Abstract
Dendritic spines arise as small protrusions from the dendritic shaft of various types of neuron and receive inputs from excitatory axons. Ever since dendritic spines were first described in the nineteenth century, questions about their function have spawned many hypotheses. In this review, we introduce understanding of the structural and biochemical properties of dendritic spines with emphasis on components studied with imaging methods. We then explore advances in in vivo imaging methods that are allowing spine activity to be studied in living tissue, from super-resolution techniques to calcium imaging. Finally, we review studies on spine structure and function in vivo. These new results shed light on the development, integration properties and plasticity of spines.
Keywords: calcium, plasticity, spine, super-resolution imaging, two-photon imaging
See the Glossary for abbreviations used in this article.
Glossary.
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CaMKII
Ca2+/calmodulin-dependent kinase II
- FRET
Förster resonance energy transfer
- HVC
hyperstriatum ventrale pars caudale/high vocal centre
- NMDA
N-methyl-D-aspartate
- OGB1
Oregon-Green-BAPTA-1
- PMCA
plasma membrane Ca2+-ATPase
Introduction
In 1888, the legendary neuroanatomist Ramón y Cajal was the first to describe dendritic spines on neurons [1]. Since this first description, technical advances have driven our knowledge of the structural and functional properties of dendritic spines but the function of spines remains the subject of intense study and debate [2,3]. Dendritic spines arise as small protrusions from the dendritic shaft of various types of neuron, including the pyramidal neurons of the neocortex, the medium spiny neurons of the striatum and the Purkinje cells of the cerebellum. Depending on the neuronal type, spines occur at various densities and are found in all vertebrates and in some invertebrates. A striking characteristic of spines is their variety of shapes and sizes, suggesting a high degree of functional diversity. Until relatively recently, it was only possible to study putative functions of the spines in vitro and in fixed tissue. In such preparations, much progress has been made on defining the structural and biochemical properties of spines. Now, however, advances in imaging techniques make it possible to investigate spine function and plasticity at high spatial resolution in living tissue.
Dendritic spines: synaptic transmission and plasticity
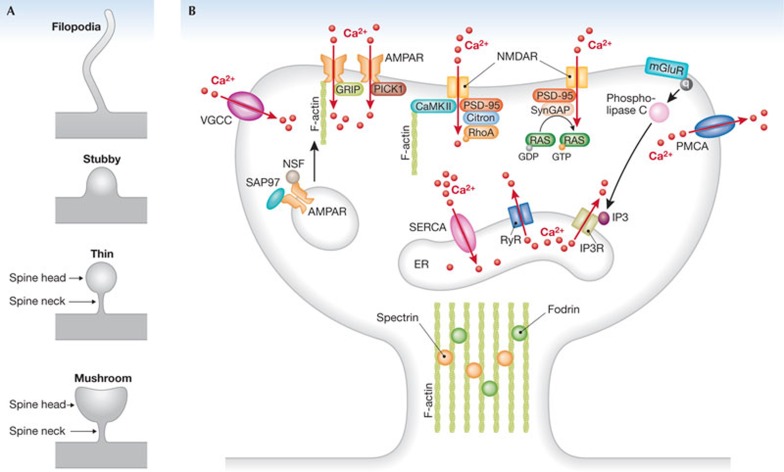
Spine morphology. The morphology of spines is highly variable and they are commonly classified into three types: thin, mushroom and stubby (Fig 1A; [4]). Thin spines have a thin, long neck and a small bulbous head, whereas mushroom spines have a larger head. Stubby spines are devoid of a neck [5] and are prominent between postnatal development [6]. The size of dendritic spines varies among brain areas, as well as between species. For example, the area of spine heads in the temporal cortex is around 0.37 μm2 in mice and 0.59 μm2 in humans, and the length of the spine neck in the same cortical area is on average 0.73 μm in mice and 0.94 μm in humans [7]. Importantly, on the same dendrite a continuum of shapes can be observed, and the morphology of a spine can change rapidly through activity-dependent and -independent mechanisms [8,9,10,11,12]. In addition, thin, hair-like protrusions called filopodia, which lack a bulbous head, are found on dendrites of developing neurons (Fig 1A). They are transient structures that might receive synaptic input and can develop into dendritic spines [13,14].
Figure 1.
Structural and molecular organization of spines. (A) Schematic drawings of spine morphologies based on the most common four-category classification. Note that on the same dendrite a continuum of shapes can be observed, and that the morphology of a spine can change rapidly. (B) Receptors and molecules related to calcium (Ca2+) signalling in spines. Red arrows indicate flux of calcium ions. AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; CaMKII, Ca2+/calmodulin-dependent kinase II; ER, endoplasmic reticulum; GAP, GTPase-activating protein; GRIP, glutamate-receptor-interacting protein; IP3(R), inositol trisphosphate (receptor); mGluR, metabotropic glutamate receptor; NMDA, N-methyl-D-aspartate; NSF, N-ethylmaleimide sensitive factor; PICK1, protein interacting with C kinase; PMCA, plasma membrane Ca2+-ATPase; PSD, postsynaptic density; RyR, ryanodine receptor; SAP97, synapse-associated protein 97; SERCA, sarco/endoplasmic reticulum Ca2+-ATPase; VGCC, voltage-gated calcium channel.
