Abstract
Context:
The link between adolescent fiber consumption, inflammation, and body fat distribution has not been investigated.
Objective:
This study investigated associations of dietary fiber intake with inflammatory-related biomarkers and robust measures of total and central adiposity in a sample of 559 adolescents aged 14–18 yr (49% female, 45% Black).
Methods:
Fasting blood samples were measured for leptin, adiponectin, resistin, C-reactive protein, and fibrinogen. Diet was assessed with four to seven 24-h recalls, and physical activity was determined by accelerometry. Fat-free soft tissue mass and fat mass were measured by dual-energy x-ray absorptiometry. Visceral adipose tissue was assessed using magnetic resonance imaging.
Results:
Multiple linear regression, adjusting for age, race, Tanner stage, fat-free soft tissue mass, energy intake, and physical activity, revealed that dietary fiber intake was inversely associated with fat mass and serum leptin in males (all P < 0.03) but not in females. In both genders, dietary fiber intake was negatively associated with visceral adipose tissue, plasma C-reactive protein, and plasma fibrinogen and positively associated with plasma adiponectin (all P < 0.05). No relations were found between dietary fiber intake and plasma resistin in either males or females.
Conclusion:
Our adolescent data suggest that greater consumption of dietary fiber is associated with lower visceral adiposity and multiple biomarkers implicated in inflammation.
A recent development in obesity research is the concept that increased adiposity is characterized by greater systemic inflammation. The basis for this notion is that inflammatory-related factors, including various cytokines and acute-phase proteins, are increased in obesity, particularly abdominal obesity (1). Because adipocytes and macrophages within fat can secrete cytokines and acute-phase proteins, it is considered that increasing visceral fat accumulation contributes to greater production of these inflammatory-related factors, thus leading to chronic low-grade systemic inflammation, a well-known risk factor for cardiovascular disease and diabetes (2).
Dietary fiber intake may play a protective role against disorders associated with obesity-related inflammation (3–6); however, the mechanism for this effect is unclear. It is plausible that dietary fiber intake may mediate the proinflammatory response, as evidenced by some studies showing beneficial effects of dietary fiber on inflammatory-related factors such as adipokines and acute-phase proteins. For instance, in a human ex vivo study, Al-Lahham and colleagues (7) observed that propionic acid, a dietary fiber derivative, stimulated expression and secretion of adipocyte-derived leptin, whereas it nearly abolished expression of macrophage-derived resistin. The investigators did not observe an effect of propionic acid on adiponectin, another adipokine. Other studies, however, have reported a beneficial effect of dietary fiber intake on adiponectin concentrations (8, 9). Investigations between dietary fiber and C-reactive protein, an acute-phase protein, have been more consistent in showing that elevated C-reactive protein levels are associated with lower dietary fiber intakes (10–13). On the other hand, the relationship between fibrinogen, another acute-phase protein, and dietary fiber intake is controversial (14, 15).
The discrepancies in the aforementioned dietary fiber and inflammatory factor investigations can be attributed, in part, to differences in the population studied and the study design and instruments used. However, it is also likely that fat depots measured (visceral vs. sc) could be an additional confounding factor. Because metabolic abnormalities, including chronic low-grade systemic inflammation, are more strongly associated with visceral, rather than sc, fat (1, 16), additional work is needed to determine whether type of adiposity could play a factor in the dietary fiber-inflammation relationship.
A limitation of the aforementioned dietary fiber-inflammation investigations was that study participants were adults. Because national data indicate that U.S. adolescents consume less than one half of the recommended adequate intake (AI) of dietary fiber (14 g/1000 kcal) (17, 18), investigations between dietary fiber intake and inflammatory factors in this population are warranted. Therefore, the purpose of this study was to determine whether adolescent fiber consumption is associated with inflammatory-related biomarkers (leptin, adiponectin, resistin, C-reactive protein, and fibrinogen) and robust measures of total and central adiposity. Furthermore, we explored whether the dietary fiber-inflammation relationship is modified by type of adiposity.
Subjects and Methods
Participants
Participants in this cross-sectional investigation were 559 adolescents who were recruited from local public high schools in the Augusta, GA, area. With approval from superintendents and school principals, flyers were distributed to all students in the high schools. Inclusion criteria for this study were White/Caucasian or Black/African-American race and age 14–18 yr. Adolescents were excluded if they were taking medications or had any chronic medical conditions that might affect growth, maturation, physical activity, nutritional status, or metabolism. Informed consent and assent were obtained from all parents and adolescents, respectively. The protocol was approved by the Human Assurance Committee at the Georgia Health Sciences University (institutional review board). All measurements were performed at the Georgia Prevention Institute at the Georgia Health Sciences University between 2001 and 2005.
