Abstract
Objectives; Pulmonary hypertension (PH) is a common and well established complication of chronic obstructive pulmonary disease (COPD). Its presence is associated with decreased survival. This study was designed to investigate the PH frequency and its relations in hospitalized tobacco and biomass related COPD patients. Methods and Results; The study was a retrospective review of inpatients with COPD defined as a history of tobacco or biomass smoking, Pulmonary function tests (PFT) within stable status, an echocardiogram within stable status. PH was defined as systolic pulmonary artery pressure (sPAP) >35 mmHg. Of the 694 individuals, 600 had suitable aspects for inclusion of study. All Females were biomass exposer and males were tobacco smoker. The Prevalence of PH was found more frequent in females than males. It was more prominent in moderate level COPD cases (56,2% and 37,5%, P<0,002). Both groups had airflow limitation, hypercapnia and hypoxemia, but no differences were found in terms of PaCO2 and PaO2. However, FEV1 % was lower in males than females (p<0,005). On the other hand, FVC % was lower in the females compared with the males (p < 0.02). When analyzing the influence of PFT and demographic parameters on PH in separate COPD level groups, the results a bit varied among the groups. Conclusion; Our study demonstrated that PH frequency is higher in female COPD cases due to biomass smoke than in male COPD cases due to tobacco smoke. The influence of FVC % on the risk of a person having PH increased with increasing COPD level.
Keywords: COPD, Pulmonary Hypertension, Environmental pollutants, Smoking.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of chronic morbidity and mortality throughout world. It is the fourth leading cause of death in the world and further increases its prevalence and mortality can be predicted in the coming decades 1, 2. Pulmonary hypertension (PH) is a common and well established complication of COPD 3. Its presence is associated with decreased survival 4, 5. In industrialized countries, tobacco smoking (TS) is responsible for more than 80 % of the incidence of COPD 6. COPD is also observed in rural areas of developing countries, affecting predominantly non-smoking women with exposure to biomass smoke (BS) during cooking. Biological fuels that produce heat are called biomass. It is predicted that half of the world population and more than 90 % of the rural population in developing countries uses biological fuels 7. Approximately 3 billion people worldwide are exposed to smoke from biomass fuel compared with 1.01 billion people who smoke tobacco, suggesting that exposure to BS might be the most important global risk factor for COPD. However, to date whether or not different between biomass exposed women and smoker men the prevalence of PH was not examined. Therefore this study was designed to investigate the PH frequency of hospitalized COPD patients with the PFT parameters, demographic aspects, smoking status, and history of use of biomass.
Materials and Methods
Subjects were chosen from the patients hospitalized in the ward of the Pulmonary Department of University Hospital. The department gives service to both economically deprived and developed population. The study was approved by the local ethics committee. A retrospective review was conducted on COPD patients who had undergone echocardiographic investigation for evaluation of pulmonary hypertension between the years of 2000 and 2010. The subjects consisted of females and males who required hospitalization in the department of pulmonary department due to COPD during the period. All patients were considered clinically stable. Patients with conditions including left heart failure, pulmonary embolus, lung cancer, sleep apnea, obesity (BMI > 35) and exposed to both of tobacco and biomass smoke were excluded. Cumulative exposure of biomass smoke was expressed as hour-years (h-yr), the product of the number of years cooking with biomass fire multiplied by the average number of hours spent daily in the traditional underground oven. Cumulative tobacco smoking was expressed as pack/years. Diagnosis of COPD was performed by assessment of functional criteria of chronic and irreversible airflow obstruction (forced expiratory volume in one second (FEV1)/(forced vital capacity) < 70 %, FEV1 < 80 % predicted) and without asthma as assessed by clinical history and response to bronchodilators (change, 12% in FEV1 following 400 μg of inhaled salbutamol).