The morphological changes of spines are tightly linked to biochemical reactions taking place inside the spine. The tiny spine head is biochemically isolated from the dendrites by the spine neck. In this small volume, an astonishingly high number of biochemical reactions take place [15]. Typically, spine heads form an asymmetric excitatory synapse with a presynaptic axon [16]. These synapses are characterized by a ‘postsynaptic density’ (PSD), which appears as an electron-dense, dark area under the electron microscope. Most proteins in the PSD are directly or indirectly involved in synaptic communication and in the regulation of synaptic strength [15]. Ultrastructural studies have shown a correlation between the size of the PSD, the spine head volume and the number of vesicles in presynaptic terminals in CA1 pyramidal neurons [9], cerebellar Purkinje cells [8] and in the olfactory cortex [10]. These results led to the idea of a causal link hypothesis between spine structure and the function of spine synapses. This was further supported by studies of calcium dynamics in spines, which revealed a close relationship between spine morphology and function [11]. Later, by using glutamate uncaging on single spines (for a review see [12]), it was demonstrated that spine morphology—mushroom compared with thin spines—correlates directly with the number of AMPA receptors [17], and that the spine–neck geometry is an important determinant of NMDA receptor-dependent calcium signalling in spine heads and dendritic shafts [18]. Furthermore, there is evidence that the induction of long-term potentiation (LTP) correlates with spine enlargement [19]. Using imaging to monitor the changes in spine shape is thus a useful way in which to study their function.
The final determinant of spine morphology is the cytoskeleton. Spine heads contain actin filaments that interact with the plasma membrane and the PSD at their barbed ends (Fig 1B). In spine necks, actin filaments form long bundles (Fig 1B; [20]). It was shown that actin polymerization occurs within seconds of LTP, underlying the enlargement of dendritic spines [21]. Thus, there is good evidence that, at least for hippocampal synapses, the reorganization of the actin cytoskeleton is tightly linked to synaptic efficacy [12,22].
Calcium imaging assessment of spine function. A powerful way to study spine function is to monitor changes in intracellular calcium concentration in this small neuronal compartment. Calcium is an intracellular secondary messenger that regulates the functional and structural properties of individual synapses, with remarkable spatio-temporal specificity [23,24]. In this section, we focus on the biochemical reactions that involve calcium influx and calcium homeostasis in spines.
A main source of calcium entry in spines is influx through ionotropic glutamate receptors and voltage-gated calcium channels (Fig 1B). NMDA and AMPA glutamate receptors are non-specific cation channels with a variable permeability for calcium ions; NMDA receptor channels are particularly calcium-permeable. Typically, the spine is first depolarized by AMPA receptor activation, which removes the blocking action of extracellular magnesium ions on NMDA receptor channels, and leads to further depolarization and calcium entry. Spine depolarization can then be further amplified by voltage-gated calcium or sodium channels [24]. Thus, spines act as NMDA-receptor-dependent coincidence detectors of pre- and postsynaptic activity [25,26]. It is well accepted that the onset of LTP, spine enlargement and an increase in receptor trafficking are coincident and mechanistically linked processes [27]. LTP induction is associated with exocytosis from endosomes and insertion of AMPA receptors into the plasma membrane [28]. Importantly, AMPA receptors diffuse laterally along the plasma membrane [29,30], reaching PSD proteins on which they can be anchored in an actin-dependent manner [31,32].
Another important form of synaptically mediated spine calcium signalling involves calcium release from internal stores, through either ryanodine receptors [33] or inositol trisphosphate (IP3) receptors (Fig 1B; [34,35]). In the cerebellar Purkinje neurons, metabotropic glutamate type 1 receptors (mGluR1s), through a signal cascade involving activation of G protein and phospholipase C, can produce IP3 and cause calcium release from the endoplasmic reticulum in dendritic spines [34,35]. This calcium release is mediated through calcium-permeable IP3 receptors. These spine calcium signals can act as coincidence detectors [36], having important roles for long-term synaptic depression [37]. Calcium levels inside the endoplasmic reticulum are tightly regulated by the sarco/endoplasmic reticulum calcium ATPase (SERCA) (Fig 1B). Interestingly, mGluR1s are located perisynaptically at some distance from the presynaptic glutamate release sites [38]. Therefore, only repetitive presynaptic activity can efficiently activate them.
Molecular mechanisms in spines assessed by live cell imaging. Live cell imaging experiments involving the use of sensors with a high specificity for target proteins have greatly advanced our understanding of the signalling mechanisms in spines. Of significant importance is the imaging of the action of CaMKII, which is a crucial component of the PSD (Fig 1B; [39,40]). CaMKII is essential for the induction and maintenance of some forms of synaptic plasticity [41,42,43,44]. For imaging, CaMKII can be tagged by fluorescent proteins [45,46], for example to directly study the dynamics of CaMKII inside spines in response to synaptic activation [47]. The activity of one of the CaMKII downstream targets, the small GTPase Ras, was also imaged after induction of LTP in the spines of hippocampal neurons [48]. Ras is active when bound to GTP, and inactive when bound to GDP (Fig 1B). This cycle is regulated through interaction with GTPase-activating proteins (GAPs) and guanine nucleotide exchange factors (GEFs)[49]. It is important to note that not only Ras, but also other members of the small GTP-binding protein superfamily, for example, Rho, Rab, Sar1/Arf and Ran, are involved in neuronal function [49]. They have been shown to regulate a wide variety of processes including gene expression, cytoskeletal reorganization and vesicle trafficking [49]. More specifically, Ras is involved in the regulation of dendritic protein synthesis and gene transcription, whereas Rho GTPases have key roles for the regulation of the actin cytoskeleton and thereby spine morphology [50,51,52]. The activation of Rho GTPases was directly determined in single spines in relation to structural changes induced by LTP [27,53].