Anthropometry and sexual maturation stage
Height (centimeters) and body weight (kilograms) were measured to calculate sex- and age-specific body mass index percentiles (19). Sexual development of the participants were measured by a five-stage scale, ranging from 1 (prepubertal) to 5 (fully mature) as described by Tanner (20). Using a gender-specific questionnaire, the subjects reported their sexual maturation stage (or Tanner stage) by comparing their own physical development to the five stages in standard sets of diagrams. A parent or research coordinator then reviewed the results with the children to make sure they understood the questionnaire. When an individual reported discordant stages of pubic hair and breast or genital development, the higher of the two stages was used.
Biochemical parameters
Fasting blood samples were obtained for measurement of serum leptin, plasma adiponectin and plasma resistin, plasma C-reactive protein, and plasma fibrinogen. Leptin was measured using serum that was assayed in duplicate using ELISA (R&D Systems, Minneapolis, MN). The intraassay coefficient of variation (CV) for leptin was 2%, and the interassay CV was 5%. Adiponectin and resistin were measured using plasma that was assayed in duplicate using ELISA (Linco Research Inc., St. Charles, MO). Adiponectin had an intraassay CV at 7.4% and an interassay CV at 8.4%. The intra- and interassay CV for resistin were 3.2 and 7.1%, respectively. C-reactive protein was measured in plasma using high-sensitivity ELISA (ALPCO Diagnostics, Windham, NH). The mean intra- and interassay CV for C-reactive protein were 10 and 10.2%, respectively. The assay procedure for fibrinogen has been described in detail in a previous publication (21).
Body composition and fat distribution measures
Fat-free soft tissue (FFST) mass (kilograms) and fat mass (kilograms) were assessed by dual-energy x-ray absorptiometry (QDR-4500W; Hologic Inc., Waltham, MA). For determination of measurement reproducibility, one-way random-effects model, single-measure intraclass correlation coefficients were calculated in participants 15–18 yr of age (n = 219). Each participant was scanned twice within a 7-d period for FFST mass and fat mass (both r ≥ 0.97).
Visceral adipose tissue (VAT) was measured by using magnetic resonance imaging (1.5-T; General Electric Medical Systems, Milwaukee, WI). Assessment of VAT is described in detail elsewhere (22). Briefly, a series of five transverse images was acquired from the lumbar region beginning at the inferior border of the fifth lumbar vertebra and proceeding toward the head; a 2-mm gap between images was used to prevent cross talk. To calculate volume for VAT, the cross-sectional area (square centimeters) from each slice was multiplied by the slice width (1 cm), and then the individual volumes (cubic centimeters) were summed. The intraclass correlation coefficients for repeat analyses of the same scans on separate days within a 7-d period was R = 0.98 for VAT.
Physical activity
The number of minutes per day spent in moderate and vigorous physical activities were assessed using MTI Actigraph monitors (model 7164; MTI Health Services, Fort Walton Beach, FL), uniaxial accelerometers that measure vertical acceleration and deceleration. With epoch length set at 1 min and expressed as counts per minute, the accelerometers were to begin recording when the subject left our laboratory after the first day of testing. The subjects were instructed to 1) wear the monitor for a period of 7 d, 2) remove it for sleep, bathing, and any activity that may cause harm to either the monitor or another person (e.g. during contact sports), and 3) bring the monitor back to us 1 wk later. Data from d 1 and 7 were discarded because a full day of information was not available for those days. Movement counts were converted to average minutes per day spent in moderate (3–6 metabolic equivalents) and vigorous (>6 metabolic equivalents) physical activity by the software accompanying the device.
Dietary intake
To assess mean daily intakes for energy (kilocalories), dietary fat (grams), dietary carbohydrate (grams), dietary protein (grams), and dietary fiber (grams), a trained registered dietitian conducted four to seven 24-h recalls (including one weekend day) using a multiple-pass, computer-assisted interview approach [Nutrition Data System for Research (NDS-R), Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN]. A total of 4, 5, 6, and 7 d of dietary information were collected in 10, 21, 22, and 47% of the adolescents, respectively, within 4 wk of the blood collection. The first two recalls were performed in person at our institute with the use of food models, portion booklets, or serving containers to assist in estimating serving size, and the remaining interviews were conducted by telephone. Participants were not interviewed on days when they had been ill or days that fell on a major holiday. To minimize the potential for undereating during the time frame for 24-h recalls, youths were blinded to the telephone recall schedule. A trained research assistant coded and analyzed dietary intake data using NDS-R software version 2006.
Statistical analyses
Descriptive statistics for raw variables are presented as mean ± sd. Normal distribution and homogeneity of variances were confirmed by Shapiro-Wilks W and Levene's tests, respectively. Sex differences for anthropometric, body composition, inflammatory markers, physical activity, and dietary intake were determined using independent-samples two-tailed t tests if data were distributed normally and Mann-Whitney U tests otherwise. Group differences in categorical variables were tested by using χ2 tests.