Pulmonary Function Tests: Post-bronchodilator spirometry was performed with a flow sensitive spirometer (Vitalograph, Vitalograph ALPHA, Maids Moreton, Buckingham, UK), which meets to the European Respiratory Society and American Thoracic Society standards. At least three reproducible maximal expiratory efforts starting from complete inspiration were obtained; the best FEV1 was used to calculate FEV1 as percent predicted. FVC and FEV1 were expressed as percentage of predicted (FVC %, FEV1 %).
Echocardiographic measurement: Echocardiography was performed by the same cardiologist (7 years of experience) using a Vivid 3 instrument (General Electric, US) and by utilizing a 2 MHz probe. The gradient between the right ventricular peak systolic pressure and right atrium pressure was measured by Doppler echocardiography at rest in cases with tricuspid insufficiency. The modified Bernoulli equation was used to calculate pulmonary artery pressure (PAP) pressure: PAP = 4 × (tricuspid systolic jet). The estimated systolic PAP (sPAP) was obtained by adding the right atrium mean pressure. Right atrial pressure is estimated to be 5 mm-Hg when the diameter of inferior vena cava (IVC) is less than 1.7 cm and a 50 % decrease in the diameter with inspiration, 10 mm-Hg when IVC is more than 1.7 cm and with normal inspiratory collapse (≥50 %), and 15 mm-Hg when IVC is more than 1.7 cm and inspiratory collapse is less than 50 % 8. When sPAP is more than 35 mm-Hg, the presence of PH is established accordingly the newly recommendations of the Working Group on Diagnosis and Assessment of Pulmonary Arterial Hypertension in the 4th World Symposium on Pulmonary Hypertension 9.
Statistical analysis: Patients were compared according to exposure using a t test for independent groups for normally distributed variables or a Mann-Whitney test or Chi-squared test (for qualitative variables). The Kolmogorov-Smirnov test was used to assess normality. Results are presented as mean ± standard deviation (SD), and frequency expressed as a percent. A multiple logistic regression analysis was used to identify factors independently associated PH in COPD. Multiple logistic regressions were performed with the dependent variable of the presence versus the absence of PH and age, gender, PaCO2, PaO2, FEV1 %, FVC % independent variables. Associations were expressed as odds ratios (OR) with 95 % confidence intervals (CI). To analyze whether risk factors for PH differ with COPD severity multiple logistic regressions were performed for moderate, severe and very severe COPD groups separately. A “p” value of < 0.05 was considered as statistically significant.
Results
Initially, 694 patients were reviewed the retrospective data analysis by our service. Of this number, 94 were excluded due to comorbidities such as obesity 14, sleep apnea 10, bronchiectasis 18, left heart failure 15 tobacco and biomass smoke exposure 21, and incomplete documentation 16. The remaining 600 patients, all with COPD with varying degrees of severity were accepted for our study and evaluated. Patients were 336 (56 %) males, and 264 (44 %) females. Males were tobacco smokers and females biomass smoke exposures. All patients were the residence of same city. The mean cumulative exposure was 233 ± 101 h-yr for the biomass group and 51 ± 32 pack-years for smokers.
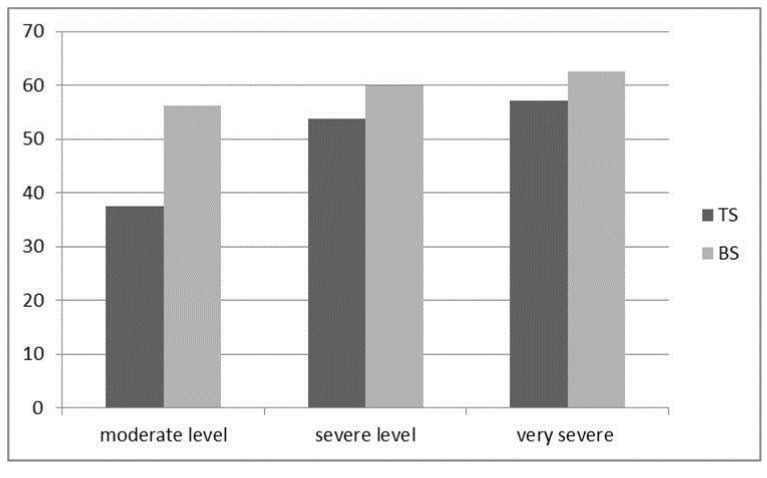
Table 1 shows the general characteristics of the patients. Presence of PH was more frequent in females than males. It was more prominent in moderate COPD cases (56,2 % and 37,5 %, P < 0,002) (Figure 1). However there was no difference between the frequency of PH in severe and in very severe COPD cases. Both groups had airflow limitation, hypercapnia and hypoxemia, but no differences were found in terms of PaCO2 and PaO2. However, FEV1 % was lower in males than females (p<0,005). On the other hand, FVC % was significantly lower in the BS group compared with the TS group (p < 0.02). Mean age of females was found to be higher than males (69 + 10, 65 + 10 p=0,001).