Accumulating evidence supports a role for dendritic mRNAs in the regulation of synaptic functions in spines. The classical view is that proteins are synthetized in the soma and then transported to appropriate locations in the dendrites. However, in addition to this mechanism, it has been shown that local translational machinery exists in dendrites, such that mRNAs can be shipped to the dendrites and then translated according to local needs, often in an activity-dependent manner [54,55]. For example, in the developing neurons of the optic tectum in Xenopus tadpoles, Bestman and Cline have imaged in vivo RNA-binding proteins tagged with fluorescent proteins [56]. They have also shown that these RNA-binding proteins are distributed throughout the developing dendritic tree and can locally regulate branch dynamics [57].
Advances in spine imaging
Recent years have seen the rapid development of molecular and cellular imaging techniques that allow spine morphology to be studied at high resolution, even in the living brain. In addition to imaging of spine morphology and localization, new methods have been developed to functionally analyse molecular interactions and spine activity.
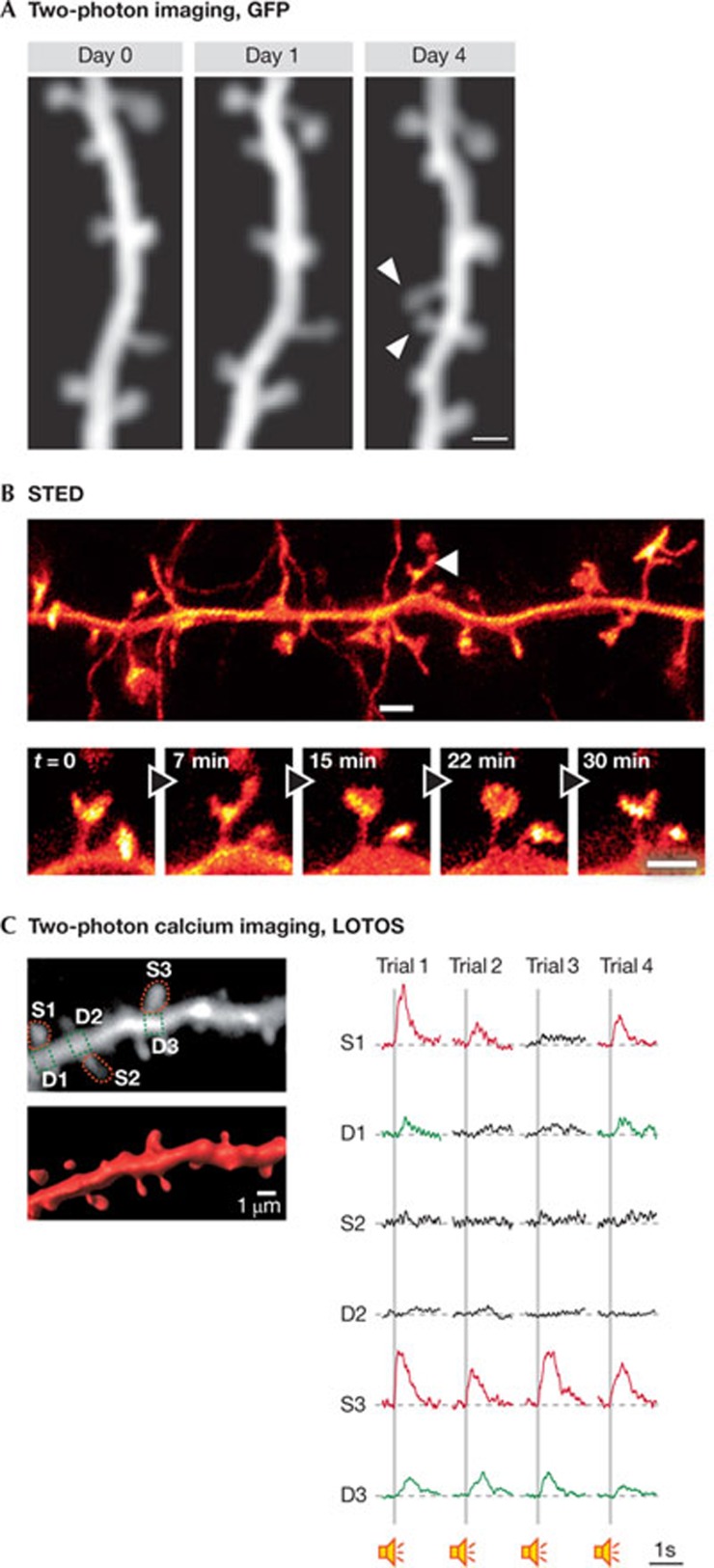
Imaging spine morphology with two-photon microscopy. Since the early work of Ramón y Cajal, the morphological properties of spines have been studied in fixed tissue at various levels of spatial resolution, ranging from light microscopy to ultrastructural studies involving electron microscopy [8,16,58]. In vital preparations, important insights into spine morphology and dynamics were obtained by using camera imaging and confocal microscopy [59,60,61]. A major step forward was the implementation of two-photon laser scanning microscopy [62], which is nowadays widely used for the imaging of spines in the highly scattering brain tissue (see reviews [63,64]). An advantage of two-photon microscopy is that the use of long wavelength-excitation light provides a depth penetration of several hundred micrometres into the intact nervous tissue. In addition, because excitation is limited to a small focal volume, photo-damage outside the focal plane is strongly reduced compared with standard one-photon microscopy (for a review see [65]). Neurons of interest are typically labelled with fluorescent proteins such as GFP or YFP (Fig 2A), either in transgenic mouse lines or after labelling through viral transduction. It is now possible to image spines in vivo up to a depth of about 800 μm [66].
Figure 2.