Multiple linear regression analyses were used to determine whether dietary fiber intake was independently related to total and central adiposity measurements (fat mass and VAT). Multiple linear regression analyses were also conducted to determine whether dietary fiber intake was associated with inflammatory markers, independent of the total and central adiposity measurements. Because body composition and biological changes emerge during puberty that influence gender-specific nutrient needs, daily AI fiber recommendations are given separately for males and females (18). For that reason, we conducted the linear regression analyses separately for the males and females. The basic model was adjusted for age, race, Tanner stage, FFST mass, energy intake, and physical activity. Second, to evaluate the effect of the total and central adiposity measurements on the dietary fiber-inflammatory marker relationships, we compared the following two different regression models by adding the two covariates (fat mass and VAT) separately and sequentially into the basic model. Before the regression analyses, leptin, adiponectin, resistin, and C-reactive protein were log-transformed so that each of these variables followed an approximate normal distribution. Data were analyzed using SPSS software (version 19.0; SPSS Inc., Chicago, IL), and statistical significance was set at P < 0.05.
Results
Descriptive characteristics of the participants are summarized in Table 1. The racial distribution, age, and Tanner stage were not different between genders. The females, however, were found to have lower levels of FFST mass, physical activity, energy intake, and macronutrient intake and higher levels of fat mass, VAT, leptin, adiponectin, resistin, and fibrinogen than males (all P < 0.05). There was a trend toward higher C-reactive protein levels in the females (P = 0.07). Furthermore, males consumed more dietary fiber than females (12.4 vs. 9.4 g/d, P < 0.01); these amounts were, respectively, 67 and 66% less than the daily recommended AI for male and female adolescents aged 14–18 yr (18).
Table 1.
Descriptive characteristics of the participants
| Variables | Females | Males | P valuea |
|---|---|---|---|
| n | 274 | 285 | |
| Age (yr) | 16.1 ± 1.2 | 16.2 ± 1.2 | 0.82 |
| Race (White/Black)b | 152/122 | 155/130 | 0.79 |
| Tanner stage (1–5) | 4.4 ± 0.7 | 4.3 ± 0.8 | 0.39 |
| BMI percentile | 61.9 ± 27.2 | 69.7 ± 15.4 | 0.81 |
| FFST mass (kg) | 39.6 ± 5.5 | 52.9 ± 8.8 | <0.01 |
| Fat mass (kg) | 18.7 ± 9.2 | 13.2 ± 9.2 | <0.01 |
| VAT (cm3) | 104.9 ± 59.2 | 87.9 ± 62.1 | <0.01 |
| Leptin (ng/ml)c | 17.4 ± 13.3 | 6.1 ± 8.3 | <0.01 |
| Adiponectin (μg/ml)c | 9.7 ± 5.5 | 7.7 ± 4.6 | <0.01 |
| Resistin (ng/ml)c | 12.6 ± 5.9 | 11.2 ± 6.1 | <0.01 |
| C-reactive protein (mg/liter)c | 1.3 ± 2.9 | 0.9 ± 1.8 | 0.07 |
| Fibrinogen (g/liter) | 2.9 ± 0.5 | 2.7 ± 0.5 | <0.01 |
| Moderate + vigorous PA (min/d) | 33.4 ± 21.6 | 52.7 ± 31.9 | <0.01 |
| Energy intake (kcal/d) | 1650 ± 498 | 2223 ± 592 | <0.01 |
| Dietary fat | |||
| g/d | 61.2 ± 21.6 | 83.2 ± 25.3 | <0.01 |
| % kcal | 33.1 ± 4.9 | 33.6 ± 4.5 | 0.26 |
| Dietary carbohydrate | |||
| g/d | 224.5 ± 71.6 | 293.5 ± 84.9 | <0.01 |
| % kcal | 54.4 ± 6.5 | 52.8 ± 6.0 | 0.02 |
| Dietary protein | |||
| g/d | 55.1 ± 17.7 | 79.5 ± 24.6 | <0.01 |
| % kcal | 13.5 ± 2.7 | 14.3 ± 2.6 | 0.02 |
| Dietary fiber | |||
| g/d | 9.4 ± 4.0 | 12.0 ± 4.5 | <0.01 |
| g/1000 kcal | 5.7 ± 1.8 | 5.4 ± 1.3 | <0.01 |
All values are means ± sd. BMI, Body mass index; PA, physical activity.
Tests of significance between groups were based on independent-samples t test.
Tests of significance between groups were based on the χ2 test.
Tests of significance between groups were based on the Mann-Whitney U test.
