Abstract
The efficacy of the Campylobacter (C.) phages NCTC12684 (group II) and CP81 (group III) and of the Yersinia (Y.) phage PY100 to reduce the numbers of Campylobacter and Y. enterocolitica in meat at 4oC applying different Multiplicities of Infection (MOIs) was analyzed. Initial experiments were carried out in broth at 4oC and 37oC to compare cell number reductions under chilling and optimized growth conditions, respectively. The results showed a 1 log10 unit reduction of Campylobacter cell numbers at 37oC in broth. However, no reduction was observed in broth and meat at 4oC. In contrast, Y. enterocolitica cell numbers were reduced in broth at 4oC (up to 3 log10 units after 24hr) and 37oC (5 log10 units after 1.5hr) and also in meat at 4oC (2 log10 units after 48hr). The highest cell number reductions were obtained at the highest MOIs.
Keywords: Campylobacter, Yersinia, bacteriophage, food safety, biocontrol
INTRODUCTION
Campylobacter causes approximately 200,000 cases of campylobacterosis in the European Union (EFSA, 2009) and thus is one of the most important bacterial foodborne pathogens. Yersinia infections are of secondary importance with about 7,500 cases yearly (EFSA, 2009). Complete elimination of both pathogens in the food chain is currently not feasible, but the quantitative load can be reduced by several pre- and post-harvest applications. Post-harvest approaches exploiting virulent phages have already been described and focus up to now on the control of Listeria (L.) monocytogenes and Salmonella. Due to the effectiveness and innocuousness of Listeria phages, two products were already approved by the FDA: ListShield, the LMP-102 phage preparation comprising six phages for the control of L. monocytogenes on ready-to-eat foods (Bren, 2007), and Listex P100 (phage P100) for the control of this species in meat and cheese products (Carlton et al, 2005). P100 is able to eliminate or reduce Listeria up to 3.5 log10 cfu/g under appropriate conditions (Carlton et al, 2005; Holck and Berg, 2009; Soni et al, 2009; Soni and Nannapaneni, 2010). With Salmonella, up to 4 log10 unit reductions of cell number have been described in vegetables, chicken, chicken products, sausages and cheese (Modi et al, 2001; Goode et al, 2003; Leverentz et al, 2003; Whichard et al, 2003; Higgins et al, 2005). Some studies demonstrated the efficacy of phages on C. jejuni. Cell numbers were reduced by approximately 1 log10 unit at 4-5°C on chicken skin, and on raw and cooked meat (Atterbury et al, 2003; Goode et al, 2003, Bigwood et al, 2008). No data are currently available on post-harvest application of Y. enterocolitica phages.
This work describes the potential of the Campylobacter phages NCTC12684 and CP81 and of the Yersinia phage PY100 to control their hosts in broth and meat.
MATERIALS AND METHODS
Bacterial strains and bacteriophages
Two Campylobacter strains and one Yersinia strain were used in this study: C. jejuni NCTC 11168 and C. coli NCTC 12668 were obtained from the National Collection of Type Cultures (NCTC), Health Protection Agency, United Kingdom. Y. enterocolitica 83/88/2 is a plasmid-cured derivative of the serogroup O:5,27, biogroup 2 strain 83/88 (Hertwig et al, 2003). Campylobacter strains were grown on Mueller-Hinton-blood (MHB) agar (Oxoid, Wesel, Germany), modified Charcoal-Cefoperazon-Desoxycholat (mCCDA) agar (Oxoid) or in Sodium-NZamines-Casaminoacids-Yeast-Magnesiumsulfate (NZCYM) medium (Roth, Karlsruhe, Germany) at 37°C under microaerobic conditions. Y. enterocolitica 83/88/2 was grown on Luria Bertani (LB) agar (Merck, Darmstadt, Germany), Yersinia selective (CIN) agar (Oxoid) or in NZCYM medium at 37oC under aerobic conditions. C. jejuni phage CP81 isolated from retail chicken meat has recently been characterized (Hammerl et al, 2011). The NCTC group II phage 12684 that infects C. jejuni and C. coli strains has also previously been described (Sails et al, 1998). PY100 is a broad host range Yersinia phage lysing strains of Y. enterocolitica, Y. pseudotuberculosis and Y. pestis (Schwudke et al, 2008).
Reduction of bacterial cell numbers in broth
Overnight cultures of bacterial strains were diluted to a final cell number of approximately 1x105 cfu/ml. 1ml phage lysate or SM buffer (negative control) was added to 1ml of the diluted culture. The mixture was incubated at 37oC (microaerobic/aerobic) or 4oC (aerobic). The initial bacterial host concentration was kept constant in all assays at approximately 1x105 cfu/ml. Bacterial counts were determined after 0, 6, 24, 27, 30, 48, 72 and 168 hours. The following MOIs were applied: high MOI for C. jejuni (102), and for C. coli and Y. enterocolitica (104); low MOI for C. jejuni and C. coli (101), and for Y. enterocolitica (102).
