Abstract
Background:
The relationship between circulating ACTH levels and cortisol secretion in Cushing's disease is not precisely known.
Hypothesis:
Chronic ACTH hyperstimulation leads to decreased adrenal potency and is restored after normalization of ACTH secretion.
Subjects:
Subjects included 20 patients with Cushing's disease, eight patients in long-term remission, and 36 healthy controls.
Outcomes:
ACTH and cortisol secretion rates and analytical dose-response estimates of endogenous ACTH efficacy (maximal cortisol secretion), dynamic ACTH potency, and adrenal sensitivity (slope term) from 24-h ACTH-cortisol profiles were evaluated.
Results:
Both basal and pulsatile secretion of ACTH and cortisol were increased in patients with active disease but normal in cured patients. ACTH, but not cortisol pulse frequency, was amplified in patients and restored after successful surgical treatment. ACTH EC50, an inverse measure of potency, was higher during pulse onset in Cushing's disease (59 ± 7.4 ng/liter) than in controls (20 ± 3.7 ng/liter) (P < 0.0001) and remitted patients after surgery [15 ± 3.2 ng/liter, P value not significant (NS) vs. controls] and during pulse recovery phases [128 ± 18 (P <0.0001), 70 ± 8.4, and 67 ± 17 ng/liter (NS vs. controls), respectively]. Efficacy was increased in active disease and normalized after surgical treatment [patients, 38 ± 8.3 nmol/liter · min, vs. controls, 21 ± 2.3 nmol/liter · min (P <0.0001), and cured patients, 15 ± 3.2 nmol/liter · min (NS vs. controls)]. Sensitivity to endogenous ACTH did not differ among the three groups.
Conclusion:
The adrenal gland in Cushing's disease exhibits decreased responsiveness to submaximal ACTH drive and amplified efficacy, with unchanged sensitivity. These target-gland abnormalities are reversible in long-term remission after pituitary surgery.
Cushing's disease is a serious disorder, caused by a hypersecreting ACTH-expressing pituitary adenoma and associated hypercortisolism, which leads to numerous detrimental effects on virtually all body systems. The only effective and rapid treatment option is selective pituitary surgery by an experienced neurosurgeon. This treatment modality often restores normal ACTH function in time, without damage to other pituitary functions, although not invariably so (1). The secretion profiles of ACTH and cortisol in Cushing's disease were first described in pioneer studies by Krieger and Allen (2), and confirmed by others later (3, 4). More recent investigations have sought to define the relationship between ACTH and cortisol secretion in physiological conditions (5, 6). By applying new mathematical analytical tools, investigations have demonstrated that gender, body mass index (BMI), and age all influence individual ACTH and cortisol secretion in normal subjects and alter the endogenous ACTH concentration-cortisol secretion effect relationship (7).
Several investigations have quantitated individual ACTH and cortisol secretion profiles in Cushing's disease using sensitive and robust hormone assays. Aside from increased hormone secretion rates, decreased secretory regularity, and erosion of circadian amplitude, these investigations raise the possibility of down-regulated adrenal responsiveness (8, 9).
In Cushing's disease, pulsatile and basal ACTH and cortisol secretion rates remain to be studied by recently developed operator-independent methods. Therefore, the first goal was to quantify secretion parameters of ACTH and cortisol in patients with active Cushing's disease and healthy controls, including secretory burst frequency, shape, regularity, and mass along with basal and hormone half-life with an automated algorithm (10). The resultant data permit dose-response estimation under conditions of allowable (but not required) down-regulation of target-gland responses. For the latter approach, the two-potency model was used, which can be described as a shift of the curve in the rightward horizontal direction during the recovery phase (i.e. during the decline of ACTH concentration pulses), compared with the rising phase. The selection of this model is based on statistical considerations (11). The third point of interest is whether the ACTH-adrenal system can recover after curative surgery, when analyzed with specific and sensitive new tools.
Subjects and Methods
Overview
Healthy volunteers and patients were hospitalized the evening before the sampling studies. On the following morning, an indwelling iv cannula was inserted in a large vein of the forearm. Blood samples (2.0 ml) were withdrawn at 10-min intervals for 24 h beginning at 0900 h. A slow iv infusion of 0.9% NaCl and heparin (1 U/ml) was used to keep the line open. Ambulation was permitted to the lavatory only. Vigorous exercise, daytime sleep, snacks, caffeinated beverages, and cigarette smoking were disallowed. Meals were provided at 0800, 1230, and 1730 h, and room lights were turned off between 2200 and 2400 h, depending upon individual sleeping habits. Blood was collected in prechilled siliconized tubes containing EDTA (ACTH) or heparin (cortisol), centrifuged at 4 C to separate plasma, and frozen at −20 C within 30 min of collection. Total blood loss was less than 360 ml. Volunteers were compensated for the time spent in the study. None of the 24-h data has been published or presented previously or analyzed in the present manner.