Pearson's bivariate analyses revealed that dietary fiber intake was associated with leptin (r = −0.21), adiponectin (r = 0.21), C-reactive protein (r = −0.24), fibrinogen (r = −0.17), fat mass (r = −0.17), and VAT (r = −0.26) in the females (all P < 0.05). In the males, dietary fiber intake was associated with leptin (r = −0.21), adiponectin (r = 0.13), C-reactive protein (r = −0.17), fibrinogen (r = −0.13), and fat mass (r = −0.16) (all P ≤ 0.05), and there was a trend toward a significant inverse relationship between dietary fiber intake and VAT (r = −0.12, P = 0.09). There were no significant associations were observed between dietary fiber intake and resistin in either gender (both P > 0.05).
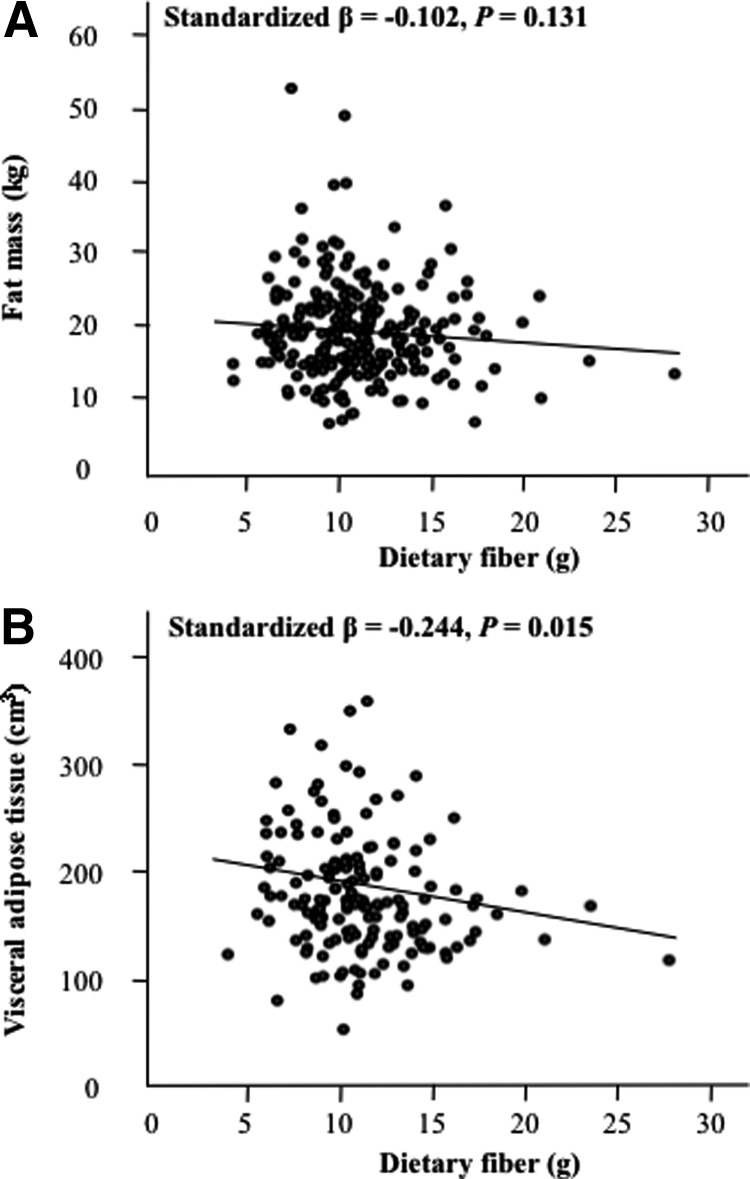
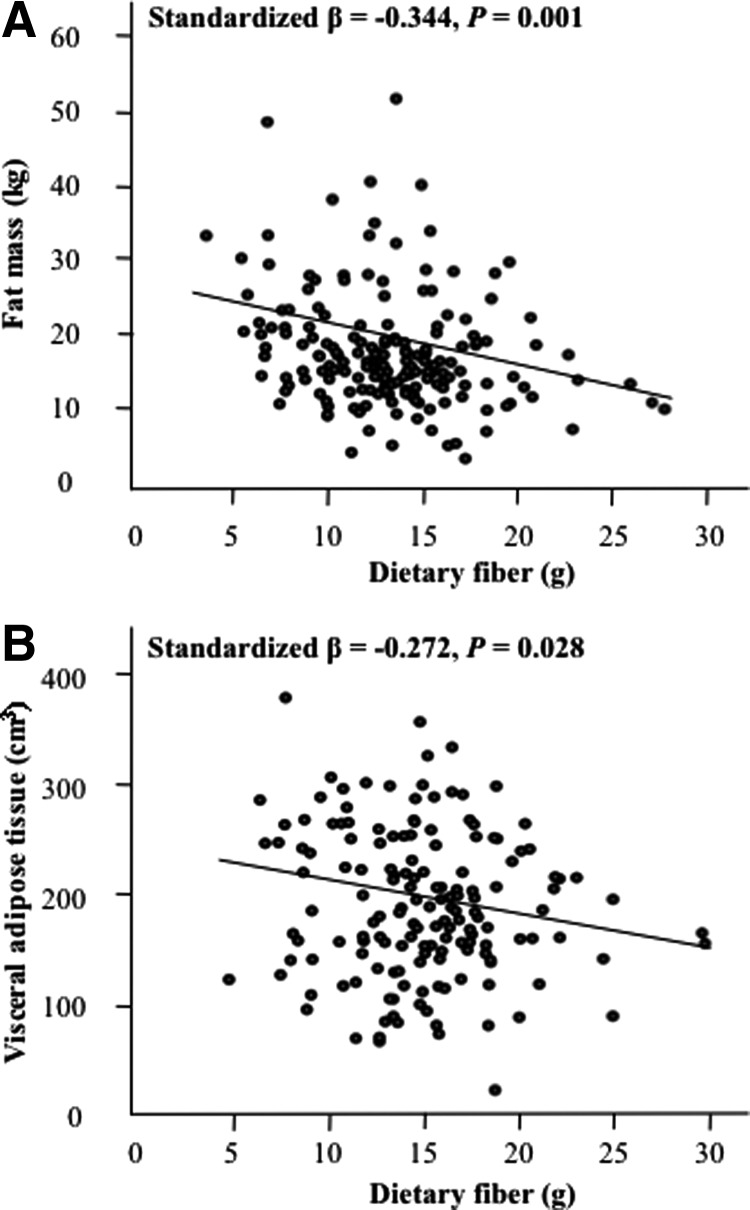
Multiple linear regression adjusting for age, race, Tanner stage, FFST, energy intake, and physical activity revealed that VAT was negatively associated with dietary fiber intake in both genders (both P < 0.03) (Figs. 1 and 2). However, fat mass was inversely associated with dietary fiber intake in males (P = 0.001) but not in females (P = 0.13).
Fig. 1.
Relationships between dietary fiber intake and total and central adiposity in adolescent females. A and B, Dietary intake vs. total fat mass (A) and dietary fiber intake vs. VAT (B). Relationships are adjusted for age, race, Tanner stage, FFST mass, energy intake, and physical activity; n = 274.
Fig. 2.
Relationships between dietary fiber intake and total and central adiposity in adolescent males. A and B, Dietary intake vs. total fat mass (A) and dietary fiber intake vs. VAT (B). Relationships are adjusted for age, race, Tanner stage, FFST mass, energy intake, and physical activity; n = 285.