Examples of spine imaging methods in vivo. (A) In vivo chronic two-photon imaging of spine dynamics in apical dendrites of layer 5 neurons in the mouse motor cortex. Repeated imaging of the same dendritic branch revealed two neighbouring new spines (arrowheads) formed between days 1 and 4 of motor-learning task training. Scale bar, 1 μm. Neurons were labelled with YFP. Reprinted by permission from Macmillan Publishers Ltd: Nature [107] © 2012. (B) In vivo STED microscopy in the molecular layer of the somatosensory cortex of a mouse with eYFP-labelled neurons. Projected volumes of dendritic and axonal structures reveal temporal dynamics of spine morphology (lower panel). Scale bars, 1 μm. Reprinted by permission from the American Association for the Advancement of Science (AAAS) [74]. (C) In vivo two-photon spine calcium imaging using the LOTOS procedure. Left panel, two-photon image (top) and three-dimensional image reconstruction (bottom) of a dendritic segment of a layer 2/3 neuron in the mouse primary auditory cortex. Right panel, four consecutive trials of subthreshold calcium transients evoked by auditory stimulation in spines (red) and corresponding dendritic shaft regions (green), as indicated in the left panel. Reprinted by permission from [82]. LOTOS, low-power temporal oversampling; STED, stimulated emission depletion; eYFP, enhanced yellow fluorescent protein.
Another area of application of two-photon excitation involves the activation of compounds at precise locations. Thus, it is possible, in a single spine, to activate proteins tagged with photoactivatable GFP to study the dynamics of these proteins after, for example, inducing LTP. This approach has been used to monitor the dynamics of PSD95—tagged with photoactivatable GFP—during activity-dependent synaptic growth [67,68]. Several studies have used two-photon uncaging of a caged glutamate compound to establish the direct structure–function relationship between spine shape and synaptic activity (see review [12]). In addition, the role of the spatial and temporal sequence of synaptic inputs can be studied by uncaging glutamate, at specific locations, in a defined temporal sequence [69,70,71]. Two-photon glutamate uncaging has also been adapted for in vivo studies [72].
Imaging spines at super-resolution. With two-photon imaging, the spatial resolution is typically limited to around 300 nm, due to Abbe's diffraction law stating that features closer than half the wavelength of light cannot be distinguished [73]. Super-resolution techniques have overcome this resolution barrier [73,74,75,76]. Two main strategies have been developed to increase spatial resolution. First, saturated stimulated emission depletion (STED) microscopy [73] has been applied to image spines at a nanoscale resolution [75]. Features such as the thickness of the spine neck or subtle changes in the shape of the spine head—for example, from thin to mushroom shape—can be studied with this approach at a resolution smaller than 70 nm. STED has been applied to image spines in the living brain (Fig 2B; [74]). So far, this technique is limited to layer 1 (around 15 μm) in conditions in which the mouse is anaesthetized and paralysed. The second strategy is based on the techniques of photoactivation localization microscopy (PALM) [77] and stochastic optical reconstruction microscopy (STORM) [78]. Both approaches rely on the sequential, statistical excitation of a fraction of fluorophores spaced at distances larger than the diffraction limit [76]. This gives a spatial resolution of around 20 nm. However, for the moment, the time needed to acquire a super-resolution image and the associated bleaching of fluorophores make the two methods suitable mostly for fixed specimens (cultured cells) and limit the temporal resolution.
Imaging spine activity in vivo. As introduced above, calcium imaging has proven to be a powerful tool for studying neuronal activity at the cellular and subcellular scale. This method has benefited from the development of sensitive fluorescent calcium indicator dyes, including synthetic dyes (e.g. Fura2, OGB1) and, more recently, FRET-based and single fluorophore genetically encoded calcium sensors [79]. In parallel, high-resolution imaging techniques have been developed to monitor changes in the fluorescence of such dyes in neuronal somata, dendrites and spines, in vivo.
The initial calcium imaging experiments performed in spines [80,81] used a CCD camera to image fluorescence changes of the calcium dye Fura2 in slices. A few years later, the use of two-photon imaging greatly improved spine calcium imaging [25]. However, until just recently, imaging calcium signals in spines was largely restricted to studies performed in vitro, mainly because of the phototoxic damages produced by strong laser light in vivo. Development of the low-power temporal oversampling (LOTOS) procedure, which uses an acousto-optic deflector-based two-photon microscope [82], minimizes phototoxic damage and allows the imaging of prolonged periods of spine activity. Thus, multiple trials of stimulus-evoked calcium signals can be recorded in the same spiny dendrite (Fig 2C). In brief, the LOTOS-based procedure relies on the acquisition of images at high frame rates (e.g. 1,000 Hz), short pixel dwell-times (50 ns) and low intensities of the excitation laser beam [82]. Another study has shown that LOTOS can also be used in combination with a resonant galvoscanner-based two-photon imaging device [83].
Determining spine structure and function in vivo
Knowing how spines are structurally modified by experience, and how single spine synaptic inputs are distributed in dendrites, is crucial for determining how neurons integrate information and generate their output signals. Thus, clustered inputs might bind behaviourally relevant clustered synapses within individual dendrites promoting the generation of local dendritic spikes, as indicated by in vitro brain slice experiments (for reviews see [84,85,86]). Alternatively, inputs with similar features might be widely distributed over multiple dendrites and integrated linearly within the dendritic tree [3,87]. Although these two modes of synaptic input arrangement on dendrites are not necessarily mutually exclusive, a specific knowledge of the functional organization of the input patterns is essential for an understanding of the neuron-type-specific algorithms of input integration.