Volunteers and patients
Thirty-six healthy community-dwelling adults of ages 18–77 yr (19 men and 17 women) served as control subjects. The mean age was 43 yr, range 18–77 yr, with BMI of 18.3–30.7 kg/m2. Criteria for exclusion included recent use of psychotropic or neuroactive drugs (within five biological half-lives); drug or alcohol abuse, psychosis, depression, mania, anorexia/bulimia, or severe anxiety; endocrinopathy, other than primary thyroidal failure receiving replacement; nightshift work or recent transmeridian travel (exceeding three time zones within 7 d of admission); acute weight change (loss or gain of >2 kg in 6 wk); abnormal hepatorenal function; glucocorticoid, anabolic steroid, or reproductive hormone therapy; and/or unwillingness to provide written informed consent. Twenty patients with Cushing's disease (14 women and six men), mean age 37 yr, range 17–74 yr, with BMI of 20.5–39 kg/m2 were studied. In all patients, the diagnosis of Cushing's disease was established by elevated 24-h urinary excretion of free cortisol, subnormal or absent overnight suppression of plasma cortisol by 1 mg oral dexamethasone, absent or subnormal suppression of urinary cortisol excretion during an oral 2-d dexamethasone test (low-dose Liddle test), suppression of plasma cortisol concentration by 190 nmol/liter or more during a 7-h iv infusion with dexamethasone at a dose of 1 mg/h (12), positive immunostaining for ACTH of the pituitary adenoma, and clinical cortisol dependency for several months after selective removal of the adenoma. Ten patients in remission of Cushing's disease (seven women and three men) were studied. Their mean age was 38 yr, range 27–63 yr, with BMI of 20.6–33 kg/m2. The diagnosis was established by criteria, described above, and was confirmed by pituitary surgery and positive ACTH immunostaining of the removed adenoma. Remission was established by the absence of signs and symptoms during long-term follow-up of 8.2 ± 1.7 yr, normalized 24-h urinary excretion of free cortisol, and suppression of the morning plasma cortisol concentration below 0.10 mmol/liter after the administration of 1 mg dexamethasone orally at 2300 h at yearly visits in the outpatient clinic. All patients needed temporary hydrocortisone substitution after surgery. The mean duration of glucocorticoid replacement therapy was 21 months (range, 12–36 months). Two females conceived after surgery and gave birth to healthy children, 2 and 3 yr after the operation. No medication was taken by any of the study subjects and normal volunteers. Premenopausal controls were studied in the follicular phase of the menstrual cycle. Controls and patients provided written informed consent. The study was approved by the Leiden University Institutional Review Board.
Assays
Plasma ACTH was measured using a two-site sandwich assay designed to detect intact ACTH (1–39) molecules. The immunoradiometric assay consisted of a soluble 125I-labeled (indicator) monoclonal antibody directed to the N terminus of ACTH as well as a second polyclonal ACTH antibody directed to the C terminus. The second antibody was covalently conjugated to biotin to react with avidin-coated plastic beads. All incubation reagents including antibodies, human ACTH-(1–39) standard, and avidin-coated beads were from Nichols Institute (San Juan Capistrano, CA; Allegro immunoradiometric assay). Each sample was assayed in duplicate, and all samples from any one subject were assayed in the same run. Sensitivity of the immunoradiometric assay was 1.0 pg/ml or 0.22 pmol/ml, and intraassay precision was 3.2–5.8% (range of median intrasample coefficients of variation in all individuals). Cross-reactivity with β-endorphin, TSH, LH, FSH, GH, or prolactin was less than 0.1%. Cross-reactivity with proopiomelanocortin was less than 0.01% (13). Plasma cortisol concentrations were measured by RIA (Sorin Biomedica, Milan, Italy). The detection limit of this assay was 25 nmol/liter. The intra- and interassay precision varied from 2–4%.
Analytical methods
Details of the dynamic dose-response methodology were described in two earlier papers (11, 14). The dose-response relationship between ACTH and cortisol was established with a four- and a six-parameter logistic regression equation with input (effector) represented by reconvolved plasma ACTH concentration and output (response) by the cortisol secretion. The six-parameter model allows the estimation of two different potencies. Whereas the four-parameter logistic function leads to a single (classical) dose-response curve, the six-parameter version estimates potency for the rising portion of the ACTH pulse and the decreasing part of the pulse. The descending phase shows decreased potency (or increased EC50) found in healthy individuals (11). The sixth parameter is the time delay for the potency switch. As a first step, the ACTH and cortisol concentration series are deconvolved with a newly MATLAB-based program (10). This operator-independent program estimates the number of pulses, the slow half-life of the hormone (minutes), the mode (time in minutes to reach the maximal secretion within a pulse), basal (nonpulsatile) secretion, and pulsatile secretion. In addition, the program estimates the λ-parameter, reflecting pulse frequency, and the γ-parameter, reflecting the pulse regularity (higher values denote increased regularity). The reconvolved (fitted) ACTH concentrations are then related to the deconvolved cortisol secretion rates (nanomoles per liter distribution volume per minute) with the logistic regression equations. The parameters of the classical logistic equation are basal secretion rate, potency, sensitivity (slope), and efficacy (maximal response). The six-parameter equation includes two additional terms, a second potency and an inflection time (minutes) representing the delay after which there is down-regulation of the ACTH-cortisol response. From the ratio of potency and sensitivity (slope), the more convenient EC50 can be calculated. The significance of potency shifts from onset to recovery was tested by signed-ranks comparison of onset and recovery potency values (before and after putative down-regulation).