Table 1.
Baseline characteristics of patients with chronic obstructive pulmonary disease.
| All | Tobacco Group | Biomass Group | p | ||
|---|---|---|---|---|---|
| n | 600 | 336 | 264 | ||
| Age | 67±10 | 65+10 | 69+10 | 0,001 | |
| Biomass smoke, h-yr | 233 ± 101 | ||||
| Tobacco smoke, pack-years | 51± 32 | ||||
| Spirometric GOLD classification | 0,03 | ||||
| Stage II, moderate, % | 27 | 28,6 | 25 | ||
| Stage III, severe, % | 51 | 48,4 | 56,8 | ||
| Stage IV, very severe, % | 22 | 25 | 18,2 | ||
| FEV1, % predicted (L/s) | 41±14 | 40+15 | 42+13 | 0,005 | |
| FVC, % predicted(L) | 55±17 | 56+15 | 55+19 | 0,02 | |
| PaO2, mm Hg | 44±10 | 44+8 | 44+11 | >0,05 | |
| PaCO2, mm Hg | 50±10 | 49+11 | 50+9 | >0,05 | |
| PH frequency % | 50 | 59,1 | 0,03 | ||
Figure 1.
Frequency (%) of PH in Different COPD levels in BS and TS groups.
The results of according to stages of COPD were investigated. In study there weren't patient with mild COPD. Moderate COPD cases included 96 males and 66 females, totally 162. Males had higher levels of FVC % and FEV1 % than females (p=0, 01). Females had more higher level of PaO2 than males (42+11, 45+7 p=0,01). PH was more frequent in females than males (63,6%, 37,5% p=0,005) (Table 2). Severe COPD cases included 156 males and 150 females, totally 306. Females had higher levels of FVC % and FEV1 % than males (p =0,000, p=0,000). The level of PaO2 was higher in females than males (p=0,007). The frequency of PH was not different between males and females (Table 3). Very severe COPD cases included 84 males and 48 females, totally 132. Males had higher levels of FVC % and FEV1 % than females (p=0,004, p=0,000). The level of PaO2 was lower in females than males (p=0,002). The frequency of PH was not different between males and females (Table 4).
Table 2.
Baseline characteristics of patients with moderate COPD cases.
| Tobacco Group | Biomass Group | p | |
|---|---|---|---|
| n | 96 | 66 | |
| Age | 70+10 | 68+3 | >0,05 |
| FEV1, % (L/s) | 61+9(1,41+0,47) | 58+7 (1,10+0,28) | 0,01 |
| FVC, % (L) | 78+16 (2,26+0,76) | 71+9 (1,62+0,32) | 0,003 |
| PaO2, mm Hg | 42±11 | 45 ± 7 | 0,01 |
| PaCO2, mm Hg | 51± 7 | 47±9 | >0,05 |
| PH frequency, % | 37,5 | 56,2 | 0,002 |
Table 3.