Reduction of bacterial cell numbers in meat
Raw chicken and pork meat was tested to be free of Campylobacter spp. and Yersinia spp. Meat was sliced aseptically into portions of 10gm, frozen at -20oC, and defrosted in a refrigerator 24hr prior to use. Campylobacter and Yersinia strains were grown overnight in NZCYM medium at 37oC under microaerobic (Campylobacter) resp. aerobic (Yersinia) conditions. Each meat portion was inoculated with 100μl of the diluted bacterial suspensions (approximately 1x106 cfu/ml). Bacteria were allowed to attach to the matrix for 30min at room temperature. Thereafter, 1ml of the corresponding phage lysate or SM buffer was added. All samples were vacuum sealed and stored either at 37oC or at 4oC, depending on the experimental setup. At each sampling time, inoculated meat pieces were diluted 1:10 with NZCYM medium, blended for 2min, and serially diluted with NZCYM medium before being spread onto mCCDA or CIN agar plates. For the enumeration of the bacteria, plates were incubated for 48hr (Campylobacter) or 24hr (Yersinia). All experiments were performed in triplicates. Statistical differences between bacterial cell numbers in the samples were assessed by using the Mann-Whitney-U test.
RESULTS
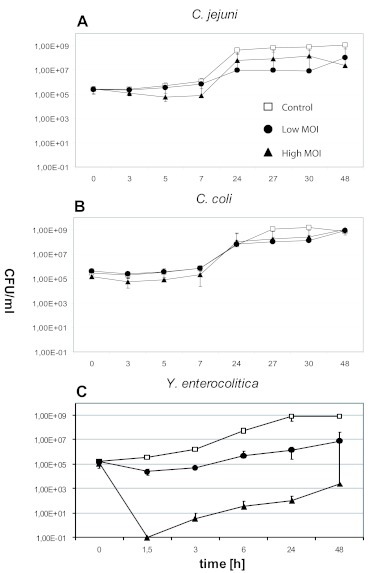
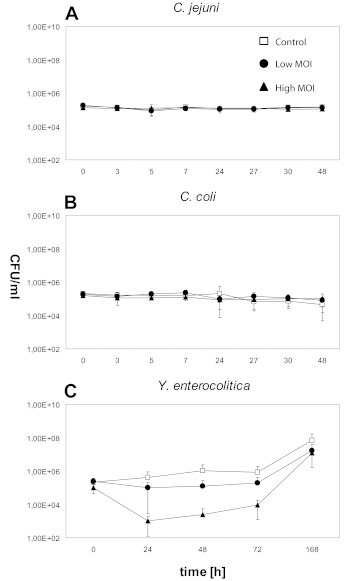
All three phages reduced the numbers of their hosts in broth at 37oC. While the Campylobacter phages CP81 and NCTC12684 yielded reductions of only 1-2 log10 units, irrespectively of the applied MOI, PY100 reduced Y. enterocolitica cell numbers at a MOI of 102 by up to 3 log10 units (after 24hr) and at a MOI of 104 by up to 5 log10 units (after 1.5hr) (Figure 1). No growth inhibition of C. jejuni and C. coli was observed at 4oC in broth at any MOI (Figure 2 A and B). At this temperature, Y. enterocolitica cell numbers were reduced by up to 1 log10 unit (low MOI) after 24hr and up to 3 log10 units (high MOI) after 24hr (Figure 2C).
Figure 1.
Phage-induced lysis of Campylobacter and Yersinia in broth at 37oC. A. C. jejuni NCTC 11168 (Phage CP81). B. C. coli NCTC 12668 (Phage NCTC12684). C. Y. enterocolitica 83/88/2 (Phage PY100).
Figure 2.
Phage-induced lysis of Campylobacter and Yersinia in broth at 4oC. A. C. jejuni NCTC 11168 (Phage CP81). B. C. coli NCTC 12668 (Phage NCTC12684). C. Y. enterocolitica 83/88/2 (Phage PY100).