Activity-dependent spine remodelling. Chronic two-photon imaging has emerged as a powerful tool for the analysis of in vivo spine remodelling over time [88,89]. These structural changes have been monitored during postnatal development or in adulthood, and after inducing plasticity through various paradigms such as sensory deprivation or motor skill learning. Chronic two-photon imaging has been used to study the development of spine motility in different cortical areas. It was shown that, at early postnatal ages, spines are highly plastic and spine turnover decreases with age [90,91]. In adults, the total spine number is globally stable over time due to comparable rates of spine formation and elimination [92,93,94]. However, spine remodelling occurs in the adult brain after induction of experience-dependent plasticity. As described below, with some examples, these activity-dependent structural changes in spines were investigated in primary sensory cortical areas, in the motor cortex and in higher cortical areas such as the frontal cortex, in both physiological and pathological conditions (Fig 3).
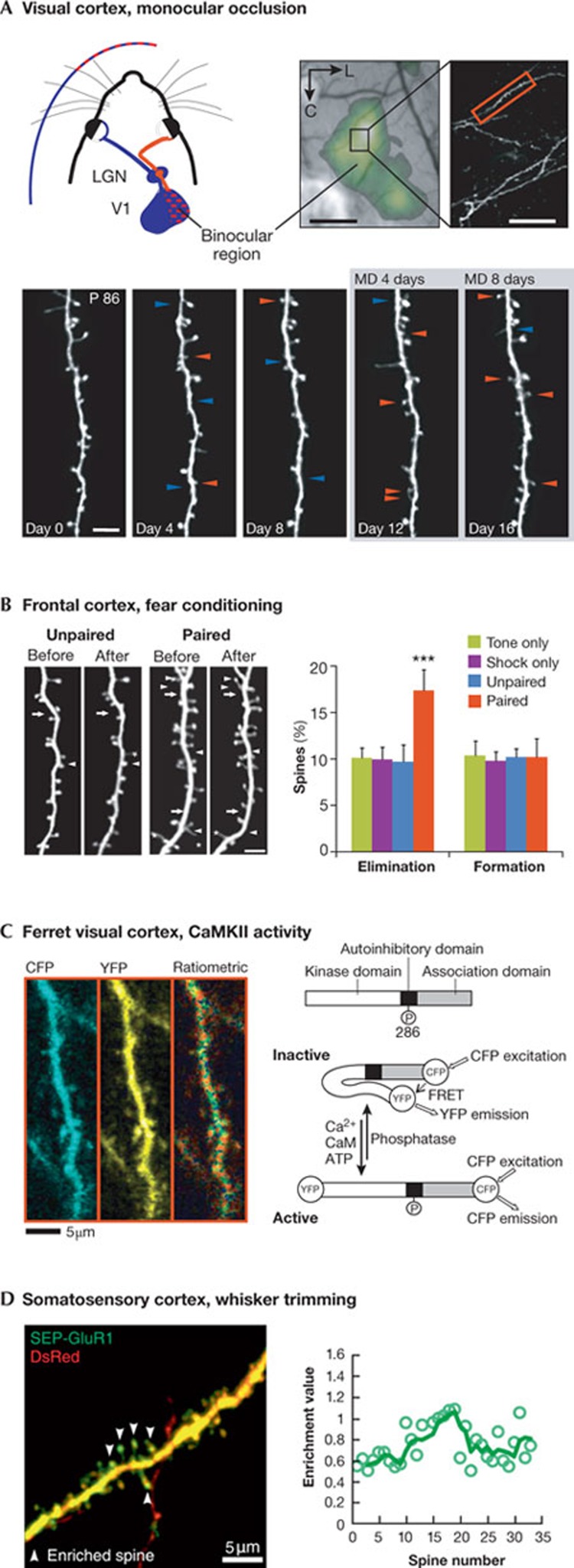
Figure 3.
Imaging activity-dependent spine structural changes in vivo. (A) Spine dynamics in apical dendrites of layer 5 neurons in binocular visual cortex after monocular deprivation in adult mice. Upper panel, schemata of the mouse visual system with intrinsic signal map of the binocular visual cortex (scale bar, 500 μm) and low-magnification image of an apical dendrite (scale bar, 50 μm). Lower panel, high-magnification view of the dendritic stretch shown above (red box), imaged in vivo every four days before and after monocular deprivation (MD). Arrows point to spines appearing (red) or disappearing (blue), compared with the previous imaging session. Scale bar, 5 μm. Reprinted by permission from Macmillan Publishers Ltd: Nature [94] © 2008. (B) Spine elimination after fear conditioning in layer 5 neurons of the mouse frontal association cortex. Left panel, representative in vivo images of dendrites before and after fear conditioning with foot shock paired or unpaired with tones. Arrows and arrowheads indicate spine formation and elimination, respectively. Asterisks mark filopodia. Scale bar, 4 μm. Percentage of spine elimination and formation 48 h after conditioning. Only the paired group showed an increase in freezing response and spine elimination. Reprinted by permission from Macmillan Publishers Ltd: Nature [109] © 2012. (C) In vivo imaging of CaMKII activity in layer 2/3 neurons of the ferret visual cortex. Schematic drawing of the conformations of the FRET-based probe for the detection of CaMKII activity (Camui), in the inactive and active form. Left panel, CFP and YFP channel images of a dendritic segment as well as a ratiometric image in intensity-modulated display mode, indicating the CFP–YFP ratio. Warm hue represents high CaMKII activity. Reprinted by permission from [122], © 2011 National Academy of Sciences, USA. (D) Left panel, example of clustered synaptic SEP–GluR1 (GluR1 tagged with a pH-sensitive form of green fluorescent protein, Super Ecliptic pHluorin) enrichment in a basal dendrite of a layer 2/3 pyramidal neuron in the somatosensory cortex of a whisker-intact mouse. Scale bar, 5 μm. Right panel, profile of SEP–GluR1 spine enrichment along the dendrite shown on the left. Neighbouring spines showed a significant positive correlation value that was significantly greater than that observed in whisker-trimmed animals. Reprinted by permission from [124], © 2011, with permission from Elsevier. CaMKII, Ca2+/calmodulin-dependent kinase II; CFP, cyan fluorescent protein; FRET, Förster resonance energy transfer; GluR1, glutamate receptor 1; LGN, lateral geniculate nucleus; SEP, super ecliptic pHluorin; YFP, yellow fluorescent protein.