Baseline characteristics of patients with severe COPD cases.
| Tobacco Group | Biomass Group | p | |
|---|---|---|---|
| n | 156 | 150 | |
| Age | 64±12 | 69±9 | 0,001 |
| FEV1, % (L/s) | 35±4 (1,04±0,24) | 43±5 (0,91±0,17) | 0,001 |
| FVC, % (L) | 51±9 (1,90±0,42) | 57±7 (1,46±0,26) | 0,001 |
| PaO2, mm Hg | 43 ± 10 | 45 ± 8 | >0,05 |
| PaCO2, mm Hg | 47 ± 12 | 52 ± 9 | 0,001 |
| PH frequency, % | 53,8 | 60 | >0,05 |
Table 4.
Baseline characteristics of patients with very severe COPD cases.
| Tobacco Group | Biomass Group | p | |
|---|---|---|---|
| n | 84 | 48 | |
| Age | 64±11 | 68±14 | >0,05 |
| FEV1, % (L/s) | 25 ± 8 (0,69±0,23) | 20±4(0,50±0,13) | 0,001 |
| FVC, % (L) | 37±5 (1,27 ± 0,29) | 33 ± 7 (0,90±0,21) | 0,004 |
| PaO2, mm Hg | 48 ±10 | 40±9 | 0,002 |
| PaCO2, mm Hg | 51±12 | 51±9 | >0,05 |
| PH frequency, % | 57,1 | 62,5 | >0,05 |
To analyze whether risk factors for PH differ with COPD level, multiple logistic regressions were performed for each COPD severity group separately. The influence of FVC % on the risk of a person having PH increased with increasing COPD level. Variables except age were significant in the development of PH in patients with moderate COPD. Blood gas parameters of PaCO2 and PaO2 were significant in the development of PH in patients with severe COPD. FVC %, FEV1 % and sex variables were significant in the development of PH in patients with very severe COPD (Table 5).
Table 5.
Risk Factors Associated of PH in patients with different COPD stages.
| Stage II, moderate | p | Stage III, severe | p | Stage IV, very severe | p | |
|---|---|---|---|---|---|---|
| FEV1, % (L/s) | 1,53 (1,29- 1,83) | 0,000 | 1,06(,99-1,12) | 0,05 | 1,20(1,05-1,36) | 0,005 |
| FVC, % (L) | 0,85 (0,77-0,94) | 0,001 | 0,98(0,95-1,02) | 0,4 | 1,12(1,05-1,19) | 0,000 |
| PaO2, mm Hg | 0,89 (0,79-0,99) | 0,04 | 1,04(1,01-1,07) | 0,001 | 1,031(,98-1,07) | 0,1 |
| PaCO2, mm Hg | 1,07 (1,01- 1,13) | 0,02 | 1,02(1,00-1,05) | 0,02 | 1,02 (0,97-1,07) | 0,2 |
| Age | 0,92(0,84-1,00) | 0,06 | 1,02(0,99-1,04) | 0,07 | 0,96 (0,93-1,00) | 0,07 |
| sex | 29 (6-128) | 0,000 | 0,71 (0,36-1,34) | 0,3 | 7 (2-24) | 0,002 |
Discussion
To our knowledge, this is the first study compares frequency of PH in the subjects exposed to biomass and tobacco smoke, and describes the possible causes of PH in these patients groups. In the present study, the most relevant findings were that patients exposed to BS had more frequent PH presence and less FVC %, whereas TS group had less frequent PH presence and FEV1 %. Finally, biomass smoke exposed group had more frequent PH especially in the moderate level COPD cases.
BS is composed of a relatively equal mixture of gases and particles and can penetrate deeply into the lung, producing a variety of morphologic and biochemical changes 10, 11. A recent meta-analysis, which reviewed risk of COPD from exposure to BS, concluded that BS exposure is a clear risk factor for COPD 12. It has reported that BS related COPD cases' clinical characteristics, quality of life, and mortality rate were similar in degree to that of tobacco smokers 13. To date, the frequency of PH was not compared in these risk groups. In the current study, we found that the biomass related COPD cases had more frequent PH than cigarette smoking related COPD cases.