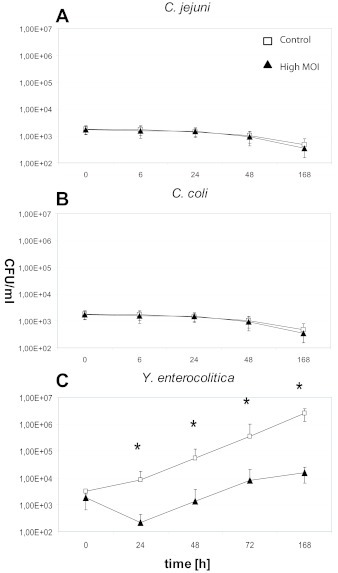
Due to restricted growth of Campylobacter at 4oC, experiments with chicken meat (Campylobacter assays) and pork meat (Y. enterocolitica assays) were exclusively carried out at high MOIs allowing lysis from without. Though, both Campylobacter phages did not lyse their host in chicken meat at 4oC (Figure 3A and B). By contrast, phage PY100 reduced the Y. enterocolitica cell numbers in pork meat significantly by approximately 2 log10 units after 24hr (Figure 3C).
Figure 3.
Phage-induced lysis of Campylobacter and Yersinia in meat at 4oC. A. C. jejuni NCTC 11168 (Phage CP81). B. C. coli NCTC 12668 (Phage NCTC12684). C. Y. enterocolitica 83/88/2 (Phage PY100). (*p <0.05)
□ control
▴ high MOI
DISCUSSION
In this study the potential of the three phages to control foodborne pathogens at the post-harvest level was analysed. We ascertained that at 37oC the Campylobacter phages CP81 and NCTC12684 reduced the cell numbers of their respective host in broth, whereas no reduction was observed at 4oC, even at a high MOI (102 resp 104). The phages probably did not cause lysis from without under these conditions. Other studies demonstrated phage-induced lysis of Campylobacter at 4oC, where an up to 1 log10 unit reduction on chicken skin and in cooked and raw meat was achieved (Atterbury et al, 2003; Goode et al, 2003; Bigwood et al, 2008). As the lysis of Campylobacter at refrigeration temperatures is rather limited, post-harvest application of phages is apparently not a promising tool to reduce the Campylobacter load on carcasses or meat. On the other hand, high Campylobacter cell number reductions (2-5 log10 units) by phage were obtained in chickens at the pre-harvest level (Loc Carrillo et al, 2005; Wagenaar et al, 2005; El-Shibiny et al, 2009).
Contrary to the Campylobacter phages, Yersinia phage PY100 significantly reduced cell numbers of its host at 4oC in broth and in pork meat. The most efficient reductions occurred at the highest MOI. The data are consistent with previously published studies, in which a control of various pathogens was accomplished by application of high phage numbers (Goode et al, 2003; Leverentz et al, 2004; O'Flynn et al, 2004; Carlton et al, 2005; Guenther et al, 2009). To our knowledge, this is the first report on an application of phage to reduce Yersinia cell numbers in food.
CONCLUSION
An application of virulent bacteriophages for the control of Y. enterocolitica at the post-harvest level seems to be promising, whereas the potential of phages to control Campylobacter in food appears to be limited.
ACKNOWLEDGEMENTS
Parts of the project were funded by the German Federal Ministry of Economics and Technology within the ZIM project (grant no. KF 2028504 SB8) and the German Federal Institute for Risk Assessment (grant no. FK 3-1329-438-5766768). The authors thank Eckhard Strauch for providing phage PY100.
COMPETING INTERESTS
None declared.
REFERENCES
- Atterbury RJ, Connerton PL, Dodd CE, Rees CE, Connerton IF. Application of host-specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Appl Environ Microbiol. 2003;69:6302–6306. doi: 10.1128/AEM.69.10.6302-6306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigwood T, Hudson JA, Billington C, Carey-Smith GV, Heinemann JA. Phage inactivation of foodborne pathogens on cooked and raw meat. Food Microbiol. 2008;25:400–406. doi: 10.1016/j.fm.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Bren L. Bacteria-eating virus approved as food additive. FDA Consum. 2007;41:20–22. [PubMed] [Google Scholar]
- Carlton RM, Noordman WH, Biswas B, De Meester ED, Loessner MJ. Bacteriophage P100 for control of Listeria monocytogenes in foods: genome sequence, bioinformatic analyses, oral toxicity study, and application. Regul Toxicol Pharmacol. 2005;43:301–312. doi: 10.1016/j.yrtph.2005.08.005. [DOI] [PubMed] [Google Scholar]
- EFSA The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2009. http://www.efsa.europa.eu/en/efsajournal/pub/2090.htm 2011 Mar; published in. [Google Scholar]
- El-Shibiny A, Scott A, Timms A, Metawea Y, Connerton P, Connerton I. Application of a group II Campylobacter bacteriophage to reduce strains of Campylobacter jejuni and Campylobacter coli colonizing broiler chickens. J Food Prot. 2009;72:733–740. doi: 10.4315/0362-028x-72.4.733. [DOI] [PubMed] [Google Scholar]
