Abstract
We have measured the numbers of genes coding for the three seed storage proteins, vicilin, convicilin and legumin, in a number of Pisum genotypes of variant protein composition. No difference in gene number existed among P. sativum genotypes for any of the proteins. There were differences in the number of genes coding for individual proteins with approximately 11 genes (per haploid genome) for vicilin, 8 genes for legumin and 1 gene for convicilin.
Full text
PDF



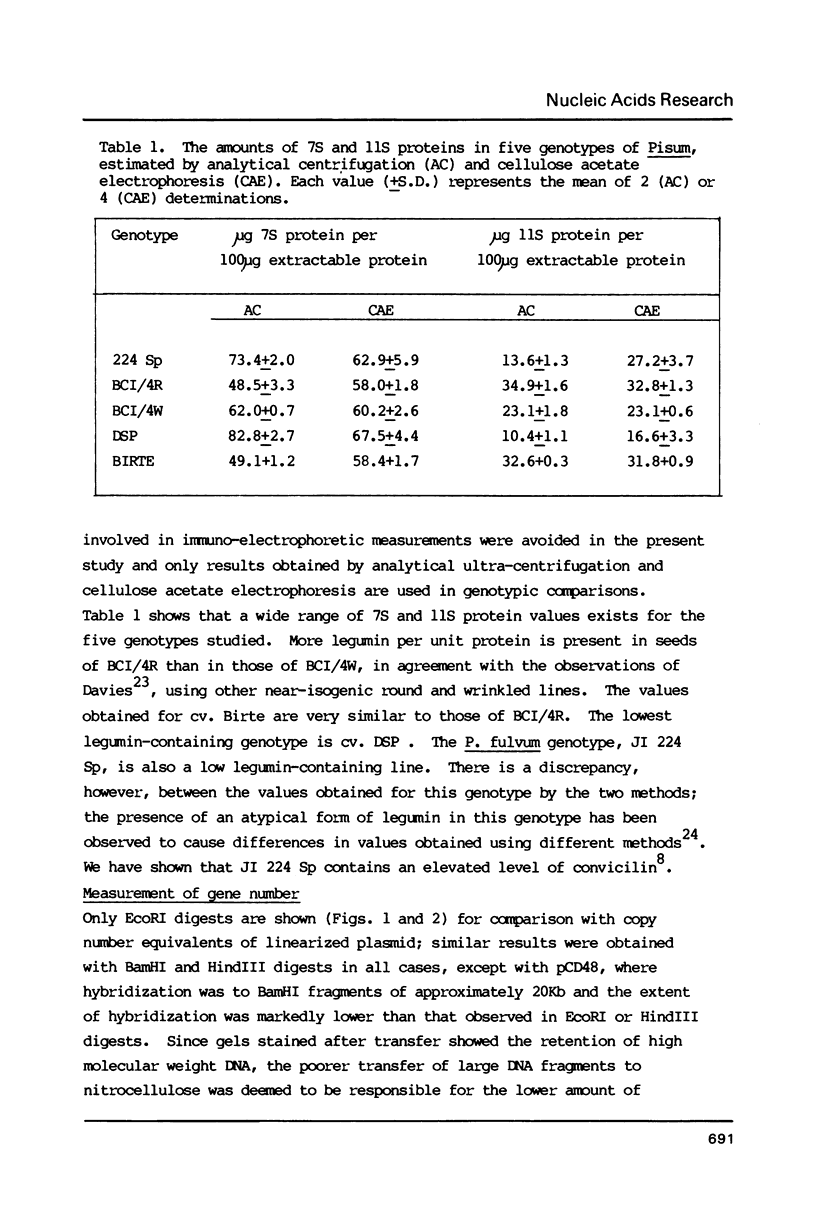
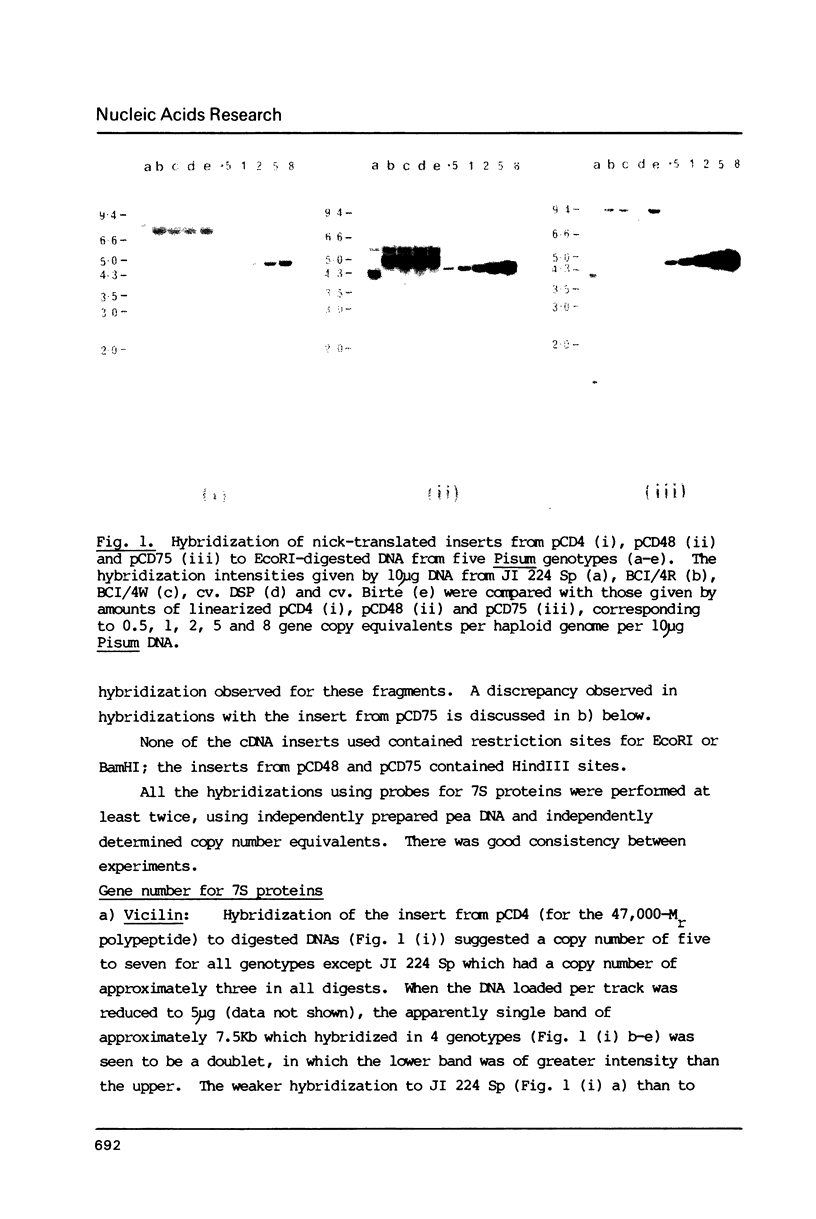
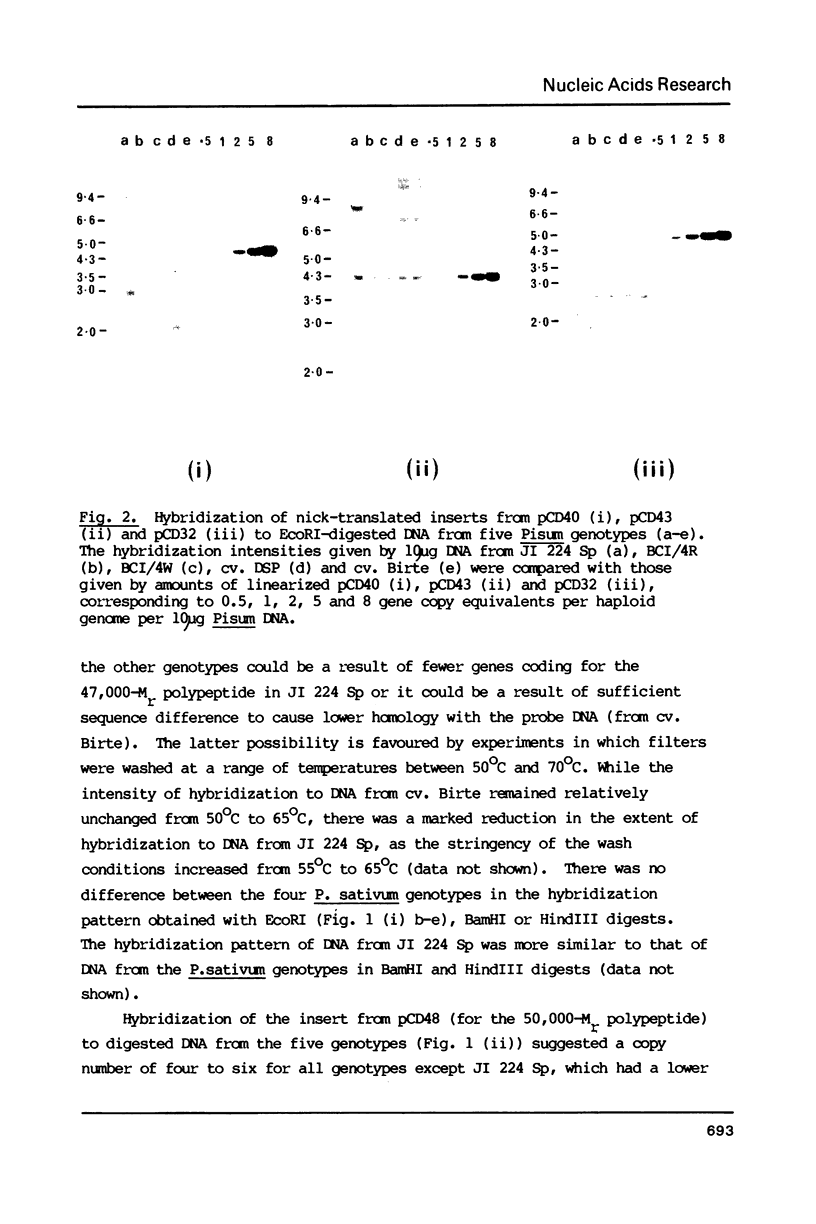






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bostock C. J., Clark E. M., Harding N. G., Mounts P. M., Tyler-Smith C., van Heyningen V., Walker P. M. The development of resistance to methotrexate in a mouse melanoma cell line. I. Characterisation of the dihydrofolate reductases and chromosomes in sensitive and resistant cells. Chromosoma. 1979;74(2):153–177. doi: 10.1007/BF00292270. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Casey R., Domoney C., Stanley J. Convicilin mRNA from pea (Pisum sativum L.) has sequence homology with other legume 7S storage protein mRNA species. Biochem J. 1984 Dec 1;224(2):661–666. doi: 10.1042/bj2240661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey R., Sanger E. Purification and some properties of a 7S seed storage protein from Pisum (pea). Biochem Soc Trans. 1980 Oct;8(5):658–658. doi: 10.1042/bst0080658. [DOI] [PubMed] [Google Scholar]