The relationship between BS exposure, and PH and cor pulmonale (CP) has long been established 14-16. An autopsy study conducted in females with CP with ages ranging from 20 to 60 years in Delhi, India. It reported that those females with CP had biomass smoke exposure history. They concluded that PH and CP development might have a correlation with biomass smoke exposure 14. A Mexican study reported a clinical picture with a chronic pulmonary disease and a significantly high PH that they have observed frequently among women exposed to wood smoke 17. An autopsy study compared the COPD cases caused by BS exposure and tobacco smoking. It reported that vascular changes were prominent in both groups, but were more severe in the biomass smoke exposed group which could explain why PH and CP in women exposed to biomass smoke is common and high 18. Also we previously reported that prevalence of PH was higher among BS exposed females than non-exposed females in a healthy cohort (48 % vs 12 %, p<0.05). The Odds Ratio (OR) for PH development with BS exposure was established as 6 (p<0.001) 19.
Independent predictors of PH were found in moderate level, severe level, and very severe level COPD cases as follow; gender, FEV1 %, FVC %, PaO2, PaCO2 , and PaO2, PaCO2 and gender, FEV1%, FVC % respectively. Some previous studies examining the relationship between spirometry and PH reported similar results 20-22. However some studies reported weak or no associations between FEV1 and sPAP 23, 24. Sandoval et al reported a correlation between pulmonary artery pressure (PAP) and PaO2 in wood smoke exposed women, but there was not any correlation regarding other factors such as FEV1 %, FVC %, and PaCO2 17. Sim et al. found that a correlation between sPAP and FEV1 %, FVC %, higher PaCO2 and lower PaO2 25. A study investigated the relation between PH and inflammation in COPD, and found that PaO2 and C-reactive protein were independent variables for PH in COPD cases 26. This mismatch results may be due to the differences in the severity of COPD between cohorts. Our finding that sex had highest value of OR for PH in moderate and very severe COPD cases. It poses that biomass smoke has a greater risk for PH in women. Also it can suggest a predisposition to PH in females. It is reported that age is an independent predictor of PH in COPD 20. It is suggest that this increasing may be relating to decreasing of FEV1 % and FVC %. In our study, however, age was not an independent factor in any COPD level although prevalence of PH was increased accordingly COPD level. This finding is a bit different from previous studies.
The pathophysiology of the development of PH in COPD is poorly understood and is likely multifactorial. The central stimulus to these processes remains chronic exposure of airways to noxious stimuli like tobacco and biomass smoke. Hypoxia has been classically considered to be the major pathogenic mechanism of pulmonary hypertension in COPD 21, 27. Chronic hypoxia induces predominant medial hypertrophy and is associated with complete reversal of pulmonary hypertension a few weeks after return to sea level 28, 29. Pathologic studies of lung specimens from patients with COPD have shown all vessel wall layers to be involved extensive pulmonary vascular remodeling with prominent intimal thickening, medial hypertrophy, and muscularization of small arterioles 30. Also pulmonary vascular remodeling has been observed in lung specimens from patients with mild to- moderate COPD without chronic hypoxemia and in smokers with normal lung function 31, 32. These very early histopathologic findings suggest that the morphologic changes in the pulmonary arteries are initiated by the toxic effects of tobacco and biomass smoke and progress in parallel with the parenchymal changes of COPD 33. There are new advances about of pathogenesis of pulmonary hypertension in COPD supporting an endothelium-derived vasoconstrictor-dilator imbalance, mainly from a decreased endothelial nitric oxide expression, increased vascular endothelial growth factor and serotonin transporter expressions 32, 34-36.
The exact prevalence of PH in patients with COPD is unclear. The routine investigation of PH is difficult in all COPD patients due to request of right heart catheterization. Estimates of the prevalence of PH in patients with COPD vary widely. The literature on the prevalence of PH in COPD is confounded by several limiting factors. Studies were different each other from definition of PH to study condition (ie rest, exercise), severity of diseases and the methods used to determine pulmonary pressures. The true prevalence of PH in patients with mild or moderate COPD is not known because of the absence of large-scale epidemiologic studies. Direct measurements of PAP obtained at right-heart catheterization have been conducted only in small series of patients with mostly severe COPD. Most studies have reported a prevalence of PH in COPD to be between 30 % and 70 % 27,37,38. Severe PH is uncommon in COPD and typically is associated with less severe respiratory function compromise 39. This “severe” PH is “disproportionate” to the degree of airflow limitation. Patients with this condition are important to identify because they may be expected to have significant clinical compromise from the PH.