In the somatosensory cortex, chronic two-photon imaging was used to study structural changes of dendritic spines after whisker potentiation in layer 5 neurons [88,95]. The results show that both structural—stabilization of new spines—and functional—somatic activity—changes were most pronounced in layer 5 neurons located at the border between spared and deprived barrel columns [96]. In the visual cortex, the turnover of spines was studied in the binocular region after monocular deprivation [97]. In adult mice, a monocular deprivation episode of four days was found to double the rate of spine formation in apical dendrites of layer 5 pyramidal neurons. The resulting increase in spine density was specific to layer 5 cells located in the binocular cortex, in which most neurons increase their responsiveness to the non-deprived eye (Fig 3A; [94]). However, the relationship between structural and functional changes is not always clear. For example, although the output of the layer 2/3 binocular neurons was strongly modified by monocular deprivation [98,99,100], no structural modification of the spines could be identified in the apical dendrites of these neurons [94]. It could be that structural changes occur in deeper parts of the dendritic trees [101], or that different mechanisms of ocular dominance plasticity take place in the upper layers. Indeed, two studies strongly suggest that inhibition is important in this experience-dependent plasticity. They both used fluorescently tagged gephyrin to label inhibitory synapses in the mouse visual cortex in vivo. They found that a short period of monocular deprivation caused the pruning of a significant number of inhibitory synapses, mainly located on dendritic spines [102,103,104].
Structural changes associated with motor skill learning have been investigated in the motor cortex [105]. Morphological changes were monitored in spines of apical dendrites of layer 5 neurons in the contralateral motor cortex (Fig 2A). New spines were formed within one hour after initiation of a forelimb reaching task or within two days after rotarod running training [92,106]. Spine formation during initial learning was followed by enhanced spine elimination, leading to a total spine count that was the same as control levels [92,106]. In addition, it was shown that one-third of new dendritic spines emerge in clusters during the initial learning phase, and that most of these clusters are neighbouring spine pairs [107]. These findings suggest that repetitive activation of cortical networks during learning induces clustering of new synapses along dendrites.
Spine dynamics have also been studied in higher order cortical areas. For example, spine dynamics were monitored in the forebrain nucleus HVC of zebra finches during song learning, which revealed that a higher level of spine turnover is correlated with a greater capacity for subsequent song imitation [108]. Another study using the mouse frontal association cortex investigated how spines of layer 5 pyramidal neurons are modified by fear learning and extinction (Fig 3B; [109]). Whereas fear conditioning increased the rate of spine elimination, fear extinction increases the rate of spine formation. In addition, extinction causes the formation of dendritic spines within a distance of 2 μm from spines eliminated after fear conditioning [109].
Finally, two-photon chronic imaging has been used to study synaptic functions in pathological conditions. The impact of strokes was assessed in the somatosensory cortex, revealing increased spine formation in the peri-infarct dendrites [110,111]. Spinal cord injury was shown to decrease spine density in the motor cortex [112], whereas retinal lesions induce massive remodelling of spines in the visual cortex [113]. Several studies have revealed spine loss in mouse models of Alzheimer disease, in hippocampal [114] and cortical pyramidal neurons [115,116]. Finally, a developmental delay in the downregulation of spine turnover and an overproduction of transient spines were revealed in a mouse model of fragile X syndrome [117,118].
To conclude, most studies so far have been performed in the apical tuft of layer 5 pyramidal neurons. Other types of plasticity might occur in other cell types. For example, it was found that a subset of inhibitory neurons carry dendritic spines that form glutamatergic synapses [119]. Given the spine changes observed in these inhibitory neurons after deprivation induced by retinal lesions, it seems that structural changes in inhibitory neurons might precede structural changes in excitatory circuitry. Thus, comprehensive studies of other neuronal cell types are essential, for a more complete understanding of synaptic plasticity, at the level of local networks. Otherwise, despite the correlations between spine dynamics and experience-dependent changes, the causality of this relationship remains unclear.
Molecular mechanisms of synaptic function in vivo. To gain functional insight into experience-dependent synaptic changes, new probes and markers are being developed in rapid sequence to image the expression of proteins known to be involved in synaptic plasticity. For example, a FRET-based CaMKII sensor [44,120] and a FRET reporter of Ras GTPase activation [121] were used for LTP studies in hippocampal cultured slices. For in vivo studies, a genetically engineered FRET probe for the detection of CaMKII activity was used to monitor CaMKII activity in single synapses of layer II/III neurons in the ferret visual cortex (Fig 3C). The results suggest that spines lost after monocular deprivation have a low basal concentration of CaMKII, whereas spines that are preserved show increased activation of CaMKII [122,123]. An elegant optical approach was also used for the in vivo study of the dynamics of AMPA receptor trafficking, after inducing synaptic plasticity through sensory experience or deprivation (Fig 3D; [124]). This approach revealed that experience-driven GluR1 incorporation into synapses is clustered on portions of dendrites, and that such clusters are eliminated when mice are deprived of sensory experience. By contrast, the incorporation of synaptic GluR2, through sensory deprivation, occurred in a distributed manner with little evidence for clustering [124,125].