Table 2 displays the associations of dietary fiber intake with serum concentrations of leptin and plasma concentrations of adiponectin, resistin, C-reactive protein, and fibrinogen after adjustment for age, race, Tanner stage, FFST, energy intake, and physical activity (base model) and further adjustment for type of adiposity. In the base model, dietary fiber intake was independently, positively associated with adiponectin but independently, inversely associated with C-reactive protein and fibrinogen in both genders (all P < 0.05). When fat mass or VAT was included as a covariate, these associations with dietary fiber intake persisted with adiponectin and C-reactive protein (all P < 0.05) but no longer remained for fibrinogen (all P > 0.05). Further analysis revealed that dietary fiber intake was independently inversely associated with leptin in males only (P < 0.001). However, when fat mass or VAT was added to the model, the significant relationship between dietary fiber and leptin no longer persisted (all P > 0.05). No significant associations were found between dietary fiber intake and resistin in either gender (all P > 0.05).
Table 2.
Associations of dietary fiber intake with serum concentrations of leptin and plasma concentrations of adiponectin, resistin, C-reactive protein, and fibrinogen in adolescents aged 14–18 yr, with adjustment of total and central adiposity
| Regression models | Leptin | Adiponectin | Resistin | C-reactive protein | fibrinogen |
|---|---|---|---|---|---|
| Females (n = 274) | |||||
| Base modela | −0.059 | 0.245c | −0.063 | −0.282c | −0.161b |
| Base model + fat mass | −0.036 | 0.237b | −0.062 | −0.215b | −0.140 |
| Base model + VAT | −0.055 | 0.217b | −0.033 | −0.230b | −0.092 |
| Males (n = 285) | |||||
| Base modela | −0.342c | 0.278c | −0.049 | −0.362c | −0.223b |
| Base model + fat mass | −0.047 | 0.246b | −0.019 | −0.237b | −0.119 |
| Base model + VAT | −0.046 | 0.237b | −0.056 | −0.308b | −0.177 |
Data are presented as standardized β-coefficients.
Base model adjusted for age, race, Tanner stage, FFST mass, energy intake, and physical activity.
P < 0.05.
P < 0.01.