A recent small cohort reported that the frequencies of PH in mild, moderate, severe, and very severe COPD were 16.67 %, 54.55 %, 60.00 %, and 83.33 %, respectively 40. In another study, the frequency of PH was also found to be 25 %, 43 %, and 68 % in mild, moderate, and severe COPD, respectively 22. We found that frequencies of PH were 44 %, 56 % and 59 %, in moderate, severe, and very severe COPD, respectively. We also observed different frequency of PH between the female and male moderate COPD cases (56,2 % and 37,5 % p < 0,00) while not observed in severe and high severe COPD cases (60 %, 53,8 %, p>0,05, 62,5 % ,57,1 % p > 0,05). We concluded that biomass smoke induced PH in females earlier than tobacco smoker. In summary, our study demonstrated that PH frequency is higher in female COPD cases due to biomass smoke than in male COPD cases due to Tobacco smoke, and this difference is prominent in moderate COPD level. Also independent factors of PH are varied among the groups according to COPD level.
References
- 1.World health report. World health organization: Geneva; http://www.who.int/whr/2000/en/statistics.htm. [Google Scholar]
- 2.Lopez AD, Mathers CD, Ezatti M, Jamison DT, Murray CJ. Global burden of disease and risk factors. Washington, DC: World Bank; 2006. [PubMed] [Google Scholar]
- 3.Burrows B, Kettel LJ, Niden AH, Rabinowitz M, Diener CF. Patterns of cardiovascular dysfunction in chronic obstructive lung disease. N Engl J Med. 1972;286:912–918. doi: 10.1056/NEJM197204272861703. [DOI] [PubMed] [Google Scholar]
- 4.Weitzenblum E, Hirth C, Ducolone A, Mirhom R, Rasaholinjanahary J, Ehrhart M. Prognostic value of pulmonary artery pressure in chronic obstructive pulmonary disease. Thorax. 1981;36:752–758. doi: 10.1136/thx.36.10.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traver GA, Cline MG, Burrows B. Predictors of mortality in chronic obstructive pulmonary disease. A 15-year follow-up study. Am Rev Respir Dis. 1979;119:895–902. doi: 10.1164/arrd.1979.119.6.895. [DOI] [PubMed] [Google Scholar]
- 6.Environmental Protection Agency. Respiratory health effects of passive smoking; lung cancer and other disorders. The report of the US Environment Protection Agency. Bethesda, MD, USA: National Institutes of Health, National Cancer Institute; 1993. [Google Scholar]
- 7.World Resources Institute/UNEP/UNDP/World Bank. 1998-99 world resources: a guide to the global environment. Oxford: Oxford University Press; 1998. [Google Scholar]
- 8.Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66:493–496. doi: 10.1016/0002-9149(90)90711-9. [DOI] [PubMed] [Google Scholar]
- 9.Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H, Oudiz RJ, Torbicki A. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:55–66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Zelikoff JT, Chi Chen L, Cohen MD, Schlesinger RB. The toxicology of inhaled wood smoke. J Toxicol Environ Health B Crit Rev. 2002;5:269–282. doi: 10.1080/10937400290070062. [DOI] [PubMed] [Google Scholar]
- 11.Montan˜ o M, Becerril C, Ruiz V, Ramos C, Sansores RH, Gonza´lez- Avila G. Matrix metalloproteinases activity in COPD associated with wood smoke. Chest. 2004;125:466–472. doi: 10.1378/chest.125.2.466. [DOI] [PubMed] [Google Scholar]
