25. Douma S, Petidis K, Doumas M, Papaefthimiou P, Triantafyllou A, Kartali N, Papadopoulos N, Vogiatzis K, Zamboulis C. 2008. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet 371:1921–1926 [DOI] [PubMed] [Google Scholar]
- 26. Fardella CE, Mosso L, Gómez-Sánchez C, Cortés P, Soto J, Gómez L, Pinto M, Huete A, Oestreicher E, Foradori A, Montero J. 2000. Primary hyperaldosteronism in essential hypertensives: prevalence, biochemical profile, and molecular biology. J Clin Endocrinol Metab 85:1863–1867 [DOI] [PubMed] [Google Scholar]
- 27. Connell JM, MacKenzie SM, Freel EM, Fraser R, Davies E. 2008. A lifetime of aldosterone excess: long-term consequences of altered regulation of aldosterone production for cardiovascular function. Endocr Rev 29:133–154 [DOI] [PubMed] [Google Scholar]
- 28. Lifton RP, Dluhy RG, Powers M, Rich GM, Gutkin M, Fallo F, Gill JR, Jr, Feld L, Ganguly A, Laidlaw JC, Murnaghan DJ, Kaufman C, Stockigt JR, Ulick S, Lalouel JM. 1992. Hereditary hypertension caused by chimaeric gene duplications and ectopic expression of aldosterone synthase. Nat Genet 2:66–74 [DOI] [PubMed] [Google Scholar]
- 29. Halperin F, Dluhy RG. 2011. Glucocorticoid-remediable aldosteronism. Endocrinol Metab Clin North Am 40:333–341, viii [DOI] [PubMed] [Google Scholar]
- 30. Mulatero P, di Cella SM, Williams TA, Milan A, Mengozzi G, Chiandussi L, Gomez-Sanchez CE, Veglio F. 2002. Glucocorticoid remediable aldosteronism: low morbidity and mortality in a four-generation Italian pedigree. J Clin Endocrinol Metab 87:3187–3191 [DOI] [PubMed] [Google Scholar]
- 31. Fallo F, Pilon C, Williams TA, Sonino N, Morra Di Cella S, Veglio F, De Iasio R, Montanari P, Mulatero P. 2004. Coexistence of different phenotypes in a family with glucocorticoid-remediable aldosteronism. J Hum Hypertens 18:47–51 [DOI] [PubMed] [Google Scholar]
- 32. Torpy DJ, Gordon RD, Lin JP, Huggard PR, Taymans SE, Stowasser M, Chrousos GP, Stratakis CA. 1998. Familial hyperaldosteronism type II: description of a large kindred and exclusion of the aldosterone synthase (CYP11B2) gene. J Clin Endocrinol Metab. 83:3214–3218 [DOI] [PubMed] [Google Scholar]
- 33. Torpy DJ, Stratakis CA, Chrousos GP. 2000. Familial hyperaldosteronism. Braz J Med Biol Res 33:1149–1155 [DOI] [PubMed] [Google Scholar]
- 34. Stowasser M, Gordon RD, Tunny TJ, Klemm SA, Finn WL, Krek AL. 1992. Familial hyperaldosteronism type II: five families with a new variety of primary aldosteronism. Clin Exp Pharmacol Physiol 19:319–322 [DOI] [PubMed] [Google Scholar]
- 35. Gordon RD, Stowasser M, Klemm SA, Tunny TJ. 1995. Primary aldosteronism—some genetic, morphological, and biochemical aspects of subtypes. Steroids 60:35–41 [DOI] [PubMed] [Google Scholar]
- 36. Gordon RD. 1995. Primary aldosteronism. J Endocrinol Invest 18:495–511 [DOI] [PubMed] [Google Scholar]
- 37. Gordon RD, Stowasser M. 1998. Familial forms broaden the horizons for primary aldosteronism. Trends Endocrinol Metab 9:220–227 [DOI] [PubMed] [Google Scholar]
- 38. Sukor N, Mulatero P, Gordon RD, So A, Duffy D, Bertello C, Kelemen L, Jeske Y, Veglio F, Stowasser M. 2008. Further evidence for linkage of familial hyperaldosteronism type II at chromosome 7p22 in Italian as well as Australian and South American families. J Hypertens 26:1577–1582 [DOI] [PubMed] [Google Scholar]
- 39. Geller DS, Zhang J, Wisgerhof MV, Shackleton C, Kashgarian M, Lifton RP. 2008. A novel form of human Mendelian hypertension featuring non-glucocorticoid remediable aldosteronism. J Clin Endocrinol Metab 93:3117–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mulatero P. 2008. A new form of hereditary primary aldosteronism: familial hyperaldosteronism type III. J Clin Endocrinol Metab 93:2972–2974 [DOI] [PubMed] [Google Scholar]