Statistics
Data are presented in tables as mean and sem and in figures as box-and-whisker plots containing the median, interquartile range, 90% confidence interval, and extreme values. Statistical comparisons between groups were performed by ANOVA, after logarithmic transformation of the data, when required. In addition, data sets were also analyzed with the Kruskal-Wallis method. P values <0.05 were considered significant. Statistical calculations were done with Systat version 11 (Systat Software, Richmond, CA).
Results
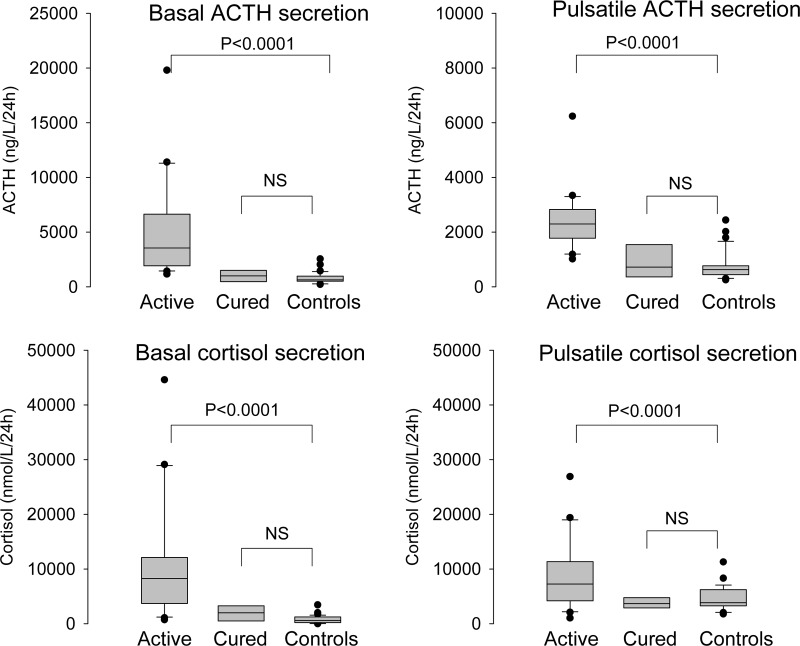
Clinical data and basal hormone concentrations are listed in Table 1. Age did not differ between the groups, but BMI was significantly higher in patients with active disease. Serum transcortin, estradiol (in women), and IGF-I levels were comparable in the three groups, but serum testosterone (in men) was diminished in patients with Cushing's disease (active and in remission). Free T4 concentration was decreased in patients with active disease, although no patient had a subnormal concentration. Results of the deconvolution analysis of the serum ACTH and cortisol concentration profiles in patients with active Cushing's disease, patients in long-term remission after pituitary surgery and healthy controls are shown in Table 2. The 24-h basal and pulsatile secretion of patients and controls are shown in Fig. 1. ACTH secretion in patients with active disease was characterized by increased secretory-burst number, decreased slow half-life, and increased basal and pulsatile secretion compared with healthy controls and patients in remission. Cortisol burst frequency was not increased in patients with active disease, whereas the slow component of the half-life was decreased. Basal and pulsatile secretion was greatly increased in patients with active disease. Differences in pulse number between controls and patient groups were mirrored by the frequency parameter λ. The regularity of cortisol, but not of ACTH, interpulse intervals, quantitated by the γ-parameter, was increased in patients compared with the other two groups. Secretion parameters were similar in controls and patients in remission, with the exception of the nocturnal cortisol secretory-burst shape (mode). The secretory burst-mode of ACTH, i.e. the time needed to reach the maximal secretion rate, was similar during the day and night, whereas for cortisol, events were shorter at night than in the day in patients, both active (P = 0.01) and in remission (P = 0.02).
Table 1.
Subject characteristics and basal hormone concentrations
| Active (n = 20) | Remission (n = 8) | Controls (n = 36) | ANOVA | |
|---|---|---|---|---|
| Age (yr) | 36.4 ± 3.3 | 37.6 ± 4.2 | 42.6 ± 2.1 | 0.12 |
| BMI (kg/m2) | 28.4 ± 1.2 | 24.8 ± 1.4 | 24.4 ± 0.6 | 0.003 |
| Transcortin (mmol/liter) | 0.69 ± 0.06 | 0.82 ± 0.10 | 0.72 ± 0.02 | 0.29 |
| Estradiol in women (pmol/liter) | 102 ± 29 | 113 ± 55 | 152 ± 36 | 0.62 |
| Testosterone in men (nmol/liter) | 11.7 ± 3.3 | 12.0 ± 2.4 | 17.4 ± 0.9 | 0.03 |
| IGF-I (nmol/liter) | 18.5 ± 1.5 | 20.6 ± 1.4 | 18.9 ± 0.9 | 0.66 |
| Free T4 (nmol/liter) | 13.6 ± 1.7 | 16.2 ± 0.6 | 16.7 ± 0.4 | 0.003 |
Data are shown as mean ± sem. Statistical comparisons were carried out with ANOVA and confirmed by the Kruskal-Wallis test.