- 12.Hu G, Zhou Y, Tian J, Yao W, Li J, Li B, Ran P. Risk of COPD from exposure to biomass smoke: a metaanalysis. Chest. 2010;138:20–31. doi: 10.1378/chest.08-2114. [DOI] [PubMed] [Google Scholar]
- 13.Ramírez-Venegas A, Sansores RH, Pérez-Padilla R, Regalado J, Velázquez A, Sánchez C, Mayar ME. Survival of patients with chronic obstructive pulmonary disease due to biomass smoke and tobacco. Am J Respir Crit Care Med. 2006;173:393–397. doi: 10.1164/rccm.200504-568OC. [DOI] [PubMed] [Google Scholar]
- 14.Padmavati S, Joshi B. Incidence and Etiology of Chronic CorPulmonale in Delhi. A Necropsy Study. Chest. 1964;46:457–63. doi: 10.1378/chest.46.4.457. [DOI] [PubMed] [Google Scholar]
- 15.Padmavatı S, Pathak SN. Chronic corpulmonale in Delhi: a study of 127 cases. Circulation. 1959;20:343–52. doi: 10.1161/01.cir.20.3.343. [DOI] [PubMed] [Google Scholar]
- 16.de Koning HW, Smith KR, Last JM. Biomass fuel combustion and health. Bull World Health Organ. 1985;63:11–26. [PMC free article] [PubMed] [Google Scholar]
- 17.Sandoval J, Salas J, Martinez-Guerra ML, Gómez A, Martinez C, Portales A, Palomar A, Villegas M, Barrios R. Pulmonary arterial hypertension and corpulmonale associated with chronic domestic woodsmoke inhalation. Chest. 1993;103:12–20. doi: 10.1378/chest.103.1.12. [DOI] [PubMed] [Google Scholar]
- 18.Rivera RM, Cosio MG, Ghezzo H, Salazar M, Pérez-Padilla R. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int J Tuberc Lung Dis. 2008;12:972–7. [PubMed] [Google Scholar]
- 19.Sertogullarindan B, Ozbay B, Asker S, Asker M, Tuncer M. An investigation of pulmonary hypertension and COPD in women exposed to biomass smoke. ERJ. 2007;51:607. [Google Scholar]
- 20.Fayngersh V, Drakopanagiotakis F, Dennis McCool F, Klinger JR. Pulmonary Hypertension in a Stable Community-Based COPD Population. Lung. 2011;189:377–82. doi: 10.1007/s00408-011-9315-2. [DOI] [PubMed] [Google Scholar]
- 21.Scharf SM, Iqbal M, Keller C, Criner G, Lee S, Fessler HE; National Emphysema Treatment Trial (NETT) Group. Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med. 2002;166:314–322. doi: 10.1164/rccm.2107027. [DOI] [PubMed] [Google Scholar]
- 22.Oswald-Mammosser M, Apprill M, Bachez P, Ehrhart M, Weitzenblum E. Pulmonary hemodynamics in chronic obstructive pulmonary disease of the emphysematous type. Respiration. 1991;58:304–310. doi: 10.1159/000195950. [DOI] [PubMed] [Google Scholar]
- 23.Higham MA, Dawson D, Joshi J, Nihoyannopoulos P, Morrell NW. Utility of echocardiography in assessment of pulmonary hypertension secondary to COPD. Eur Respir J. 2001;17:350–355. doi: 10.1183/09031936.01.17303500. [DOI] [PubMed] [Google Scholar]
- 24.Bishop JM, Csukas M. Combined use of non-invasive techniques to predict pulmonary arterial pressure in chronic respiratory disease. Thorax. 1989;44:85–96. doi: 10.1136/thx.44.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sims MW, Margolis DJ, Localio AR, Panettieri RA, Kawut SM, Christie JD. Impact of pulmonary artery pressure on exercise function in severe COPD. Chest. 2009;136:412–419. doi: 10.1378/chest.08-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joppa P, Petrasova D, Stancak B, Tkacova R. Systemic inflammation in patients with COPD and pulmonary hypertension. Chest. 2006;130:326–33. doi: 10.1378/chest.130.2.326. [DOI] [PubMed] [Google Scholar]
- 27.Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J. 2008;32:1371–1385. doi: 10.1183/09031936.00015608. [DOI] [PubMed] [Google Scholar]