Discussion
To our knowledge, this is the first study to assess relations between dietary fiber intake and inflammatory markers in adolescents. We found that greater fiber consumption was associated with lower C-reactive protein and fibrinogen concentrations and higher adiponectin concentrations. These relationships were independent of potential confounding factors including age, sex, race, Tanner stage, body composition, energy intake, and physical activity. Our study findings in adolescents suggest that greater fiber consumption may reduce biomarkers implicated in inflammation.
Although several studies have investigated the role of dietary fiber intake on inflammatory markers, the findings have been disparate (7–15). The disparate results in the studies may be due to differing levels of type of fat (total vs. central) in the different subject groups. Based on our findings, it seems that greater fiber consumption may be more relevant to people with greater amounts of visceral, rather than general, adiposity, because negative relationships were found between dietary fiber intake and VAT, independent of sex. In support of this notion, Davis and colleagues (23) reported that increases in dietary fiber intake over a 2-yr period were associated with less visceral fat accumulation in overweight Latino youth. The mechanism for how dietary fiber might affect visceral adiposity is unknown; however, some hypotheses include 1) increased fecal bulk and less transit time, thus allowing less time for digestion and absorption of nutrients (24); 2) increased insulin sensitivity (25); and 3) increased intakes of phytoestrogens (i.e. isoflavones and lignans) that are generally found in foods high in fiber such as fruits and vegetables (26). It is important to note that we also linked dietary fiber intake with general adiposity, but this relationship was sex dependent, because dietary fiber intake was negatively associated with total fat levels in males only. Given that sex differences in the relationship between dietary fiber intake and general adiposity have been reported in previous investigations (3, 27–30), additional work is warranted to understand the role of sex in the dietary fiber and adiposity relationship.
Numerous studies have demonstrated that increased adiposity, particularly visceral adiposity, contributes directly toward an increase in systemic inflammation with the production of various inflammatory cytokines by adipocytes (31–35). The mechanism by which dietary fiber may affect inflammation remains to be determined. However, one hypothesis is that a diet high in fiber may help reduce visceral adiposity (25–27), as previously mentioned, and therefore reduce systemic inflammation. Interestingly, including VAT as a covariate did not attenuate the relationship between dietary fiber intake and adiponectin or C-reactive protein, suggesting that other factors are at play. A recent study comparing 18-wk isoenergetic diets varying in cereal fiber and protein content observed greater changes in insulin sensitivity after intake of the high-cereal-fiber diet than after intake of the high-protein diet (36), which the investigators explain may be due to fiber-induced interference with dietary protein digestion and prevention of the mammalian target of rapamycin/S6 kinase 1 signaling pathway (37). Importantly, there were no diet-induced differences in inflammatory markers (i.e. leptin, adiponectin, and C-reactive protein) and visceral adiposity between groups (36). Taken together, these findings suggest that the observed associations between dietary fiber and inflammatory markers and visceral adiposity in the present study could be a consequence of dietary fiber-induced improvement in insulin sensitivity. However, because we did not assess insulin sensitivity, this proposition warrants further investigation. Other factors worth exploring that may be linked to dietary fiber intake are satiety, gastric emptying, and gastrointestinal hormones, such as glucagon-like peptide and glucose-dependent insulinotropic polypeptide (38).
Strengths of the study include the consideration of confounding variables in the relationship between dietary fiber intake and inflammation, including the high-quality assessments of general and central adiposity using dual-energy x-ray absorptiometry and magnetic resonance imaging, respectively. However, we acknowledge study limitations. Given that our study used cross-sectional data, we cannot be certain that dietary fiber intake has a direct effect on inflammatory markers and adiposity. Second, our study findings are limited to male and female, Black and White adolescents living in the southeastern United States; thus, differences in socioeconomic status, geographic location, social environment, lifestyle, or food habits of the study population may limit generalizability of the study findings. However, given that the mean daily fiber intake of the 14- to 18-yr-old males (12 g) and females (9 g) in our study samples are comparable to the most recent national average in 14- to 18-yr-old males (15 g) and females (12 g) (39), our findings are likely generalizable to many other settings.
In conclusion, our study findings in adolescents suggest that greater consumption of dietary fiber is associated with lower visceral adiposity and multiple biomarkers implicated in inflammation. Additional research is needed in youth to assess the long-term implications of increasing dietary fiber intake on obesity-related inflammation.
Acknowledgments
We gratefully acknowledge the study participants and parents as well as the research staff who made this project possible.
This study was supported by Grants HL077230, HL64157, and HL 87923-03S1 from the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AI
- Adequate intake
- CV
- coefficient of variation
- FFST
- fat-free soft tissue
- VAT
- visceral adipose tissue.