Table 2.
Deconvolution of ACTH and cortisol profiles in patients with active Cushing's disease, patients in remission, and healthy controls
| Active (A) | Remission (B) | Controls (C) | ANOVA | P, C vs. B | P, A vs. C | |
|---|---|---|---|---|---|---|
| ACTH | ||||||
| Pulse number (per 24 h) | 23.9 ± 0.9 | 20.5 ± 1.7 | 19.6 ± 0.7 | 0.002 | 0.59 | 0.003 |
| Slow half-life (min) | 13.1 ± 0.8 | 15.4 ± 1.5 | 16.4 ± 0.7 | 0.014 | 0.51 | 0.02 |
| Mode day (min) | 10 ± 1.3 | 7.9 ± 2.0 | 7.9 ± 1.0 | 0.41 | ||
| Mode night (min) | 9.9 ± 1.6 | 7.9 ± 1.8 | 7.4 ± 0.8 | 0.25 | ||
| Basal secretion (ng/liter · 24 h) | 5110 ± 1010 | 1160 ± 305 | 800 ± 82 | <0.0001 | 0.72 | <0.0001 |
| Pulsatile secretion (ng/liter · 24 h) | 2380 ± 250 | 960 ± 270 | 760 ± 85 | <0.0001 | 0.52 | <0.0001 |
| Mean pulse mass (ng/liter) | 106 ± 15 | 45 ± 12 | 40 ± 4.5 | <0.0001 | 0.79 | <0.0001 |
| λ (frequency) | 21.7 ± 0.7 | 18.1 ± 1.5 | 18.1 ± 0.7 | 0.004 | 0.96 | 0.002 |
| γ (regularity) | 2.01 ± 0.11 | 1.87 ± 0.14 | 1.80 ± 0.06 | 0.16 | ||
| Cortisol | ||||||
| Pulse number (per 24 h) | 18.8 ± 1.1 | 19.8 ± 2.0 | 19.4 ± 0.8 | 0.88 | ||
| Slow half-life (min) | 44.9 ± 1.6 | 56.5 ± 4.4 | 55.6 ± 1.8 | 0.001 | 0.84 | <0.0001 |
| Mode day (min) | 23.9 ± 4.0 | 25.2 ± 6.3 | 13.8 ± 1.7 | 0.016 | 0.04 | 0.01 |
| Mode night (min) | 11.1 ± 1.6 | 6.9 ± 1.8 | 15.3 ± 2.3 | 0.09 | ||
| Basal secretion (nmol/liter · 24 h) | 11100 ± 2440 | 1970 ± 500 | 740 ± 120 | <0.0001 | 0.61 | <0.0001 |
| Pulsatile secretion (nmol/liter · 24 h) | 8580 ± 1430 | 3840 ± 570 | 4590 ± 340 | <0.0001 | 0.63 | <0.0001 |
| Mean pulse mass (nmol/liter) | 540 ± 102 | 202 ± 34 | 250 ± 21 | 0.001 | 0.67 | <0.0001 |
| λ (frequency) | 16.8 ± 1.0 | 17.6 ± 1.8 | 17.5 ± 0.7 | 0.85 | ||
| γ (regularity) | 2.15 ± 0.12 | 1.72 ± 0.06 | 1.86 ± 0.06 | 0.014 | 0.36 | 0.004 |
Data are shown as mean ± sem. Groups were compared with ANOVA, and post hoc contrasts were calculated between patients with active disease and controls and between patients in remission and controls. Basal and pulsatile secretion and mean pulse mass are expressed per liter distribution volume of the hormone. Higher γ denotes more uniform interpulse intervals.
Fig. 1.
Deconvolution of 24-h ACTH and cortisol plasma profiles in 30 patients with active Cushing's disease, 10 patients in long-term remission, and 36 healthy controls. Results are given as box-and-whisker plots, showing the median, interquartile range, 90% confidence interval, and extreme values. Statistical comparisons were performed with ANOVA and appropriate post hoc contrasts. NS, Not significant.
The endogenous plasma ACTH concentration-cortisol secretion dose-response results are shown in Table 3 and graphically shown in Fig. 2. No differences were found between healthy controls and patients who had undergone successful resection of the pituitary adenoma. The most striking differences between patients with active Cushing's disease and healthy controls were 1) higher EC50 values during the initial secretion phase (ACTH-cortisol pulse onset) and during the delayed (recovery) phase and 2) increased basal (unstimulated) and maximal (efficacy) secretion of cortisol. There were no differences in the slope (sensitivity) of the curves.