- Goode D, Allen VM, Barrow PA. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl Environ Microbiol. 2003;69:5032–5036. doi: 10.1128/AEM.69.8.5032-5036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther S, Huwyler D, Richard S, Loessner MJ. Virulent bacteriophage for efficient biocontrol of Listeria monocytogenes in ready-to-eat foods. Appl Environ Microbiol. 2009;75:93–100. doi: 10.1128/AEM.01711-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerl JA, Jackel C, Reetz J, Beck S, Alter T, Lurz R, Barretto C, Brussow H, Hertwig S. Campylobacterjejuni group III phage CP81 contains many T4-like genes without belonging to the T4-type phage group: implications for the evolution of T4 phages. J Virol. 2011;85:8597–8605. doi: 10.1128/JVI.00395-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertwig S, Klein J, Schmidt V, Beck S, Hammerl JA, Appel B. Sequence analysis of the genome of the temperate Yersinia enterocolitica phage PY54. J Mol Biol. 2003;331:605–622. doi: 10.1016/s0022-2836(03)00763-0. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Higgins SE, Guenther KL, Huff W, Donoghue AM, Donoghue DJ, Hargis BM. Use of a specific bacteriophage treatment to reduce Salmonella in poultry products. Poult Sci. 2005;84:1141–1145. doi: 10.1093/ps/84.7.1141. [DOI] [PubMed] [Google Scholar]
- Holck A, Berg J. Inhibition of Listeria monocytogenes in cooked ham by virulent bacteriophages and protective cultures. Appl Environ Microbiol. 2009;75:6944–6946. doi: 10.1128/AEM.00926-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverentz B, Conway WS, Camp MJ, Janisiewicz WJ, Abuladze T, Yang M, Saftner R, Sulakvelidze A. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl Environ Microbiol. 2003;69:4519–4526. doi: 10.1128/AEM.69.8.4519-4526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverentz B, Conway WS, Janisiewicz W, Camp MJ. Optimizing concentration and timing of a phage spray application to reduce Listeria monocytogenes on honeydew melon tissue. J Food Prot. 2004;67:1682–1686. doi: 10.4315/0362-028x-67.8.1682. [DOI] [PubMed] [Google Scholar]
- Loc Carrillo C, Atterbury RJ, El-Shibiny A, Connerton PL, Dillon E, Scott A, Connerton IF. Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl Environ Microbiol. 2005;71:6554–6563. doi: 10.1128/AEM.71.11.6554-6563.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi R, Hirvi Y, Hill A, Griffiths MW. Effect of phage on survival of Salmonella enteritidis during manufacture and storage of cheddar cheese made from raw and pasteurized milk. J Food Prot. 2001;64:927–933. doi: 10.4315/0362-028x-64.7.927. [DOI] [PubMed] [Google Scholar]
- Sails AD, Wareing DR, Bolton FJ, Fox AJ, Curry A. Characterisation of 16 Campylobacter jejuni and C. coli typing bacteriophages. J Med Microbiol. 1998;47:123–128. doi: 10.1099/00222615-47-2-123. [DOI] [PubMed] [Google Scholar]
- Schwudke D, Ergin A, Michael K, Volkmar S, Appel B, Knabner D, Konietzny A, Strauch E. Broad-host-range Yersinia phage PY100: genome sequence, proteome analysis of virions, and DNA packaging strategy. J Bacteriol. 2008;190:332–342. doi: 10.1128/JB.01402-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni KA, Nannapaneni R. Bacteriophage significantly reduces Listeria monocytogenes on raw salmon fillet tissue. J Food Prot. 2010;73:32–38. doi: 10.4315/0362-028x-73.1.32. [DOI] [PubMed] [Google Scholar]
- Soni KA, Nannapaneni R, Hagens S. Reduction of Listeria monocytogenes on the surface of fresh channel catfish fillets by bacteriophage Listex P100. Foodborne Pathog Dis. 2009;4:427–434. doi: 10.1089/fpd.2009.0432. [DOI] [PubMed] [Google Scholar]
- Wagenaar JA, Van Bergen MA, Mueller MA, Wassenaar TM, Carlton RM. Phage therapy reduces Campylobacter jejuni colonization in broilers. Vet Microbiol. 2005;109:275–283. doi: 10.1016/j.vetmic.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Whichard JM, Sriranganathan N, Pierson FW. Suppression of Salmonella growth by wild-type and large-plaque variants of bacteriophage Felix O1 in liquid culture and on chicken frankfurters. J Food Prot. 2003;66:220–225. doi: 10.4315/0362-028x-66.2.220. [DOI] [PubMed] [Google Scholar]