- 28.Heath D, Williams DR. High-altitude medicine and pathology. London: Butterworths; 1989. pp. 102–114. [Google Scholar]
- 29.Peinado VI, Barbera JA, Abate P, Ramirez J, Roca J, Santos S, Rodriguez-Roisin R. Inflammatory reaction in pulmonary muscular arteries of patients with mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;59:1605–1611. doi: 10.1164/ajrccm.159.5.9807059. [DOI] [PubMed] [Google Scholar]
- 30.Wright JL, Petty T, Thurlbeck WM. Analysis of the structure of the muscular pulmonary arteries in patients with pulmonary hypertension and COPD: National Institutes of Healthnocturnal oxygen therapy trial. Lung. 1992;170:109–124. doi: 10.1007/BF00175982. [DOI] [PubMed] [Google Scholar]
- 31.Santos S, Peinado VI, Ramírez J. et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002;19:632– 638. doi: 10.1183/09031936.02.00245902. [DOI] [PubMed] [Google Scholar]
- 32.Peinado VI, Barbera` JA, Ramı´rez J. et al. Endothelial dysfunction in pulmonary arteries of patients with mild COPD. Am J Physiol Lung Cell Mol Physiol. 1998;18:908–913. doi: 10.1152/ajplung.1998.274.6.L908. [DOI] [PubMed] [Google Scholar]
- 33.Hale KA, Niewoehner DE, Cosio MG. Morphologic changes in the muscular pulmonary arteries: relationship to cigarette smoking, airway disease, and emphysema. Am Rev Respir Dis. 1980;122:273–8. doi: 10.1164/arrd.1980.122.2.273. [DOI] [PubMed] [Google Scholar]
- 34.Dinh-Xuan AT, Higenbottam TW, Clelland CA, Pepke-Zaba J, Cremona G, Butt AY, Large SR, Wells FC, Wallwork J. Impairment of endothelium- dependent pulmonary artery relaxation in chronic obstructive lung disease. N Engl J Med. 1991;324:1539–1547. doi: 10.1056/NEJM199105303242203. [DOI] [PubMed] [Google Scholar]
- 35.Santos S, Peinado VI, Ramirez J, Morales-Blanhir J, Bastos R, Roca J, Rodriguez-Roisin R, Barbera JA. Enhanced expression of vascular endothelial growth factor in pulmonary arteries of smokers and patients with moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1250–1256. doi: 10.1164/rccm.200210-1233OC. [DOI] [PubMed] [Google Scholar]
- 36.Eddahibi S, Chaouat A, Morrell N, Fadel E, Fuhrman C, Bugnet AS, Dartevelle P, Housset B, Hamon M, Weitzenblum E. et al. Polymorphism of the serotonin transporter gene and pulmonary hypertension in chronic obstructive pulmonary disease. Circulation. 2003;108:1839–1844. doi: 10.1161/01.CIR.0000091409.53101.E8. [DOI] [PubMed] [Google Scholar]
- 37.Chatila WM, Thomashow BM, Minai OA, Criner GJ, Make BJ. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:549– 555. doi: 10.1513/pats.200709-148ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falk JA, Kadiev S, Criner GJ, Scharf SM, Minai OA, Diaz P. Cardiac disease in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:543– 548. doi: 10.1513/pats.200708-142ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaouat A, Bugnet A-S, Kadaoui N. et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:189– 194. doi: 10.1164/rccm.200401-006OC. [DOI] [PubMed] [Google Scholar]
- 40.Gupta NK, Agrawal RK, Srivastav AB, Ved ML. Echocardiographic evaluation of heart in chronic obstructive pulmonary disease patient and its co-relation with the severity of disease. Lung India. 2011;28:105–9. doi: 10.4103/0970-2113.80321. [DOI] [PMC free article] [PubMed] [Google Scholar]