Table 3.
Concentration-response (ACTH-cortisol) curve parameters of patients with active Cushing disease, patients in remission after pituitary surgery, and healthy controls
| Active (A) | Remission (B) | Controls (C) | ANOVA | P, C vs. B | P, A vs. C | |
|---|---|---|---|---|---|---|
| Sensitivity (slope) | 0.43 ± 0.06 | 0.58 ± 0.15 | 0.59 ± 0.07 | 0.26 | ||
| Efficacy (nmol/liter · min) | 38 ± 8.3 | 15 ± 3.3 | 21 ± 2.3 | 0.015 | 0.54 | 0.01 |
| Basal secretion (nmol/liter · min) | 6.2 ± 1.2 | 2.3 ± 0.48 | 1.7 ± 0.17 | <0.0001 | 0.61 | <0.0001 |
| Inflection point (min) | 21 ± 2.9 | 24 ± 4.9 | 22 ± 2.0 | 0.73 | ||
| EC50 onset (ng/liter) | 59 ± 7.9 | 15 ± 3.2 | 20 ± 3.7 | <0.0001 | 0.64 | <0.0001 |
| EC50 recovery (ng/liter) | 128 ± 18 | 67 ± 17 | 70 ± 8.4 | 0.004 | 0.91 | 0.001 |
Data are shown as mean ± sem. Groups were compared with ANOVA, and post hoc contrasts were calculated between patients with active disease and controls and between patients in remission and controls.
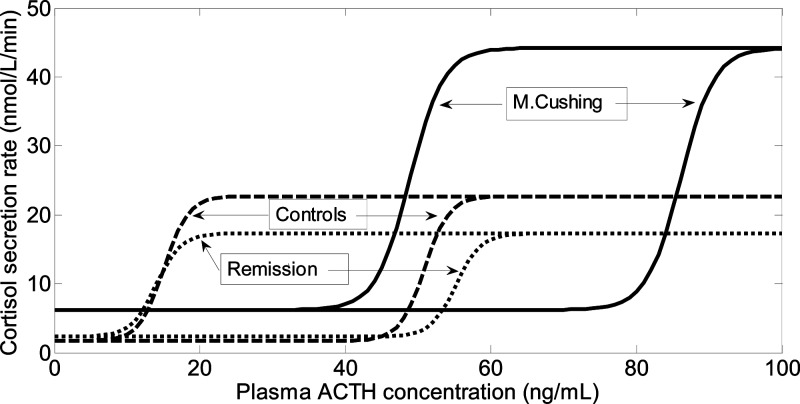
Fig. 2.
Dose-response curves of the plasma ACTH concentration and cortisol secretion rate in 30 patients with active Cushing's disease, 10 patients in long-term remission, and 36 healthy controls. The lines shown are based on the mean values of the logistic regression function. The model shown here is the two-potency model (see Subjects and Methods), so that for each group, the dose-response curve consisted of two lines, one for the phase during which the ACTH concentration rose, and one during which the ACTH concentration fell. The two curves are parallel but display different EC50 values. No statistical difference was found between cured patients and controls. In patients with active disease, the curves were displaced to the right, denoting decreased potency (increased EC50), but with a higher maximal value, denoting increased efficacy. The slopes of the curves were similar, indicating unchanged sensitivity.
Discussion
Novel findings in this study were a marked rightward shift in the endogenous ACTH-cortisol dose-response curve in Cushing's patients, elevated basal (nonpulsatile) cortisol secretion, and increased ACTH efficacy (maximal cortisol secretion). In surgically treated patients in long-term remission, ACTH-cortisol response curves were indistinguishable from those in healthy subjects, indicating complete recovery from and reversibility of partial ACTH-cortisol down-regulation.
The first publications on plasma ACTH and serum cortisol concentration profiles were descriptive (2, 3) or used a simple pulse detection algorithm (4). Newer deconvolution methods dissect the profile into a basal secretion component, a series of hormone pulses (pulsatile component), and the associated pulse wave-forms and half-lives. Compared with previous publications, the present deconvolution technology estimates lower ACTH secretory burst frequency both in patients and controls, although increased ACTH frequency was still noted in the tumorous condition (8). Lower ACTH pulse frequency can be explained by the use of the automated operator-independent time-intensive calculations and rigid statistical evaluation. In contrast to ACTH, cortisol frequency was similar in all three studied cohorts. As we described earlier, not every ACTH pulse is followed by a cortisol pulse in Cushing's disease for unknown reasons (8). A possibility requiring evaluation of larger cohorts is that subgroups of patients may exist with high and low pulsatility, as others have described (15). In the present study, we also noted decreased half-lives for ACTH and cortisol in patients. It is conceivable that under an overload of cortisol, its metabolism is greatly enhanced, thus leading to increased clearance from the circulation and decreased half-life. Whether such a mechanism can explain a similar picture for ACTH is not certain and requires additional study.