In parallel to the development of molecular target-specific fluorescent probes, an increasing number of transgenic mice are available to monitor the expression or localization of synaptic components. For example, adult transgenic mice in which GFP–GluR1 is expressed under control of the c-fos promoter have been used to probe the insertion of newly synthesized AMPA receptors after fear conditioning [126] and behavioural exploration in vivo [127]. The results indicate that glutamate receptors are preferentially inserted into neighbouring spines [127].
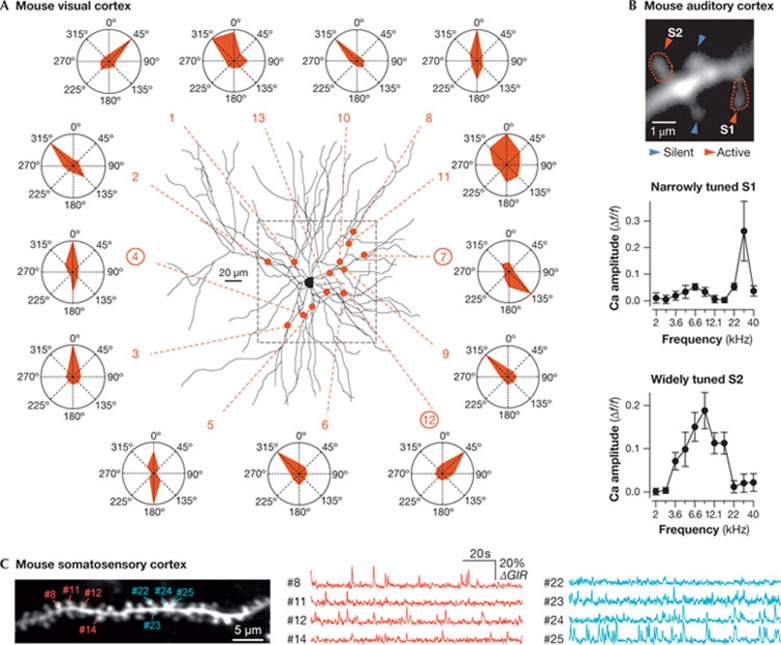
Functional neuroanatomy in vivo with calcium imaging. In vivo two-photon calcium imaging was first used to map functional inputs in dendrites in invertebrates. In the cricket cercal system, simultaneous pre- and postsynaptic calcium imaging revealed different topographical organizations of sensory inputs into interneuron dendrites, underlying different computation processes of these inputs [128]. Further studies in the visual systems of locusts [129] and Xenopus tadpoles [130] indicated that the topographical organization of sensory inputs on dendrites has an influence on the integration of visual information. Similar approaches were implemented for the functional analysis of dendrites in the mammalian brain. Studies performed in the mouse visual and somatosensory cortices identified dendritic NMDA-dependent calcium hotspots driven by visual (Fig 4A; [131,132]) or whisker stimulation [83] in layer 2/3 neurons. A common conclusion for both sensory modalities is that sensory input coding for the same feature is heterogeneously distributed throughout the entire dendritic tree, with no evidence for clustering on individual dendrites.
Figure 4.
Imaging spine activity in vivo. (A) In vivo two-photon calcium imaging of synaptic inputs evoked by visual stimulation in a layer 2/3 pyramidal neuron of the mouse visual cortex. Red dots indicate the location of each hotspot of local dendritic calcium signal, on the Z-projection of the reconstructed dendritic tree. Red dashed lines point to the polar plot obtained for the corresponding local dendritic calcium signal. The frame (grey dashed line) indicates the area of imaging. Reprinted by permission from Macmillan Publishers Ltd: Nature [131] © 2010. (B) In vivo two-photon calcium imaging of dendritic spines of a layer 2/3 neuron in the mouse auditory cortex, using the LOTOS procedure. Frequency tuning curves of the narrowly tuned spine S1 and of the widely tuned spine S2, shown in the two-photon image in the left panel. Error bars, s.e.m. Reprinted by permission from Macmillan Publishers Ltd: Nature [82] © 2011. (C) In vivo two-photon calcium imaging of dendritic spines using conventional two-photon imaging. Left panel, a stack image of dendrites of a layer 2/3 pyramidal cell in the mouse somatosensory cortex in vivo. Right panels, typical traces of spontaneous calcium activity from eight spines detected as functionally clustered and indicated in the left panel. Reprinted by permission from the American Association for the Advancement of Science (AAAS) [127]. LOTOS, low-power temporal oversampling.
Recently, in vivo calcium imaging in single spines in the intact mouse brain became feasible. The first report of this approach involved the LOTOS two-photon imaging procedure [82] to detect sound-evoked responses in single spines in the auditory cortex (Fig 4B). Similarly to the dendritic ‘hotspots’ mentioned above, these results demonstrated a widespread distribution of afferent sensory inputs throughout the dendritic tree of a given neuron [82]. Although in a few instances spines with similar frequency tuning were locally clustered, most other similarly tuned spines were located on remote sites throughout both apical and basal dendrites. Another study showed that, at least when recording spontaneous activity, conventional two-photon imaging can also be used for in vivo spine calcium imaging [127]. The results provide evidence for local clustering of synaptic inputs in the dendrites of layer 2/3 pyramidal neurons in the mouse somatosensory cortex (Fig 4C).