- Chandler P. M., Spencer D., Randall P. J., Higgins T. J. Influence of Sulfur Nutrition on Developmental Patterns of Some Major Pea Seed Proteins and Their mRNAs. Plant Physiol. 1984 Jul;75(3):651–657. doi: 10.1104/pp.75.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy R. R., Gatehouse J. A., Tyler M., Boulter D. The purification and characterization of a third storage protein (convicilin) from the seeds of pea (Pisum sativum L.). Biochem J. 1980 Nov 1;191(2):509–516. doi: 10.1042/bj1910509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson C. E. Seed globulins of the Gramineae and Leguminosae. Biochem J. 1949;44(4):387–400. doi: 10.1042/bj0440387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. R. The ra locus and legumin synthesis in Pisum sativum. Biochem Genet. 1980 Dec;18(11-12):1207–1219. doi: 10.1007/BF00484348. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Domoney C., Casey R. Storage protein precursor polypeptides in cotyledons of Pisum sativum L. Identification of, and isolation of a cDNA clone for, an 80000-Mr legumin-related polypeptide. Eur J Biochem. 1984 Mar 1;139(2):321–327. doi: 10.1111/j.1432-1033.1984.tb08010.x. [DOI] [PubMed] [Google Scholar]
- Gatehouse J. A., Croy R. R., Morton H., Tyler M., Boulter D. Characterisation and subunit structures of the vicilin storage proteins of pea (Pisum sativum L.). Eur J Biochem. 1981 Sep 1;118(3):627–633. doi: 10.1111/j.1432-1033.1981.tb05565.x. [DOI] [PubMed] [Google Scholar]
- Gatehouse J. A., Lycett G. W., Delauney A. J., Croy R. R., Boulter D. Sequence specificity of the post-translational proteolytic cleavage of vicilin, a seed storage protein of pea (Pisum sativum L.). Biochem J. 1983 May 15;212(2):427–432. doi: 10.1042/bj2120427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R. B., Hoschek G., Ditta G. S., Breidenbach R. W. Developmental regulation of cloned superabundant embryo mRNAs in soybean. Dev Biol. 1981 Apr 30;83(2):218–231. doi: 10.1016/0012-1606(81)90468-1. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Hoschek G., Vodkin L. O. An insertion sequence blocks the expression of a soybean lectin gene. Cell. 1983 Jun;33(2):465–475. doi: 10.1016/0092-8674(83)90428-2. [DOI] [PubMed] [Google Scholar]
- Kreis M., Shewry P. R., Forde B. G., Rahman S., Miflin B. J. Molecular analysis of a mutation conferring the high-lysine phenotype on the grain of barley (Hordeum vulgare). Cell. 1983 Aug;34(1):161–167. doi: 10.1016/0092-8674(83)90146-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lycett G. W., Croy R. R., Shirsat A. H., Boulter D. The complete nucleotide sequence of a legumin gene from pea (Pisum sativum L.). Nucleic Acids Res. 1984 Jun 11;12(11):4493–4506. doi: 10.1093/nar/12.11.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycett G. W., Delauney A. J., Gatehouse J. A., Gilroy J., Croy R. R., Boulter D. The vicilin gene family of pea (Pisum sativum L.): a complete cDNA coding sequence for preprovicilin. Nucleic Acids Res. 1983 Apr 25;11(8):2367–2380. doi: 10.1093/nar/11.8.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Cuellar R. E., Thompson W. F. DNA sequence organization in the pea genome. Biochemistry. 1978 Dec 26;17(26):5781–5790. doi: 10.1021/bi00619a027. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Sun S. M., Hall T. C. Complete nucleotide sequence of a French bean storage protein gene: Phaseolin. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1897–1901. doi: 10.1073/pnas.80.7.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]