Interesting, but not readily explained, was a day-night difference in the latency for attaining the maximal cortisol secretion rates within bursts (mode), such that at night bursts were narrower with an earlier maximum than during the daytime in both cured and active patients. This finding suggests that long-term exposure to ACTH may alter permanently the cortisol secretion process per se.
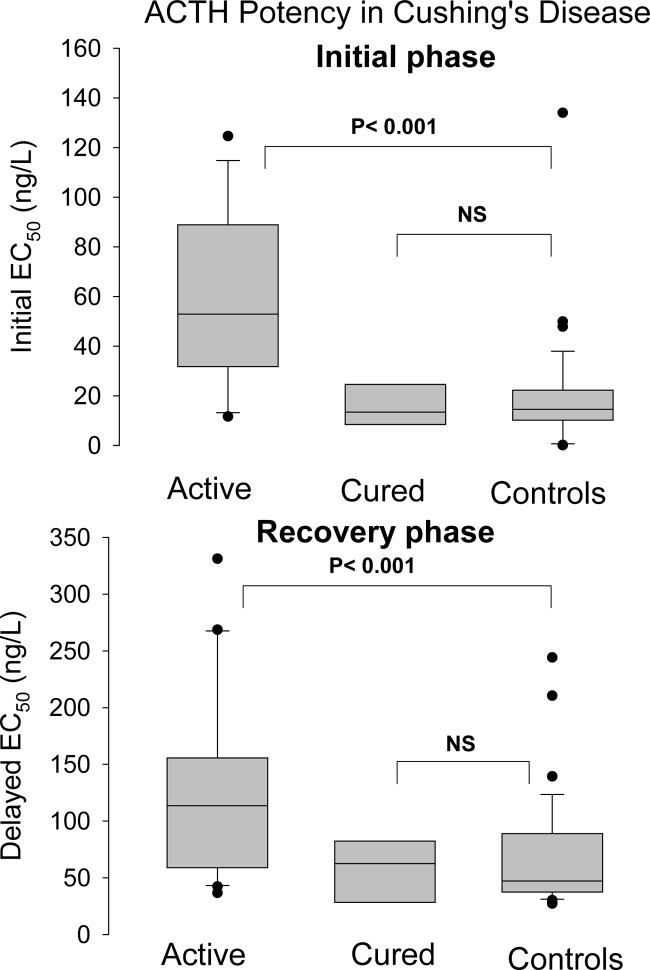
Fig. 3.
ACTH potency during the initial and recovery phases in 30 patients with active Cushing's disease, 10 patients in long-term remission, and 36 healthy controls. Results are given as box-and-whisker plots, showing the median, interquartile range, 90% confidence interval, and extreme values. Statistical comparisons were performed with ANOVA and appropriate post hoc contrasts. NS, Not significant.
A striking finding of this study was the combination of decreased ACTH potency and increased ACTH efficacy without change in sensitivity. The change of potency, here quantitated by EC50, was present during both phases of cortisol secretory bursts, i.e. the initial and recovery phases. Previous investigations raised the possibility of diminished responsiveness of the adrenal glands in patients with Cushing's syndrome, but this postulate was based on the ratio of 24-h secretion rates of cortisol and ACTH (8). The formal dose-response model applied here for the first time provides a mechanistic basis for adrenal down-regulation, viz. reduced ACTH pulse potency. The question arises of what factors produce the observed changes in the ACTH-cortisol relationship in Cushing's disease. It seems logical to attribute the increased efficacy (maximal cortisol concentration) to the expanded adrenal cell mass and increased number of melanocortin-2 receptors on adrenocortical cells, which is a hallmark of long-term stimulation by ACTH, irrespective of its source (16). The rightward shift of the dose-response curves suggests competitive inhibition of ACTH-receptor signaling (17). Because circulating ACTH molecules in Cushing's disease do not differ from those in normal subjects, it is likely that postreceptor mechanisms are involved (18). This would be consistent with demonstrable down-regulation of ACTH action in vitro. The clinical consequence of the altered nocturnal secretion pattern in cured patients is not known.
During the last two decades, it has become increasingly clear that the adrenal gland is not an unregulated target for ACTH but that the autonomic nervous system and intraadrenal hormone signals can modify steroidogenesis (19). However, circulating hormones other than ACTH may also modulate cortisol secretion. Indeed, leptin can directly inhibit glucocorticoid synthesis of the normal human and rat adrenal gland in vitro (20, 21). It is thus possible that a leptin-dependent protective mechanism operates in Cushing's disease. Interestingly, obesity is also associated with decreased ACTH potency, but with unchanged efficacy (22), as in Cushing's disease. Increased visceral fat mass characterizes both conditions, resulting in insulin resistance and hypersecretion of various adipokines. Another pathway for regulating glucocorticoid secretion and adrenal gland responsiveness to ACTH in the human and experimental animals is the functional suprachiasmatic-adrenal neuronal pathway. This route is responsible for the acute rise of serum cortisol concentration without a rise in ACTH after bright light exposure and contributes to modulating responsiveness of the adrenal gland to ACTH (23). Whether this pathway diminishes ACTH-cortisol coupling in obesity and Cushing's disease with its associated increased sympathetic activity is not yet known (24, 25).