References
- 1. Trujillo ME, Scherer PE. 2006. Adipose tissue-derived factors: impact on health and disease. Endocr Rev 27:762–778 [DOI] [PubMed] [Google Scholar]
- 2. Kershaw EE, Flier JS. 2004. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89:2548–2556 [DOI] [PubMed] [Google Scholar]
- 3. Davis JN, Hodges VA, Gillham MB. 2006. Normal-weight adults consume more fiber and fruit than their age- and height-matched overweight/obese counterparts. J Am Diet Assoc 106:833–840 [DOI] [PubMed] [Google Scholar]
- 4. Salmerón J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. 1997. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 277:472–477 [DOI] [PubMed] [Google Scholar]
- 5. Salmerón J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL, Willett WC. 1997. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 20:545–550 [DOI] [PubMed] [Google Scholar]
- 6. Weickert MO, Möhlig M, Schöfl C, Arafat AM, Otto B, Viehoff H, Koebnick C, Kohl A, Spranger J, Pfeiffer AF. 2006. Cereal fiber improves whole-body insulin sensitivity in overweight and obese women. Diabetes Care 29:775–780 [DOI] [PubMed] [Google Scholar]
- 7. Al-Lahham SH, Roelofsen H, Priebe M, Weening D, Dijkstra M, Hoek A, Rezaee F, Venema K, Vonk RJ. 2010. Regulation of adipokine production in human adipose tissue by propionic acid. Eur J Clin Invest 40:401–407 [DOI] [PubMed] [Google Scholar]
- 8. Qi L, Rimm E, Liu S, Rifai N, Hu FB. 2005. Dietary glycemic index, glycemic load, cereal fiber, and plasma adiponectin concentration in diabetic men. Diabetes Care 28:1022–1028 [DOI] [PubMed] [Google Scholar]
- 9. Qi L, Meigs JB, Liu S, Manson JE, Mantzoros C, Hu FB. 2006. Dietary fibers and glycemic load, obesity, and plasma adiponectin levels in women with type 2 diabetes. Diabetes Care 29:1501–1505 [DOI] [PubMed] [Google Scholar]
- 10. King DE, Egan BM, Woolson RF, Mainous AG, 3rd, Al-Solaiman Y, Jesri A. 2007. Effect of a high-fiber diet vs a fiber-supplemented diet on C-reactive protein level. Arch Intern Med 167:502–506 [DOI] [PubMed] [Google Scholar]
- 11. King DE, Mainous AG, 3rd, Egan BM, Woolson RF, Geesey ME. 2008. Effect of psyllium fiber supplementation on C-reactive protein: the trial to reduce inflammatory markers (TRIM). Ann Fam Med 6:100–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wannamethee SG, Whincup PH, Thomas MC, Sattar N. 2009. Associations between dietary fiber and inflammation, hepatic function, and risk of type 2 diabetes in older men: potential mechanisms for the benefits of fiber on diabetes risk. Diabetes Care 32:1823–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. North CJ, Venter CS, Jerling JC. 2009. The effects of dietary fibre on C-reactive protein, an inflammation marker predicting cardiovascular disease. Eur J Clin Nutr 63:921–933 [DOI] [PubMed] [Google Scholar]
- 14. Fehily AM, Milbank JE, Yarnell JW, Hayes TM, Kubiki AJ, Eastham RD. 1982. Dietary determinants of lipoproteins, total cholesterol, viscosity, fibrinogen, and blood pressure. Am J Clin Nutr 36:890–896 [DOI] [PubMed] [Google Scholar]
- 15. Djoussé L, Ellison RC, Zhang Y, Arnett DK, Sholinsky P, Borecki I. 1998. Relation between dietary fiber consumption and fibrinogen and plasminogen activator inhibitor type 1: The National Heart, Lung, and Blood Institute Family Heart Study. Am J Clin Nutr 68:568–575 [DOI] [PubMed] [Google Scholar]
- 16. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB, Sr, O'Donnell CJ. 2007. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116:39–48 [DOI] [PubMed] [Google Scholar]
- 17. Alaimo K, McDowell MA, Briefel RR, Bischof AM, Caughman CR, Loria CM, Johnson CL. 1994. Dietary intake of vitamins, minerals, and fiber of persons ages 2 months and over in the United States: Third National Health and Nutrition Examination Survey, Phase 1, 1988–91. Adv Data 258:1–28 [PubMed] [Google Scholar]
- 18. Institute of Medicine, Food and Nutrition Board 2002. Dietary Reference Intakes for energy, carbohydrates, fiber, fat, fatty acid, cholesterol, protein, amino acids. Washington, DC: The National Academies Press [Google Scholar]
- 19. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. 2000. CDC growth charts: United States. Adv Data 314:1–27 [PubMed] [Google Scholar]
- 20. Tanner J. 1962. Growth and adolescence. 2nd ed. Oxford, UK: Blackwell Scientific Publications [Google Scholar]