It is well known that the hypothalamo-ACTH-cortisol axis is activated in many patients with major depression, which was demonstrated in the 1980s (26, 27). Several arguments, both direct and indirect, suggest that the central CRH system is activated (28, 29). In addition, relatives of patients with major depression have subtle abnormalities of the hypothalamo-ACTH-cortisol axis before developing depression (30). Aberrations of the ACTH-cortisol secretion in depression are thus common and in Cushing's disease by definition. On the other hand, although in major depression, ACTH is activated by hypothalamic abnormalities (e.g. CRH with a possible contribution by arginine vasopressin), in Cushing's disease with its accompanying atypical depression, this is accomplished by unregulated tumorous ACTH production. One might wonder whether the change in adrenal responsiveness as found in Cushing's disease could be related to depression per se. This question cannot be resolved yet, because there are no data on the ACTH-cortisol relation in major depression studied with the tools applied here.
Compared with classical pharmacological dose-effect studies of the hypothalamo-pituitary-adrenal system, the major advantage of the current noninvasive analytical approach is that it does not interfere with the regulatory system being examined. This is critical for physiological validity. The EC50 in controls (20 ± 3.7 ng/liter ACTH) mirrors the magnitude found in vitro (31) and in a large cohort of more than 100 normal subjects sampled during the night (7). In contrast, EC50 values in the range of 25–59 ng/liter have been in indirect studies, as discussed by Keenan et al. (6). The noninvasive dose-response estimation approach used here may have merit in evaluating other pathophysiology, especially in the human.
In summary, this study identifies decreased ACTH potency (increased EC50) and increased efficacy (maximal effect) with unchanged sensitivity (slope) in patients suffering from Cushing's disease. These abnormalities are completely reversed after curative pituitary surgery.
Acknowledgments
Disclosure Summary: The authors have nothing to declare.
Footnotes
- BMI
- Body mass index.
References
- 1. Reitmeyer M, Vance ML, Laws ER., Jr 2002. The neurosurgical management of Cushing's disease. Mol Cell Endocrinol 197:73–79 [DOI] [PubMed] [Google Scholar]
- 2. Krieger DT, Allen W. 1975. Relationship of bioassayable and immunoassayable plasma ACTH and cortisol concentrations in normal subjects and in patients with Cushing's disease. J Clin Endocrinol Metab 40:675–687 [DOI] [PubMed] [Google Scholar]
- 3. Boyar RM, Witkin M, Carruth A, Ramsey J. 1979. Circadian cortisol secretory rhythms in Cushing's disease. J Clin Endocrinol Metab 48:760–765 [DOI] [PubMed] [Google Scholar]
- 4. Liu JH, Kazer RR, Rasmussen DD. 1987. Characterization of the twenty-four hour secretion patterns of adrenocorticotropin and cortisol in normal women and patients with Cushing's disease. J Clin Endocrinol Metab 64:1027–1035 [DOI] [PubMed] [Google Scholar]
- 5. Veldhuis JD, Roelfsema F, Iranmanesh A, Carroll BJ, Keenan DM, Pincus SM. 2009. Basal, pulsatile, entropic (patterned), and spiky (staccato-like) properties of ACTH secretion: impact of age, gender, and body mass index. J Clin Endocrinol Metab 94:4045–4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keenan DM, Roelfsema F, Carroll BJ, Iranmanesh A, Veldhuis JD. 2009. Sex defines the age dependence of endogenous ACTH-cortisol dose responsiveness. Am J Physiol Intergr Comp Physiol 297:R515–R523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Veldhuis JD, Iranmanesh A, Roelfsema F, Miles JM., Aoun P, Takahashi P, Keenan DM. 13 July 2011. Tripartite control of dynamic ACTH-cortisol dose-responsiveness by age, body mass index and gender in 111 healthy adults. J Clin Endocrinol Metab 10.1210/jc.2011.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van den Berg G, Frölich M, Veldhuis JD, Roelfsema F. 1995. Combined amplification of the pulsatile and basal modes of adrenocorticotropin and cortisol secretion in patients with Cushing's disease: evidence for decreased responsiveness of the adrenal glands. J Clin Endocrinol Metab 80:3750–3757 [DOI] [PubMed] [Google Scholar]
- 9. van den Berg G, Pincus SM, Veldhuis JD, Frölich M, Roelfsema F. 1997. Greater disorderliness of ACTH and cortisol release accompanies pituitary-dependent Cushing's disease. Eur J Endocrinol 136:394–400 [DOI] [PubMed] [Google Scholar]