- 21. Gutin B, Johnson MH, Humphries MC, Hatfield-Laube JL, Kapuku GK, Allison JD, Gower BA, Daniels SR, Barbeau P. 2007. Relationship of visceral adiposity to cardiovascular disease risk factors in black and white teens. Obesity (Silver Spring) 15:1029–1035 [DOI] [PubMed] [Google Scholar]
- 22. Owens S, Gutin B, Allison J, Riggs S, Ferguson M, Litaker M, Thompson W. 1999. Effect of physical training on total and visceral fat in obese children. Med Sci Sports Exerc 31:143–148 [DOI] [PubMed] [Google Scholar]
- 23. Davis JN, Alexander KE, Ventura EE, Toledo-Corral CM, Goran MI. 2009. Inverse relation between dietary fiber intake and visceral adiposity in overweight Latino youth. Am J Clin Nutr 90:1160–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jenkins DJ, Jenkins AL, Wolever TM, Rao AV, Thompson LU. 1986. Fiber and starchy foods: gut function and implications in disease. Am J Gastroenterol 81:920–930 [PubMed] [Google Scholar]
- 25. Lundin EA, Zhang JX, Lairon D, Tidehag P, Aman P, Adlercreutz H, Hallmans G. 2004. Effects of meal frequency and high-fibre rye-bread diet on glucose and lipid metabolism and ileal excretion of energy and sterols in ileostomy subjects. Eur J Clin Nutr 58:1410–1419 [DOI] [PubMed] [Google Scholar]
- 26. de Kleijn MJ, van der Schouw YT, Wilson PW, Grobbee DE, Jacques PF. 2002. Dietary intake of phytoestrogens is associated with a favorable metabolic cardiovascular risk profile in postmenopausal U.S.women: the Framingham study. J Nutr 132:276–282 [DOI] [PubMed] [Google Scholar]
- 27. Rose N, Hosig K, Davy B, Serrano E, Davis L. 2007. Whole-grain intake is associated with body mass index in college students. J Nutr Educ Behav 39:90–94 [DOI] [PubMed] [Google Scholar]
- 28. Howarth NC, Saltzman E, Roberts SB. 2001. Dietary fiber and weight regulation. Nutr Rev 59:129–139 [DOI] [PubMed] [Google Scholar]
- 29. Ludwig DS, Pereira MA, Kroenke CH, Hilner JE, Van Horn L, Slattery ML, Jacobs DR., Jr 1999. Dietary fiber, weight gain, and cardiovascular disease risk factors in young adults. JAMA 282:1539–1546 [DOI] [PubMed] [Google Scholar]
- 30. Byrd-Williams CE, Strother ML, Kelly LA, Huang TT. 2009. Dietary fiber and associations with adiposity and fasting insulin among college students with plausible dietary reports. Nutrition 25:896–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, Yamashita S, Miura M, Fukuda Y, Takemura K, Tokunaga K, Matsuzawa Y. 1996. Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat Med 2:800–803 [DOI] [PubMed] [Google Scholar]
- 32. Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. 2001. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 280:E745–E751 [DOI] [PubMed] [Google Scholar]
- 33. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. 2003. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berg AH, Scherer PE. 2005. Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96:939–949 [DOI] [PubMed] [Google Scholar]
- 35. Polak J, Kovacova Z, Jacek M, Klimcakova E, Kovacikova M, Vitkova M, Kuda O, Sebela M, Samcova E, Stich V. 2007. An increase in plasma adiponectin multimeric complexes follows hypocaloric diet-induced weight loss in obese and overweight pre-menopausal women. Clin Sci (Lond) 112:557–565 [DOI] [PubMed] [Google Scholar]
- 36. Weickert MO, Roden M, Isken F, Hoffmann D, Nowotny P, Osterhoff M, Blaut M, Alpert C, Gögebakan O, Bumke-Vogt C, Mueller F, Machann J, Barber TM, Petzke KJ, Hierholzer J, Hornemann S, Kruse M, Illner AK, Kohl A, Loeffelholz CV, Arafat AM, Möhlig M, Pfeiffer AF. 2011. Effects of supplemented isoenergetic diets differing in cereal fiber and protein content on insulin sensitivity in overweight humans. Am J Clin Nutr 94:459–471 [DOI] [PubMed] [Google Scholar]
- 37. Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, Nowotny P, Waldhäusl W, Marette A, Roden M. 2005. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 54:2674–2684 [DOI] [PubMed] [Google Scholar]
- 38. Weickert MO, Pfeiffer AF. 2008. Metabolic effects of dietary fiber consumption and prevention of diabetes. J Nutr 138:439–442 [DOI] [PubMed] [Google Scholar]
- 39. National Cancer Institute, Applied Research Program 2010. Risk factor monitoring and methods: sources of fiber among US children & adolescents, 2005–06. http://riskfactor.cancer.gov/diet/foodsources/fiber/ Accessed August 18, 2011