- 10. Liu PY, Keenan DM, Kok P, Padmanabhan V, O'Byrne KT, Veldhuis JD. 2009. Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endocrinol Metab 297:E538–E544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keenan DM, Roelfsema F, Veldhuis JD. 2010. Dose-response downregulation within the span of single interpulse intervals. Am J Physiol 299:R11–R18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Biemond P, de Jong FH, Lamberts SWJ. 1990. Continuous dexamethasone infusion for seven hours in patients with Cushing syndrome. Ann Intern Med 112:738–742 [DOI] [PubMed] [Google Scholar]
- 13. Iranmanesh a, Lizarralde G, Veldhuis JD. 1993. Coordinate activation of the corticotropic axis by insulin-induced hypogycemia: simultaneous estimates of β-endorphin, ACTH, and cortisol secretion and disappearance in normal men. Acta Endocrinol (Copenh) 128:521–528 [DOI] [PubMed] [Google Scholar]
- 14. Keenan DM, Iranmanesh A, Veldhuis JD. 2011. Analytical construct of reversible desensitization of pituitary-testicular signaling: illustrative application in aging. Am J Physiol Reg Integr Comp Physiol 300:R349–R360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Cauter E, Refetoff S. 1985. Evidence for two subtypes of Cushing's disease based on the analysis of episodic cortisol secretion. N Engl J Med 312:1343–1349 [DOI] [PubMed] [Google Scholar]
- 16. Imai T, Seo H, Murata Y, Ohno M, Satoh Y, Funahashi H, Takagi H, Matsui N. 1990. Adrenocorticotropin increases expression of c-fos and β-actin genes in rat adrenals. Endocrinology 127:1742–1747 [DOI] [PubMed] [Google Scholar]
- 17. Kenakin TP. 2009. A pharmacology primer. 3rd ed San Diego: Elsevier Academic Press [Google Scholar]
- 18. Bertagna X, Stone WJ, Nicholson WE, Mount CD, Orth DN. 1981. Simultaneous assay of immunoreactive β-lipotropin, γ-lipotropin, and β-endorphin in plasma of normal human subjects, patients with ACTH/LPH hypersecretory syndromes, and patients undergoing chronic hemodialysis. J Clin Invest 67:124–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. 1998. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev 19:101–143 [DOI] [PubMed] [Google Scholar]
- 20. Pralong FP, Roduit R, Waeber G, Castillo E, Mosimann F, Thorens B, Gaillard RC. 1998. Leptin inhibits directly glucocorticoid secretion by normal human and rat adrenal gland. Endocrinology 139:4264–4268 [DOI] [PubMed] [Google Scholar]
- 21. Bornstein SR, Uhlmann K, Haidan A, Ehrhart-Bornstein M, Scherbaum WA. 1997. Evidence for a novel peripheral action of leptin as a metabolic signal to the adrenal gland: leptin inhibits cortisol release directly. Diabetes 46:1235–1238 [DOI] [PubMed] [Google Scholar]
- 22. Roelfsema F, Kok P, Frolich M, Pereira AM, Pijl H. 2009. Disordered and increased adrenocorticotropin secretion with diminished adrenocorticotropin potency in obese premenopausal women. J Clin Endocrinol Metab 94:2991–2997 [DOI] [PubMed] [Google Scholar]
- 23. Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. 1999. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci 11:1535–1544 [DOI] [PubMed] [Google Scholar]
- 24. Leproult R, Colecchia EF, L'Hermite-Balériaux M, Van Cauter E. 2001. Transition from dim to bright light in the morning induces an immediate elevation of cortisol levels. J Clin Endocrinol Metab 86:151–157 [DOI] [PubMed] [Google Scholar]
- 25. Alvarez GE, Beske SD, Ballard TP, Davy KP. 2002. Sympathetic neural activation in visceral obesity. Circulation 106:2533–2536 [DOI] [PubMed] [Google Scholar]
- 26. Mortola JF, Liu JH, Gillin JC, Rasmussen DD, Yen SS. 1987. Pulsatile rhythms of adrenocorticotropin (ACTH) and cortisol in women with endogenous depression: evidence for increased ACTH pulse frequency. J Clin Endocrinol Metab 65:962–968 [DOI] [PubMed] [Google Scholar]
- 27. Linkowski P, Mendlewicz J, Kerkhofs M, Leclercq R, Golstein J, Brasseur M, Copinschi G, Van Cauter E. 1987. 24-hour profiles of adrenocortiotropin, cortisol, and growth hormone in major depressive illness: effect of antidepressant treatment. J Clin Endocrinol Metab 65:141–152 [DOI] [PubMed] [Google Scholar]
- 28. Young EA, Veldhuis JD. 2006. Disordered adrenocorticotropin secretion in women with major depression. J Clin Endocrinol Metab 91:1924–1928 [DOI] [PubMed] [Google Scholar]
- 29. Bao AM, Meynen G, Swaab DF. 2008. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev 57:531–553 [DOI] [PubMed] [Google Scholar]
- 30. Holsboer F. 2000. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23:477–501 [DOI] [PubMed] [Google Scholar]
- 31. Catalano RD, Stuve L, Ramachandran J. 1986. Characterization of corticotropin receptors in human adrenocortical cells. J Clin Endocrinol Metab 62:300–304 [DOI] [PubMed] [Google Scholar]