Finally, it should be remembered that synaptically evoked spine calcium signals have been detected unambiguously only in the absence of the back-propagation of action action potentials, or in neurons that had low resting membrane potentials and were not firing [82], or that were hyperpolarized [83,131], or in the presence of intracellular sodium channel blockers [127]. In this last study, the neurons were additionally voltage-clamped at −30–0 mV. These recording conditions might have influenced the probability of occurrence of the spine calcium events that were detected. Thus, improved methods of spine activity detection might reveal synaptic input sites in conditions of spiking activity, which might help to increase our understanding of the mechanisms by which specific inputs drive the output signals.
Conclusions
The insights into dendritic spine structure and function, resulting from the use of modern imaging techniques, have significantly increased our knowledge of synaptic function, ranging from a better understanding of the molecular mechanisms of experience-dependent plasticity to the dendritic organization of sensory inputs in the intact brain in vivo. As we have described, it is the technical developments that have driven forward studies of spine function, involving both new imaging technology—for example, two-photon microscopy, STED and LOTOS—and the development of a large variety of new fluorescent sensors. The main challenges that remain include questions related to spine function during the formation of circuits in vivo (Sidebar A), the mechanisms of single spine-dependent experience plasticity in conditions of behaviourally relevant learning processes and the changes that occur in pathophysiological conditions, such as stroke and Alzheimer disease.
Sidebar A | In need of answers.
In spite of the strong evidence for distributed inputs, how relevant are clustered inputs in vivo for neuronal function?
What is the functional circuit map of the presynaptic neurons contacting dendritic spines?
How are spine signals integrated by dendrites?
What is the relation between structural and functional spine plasticity in vivo?
What is the molecular machinery underlying spine function and plasticity in vivo?
Acknowledgments
We thank Jia Lou for excellent technical assistance. The authors are supported by the German Research Foundation, ERAnet and the Friedrich Schiedel Foundation. A.K. is a Carl von Linde Senior Fellow of the Institute for Advanced Study of the TUM. N.L.R. was supported by the German Research Foundation (IRTG 1373).
Footnotes
The authors declare that they have no conflict of interest.
References
- García-López P, García-Marín V, Freire M (2007) The discovery of dendritic spines by Cajal in 1888 and its relevance in the present neuroscience. Prog Neurobiol 83: 110–130 [DOI] [PubMed] [Google Scholar]
- Shepherd GM (1996) The dendritic spine: a multifunctional integrative unit. J Neurophysiol 75: 2197–2210 [DOI] [PubMed] [Google Scholar]
- Yuste R (2011) Dendritic spines and distributed circuits. Neuron 71: 772–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Kaiserman-Abramof IR (1970) The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am J Anat 127: 321–355 [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TP (1969) Morphological variations in the dendritic spines of the neocortex. J Cell Sci 5: 509–529 [DOI] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B (1992) Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci 12: 2685–2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides-Piccione R, Ballesteros-Yáñez I, DeFelipe J, Yuste R (2002) Cortical area and species differences in dendritic spine morphology. J Neurocytol 31: 337–346 [DOI] [PubMed] [Google Scholar]
- Harris KM, Stevens JK (1988) Dendritic spines of rat cerebellar Purkinje cells: serial electron microscopy with reference to their biophysical characteristics. J Neurosci 8: 4455–4469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Stevens JK (1989) Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci 9: 2982–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF (1999) Quantitative fine-structural analysis of olfactory cortical synapses. Proc Natl Acad Sci USA 96: 4107–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Majewska A, Holthoff K (2000) From form to function: calcium compartmentalization in dendritic spines. Nat Neurosci 3: 653–659 [DOI] [PubMed] [Google Scholar]
- Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J (2010) Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci 33: 121–129 [DOI] [PubMed] [Google Scholar]
- Miller M, Peters A (1981) Maturation of rat visual cortex. IIA combined Golgi-electron microscope study of pyramidal neurons. J Comp Neurol 203: 555–573 [DOI] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM (1998) Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci 18: 8900–8911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Yuste R (2003) Structure and molecular organization of dendritic spines. Histol Histopathol 18: 617–634 [DOI] [PubMed] [Google Scholar]
- Gray EG (1959) Electron microscopy of synaptic contacts on dendrite spines of the cerebral cortex. Nature 183: 1592–1593 [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H (2001) Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci 4: 1086–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi J, Matsuzaki M, Ellis-Davies GC, Kasai H (2005) Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron 46: 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H (2004) Structural basis of long-term potentiation in single dendritic spines. Nature 429: 761–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifkova E, Delay RJ (1982) Cytoplasmic actin in neuronal processes as a possible mediator of synaptic plasticity. J Cell Biol 95: 345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkura N, Matsuzaki M, Noguchi J, Ellis-Davies GC, Kasai H (2008) The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron 57: 719–729 [DOI] [PubMed] [Google Scholar]
- Cingolani LA, Goda Y (2008) Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci 9: 344–356 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529 [DOI] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL (2007) Ca2+ signaling in dendritic spines. Curr Opin Neurobiol 17: 345–351 [DOI] [PubMed] [Google Scholar]
- Yuste R, Denk W (1995) Dendritic spines as basic functional units of neuronal integration. Nature 375: 682–684 [DOI] [PubMed] [Google Scholar]
- Yuste R, Majewska A, Cash SS, Denk W (1999) Mechanisms of calcium influx into hippocampal spines: heterogeneity among spines, coincidence detection by NMDA receptors, and optical quantal analysis. J Neurosci 19: 1976–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H, Yasuda R (2011) Postsynaptic signaling during plasticity of dendritic spines. Trends Neurosci 35: 135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R (2009) Synaptic AMPA receptor plasticity and behavior. Neuron 61: 340–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine M, Groc L, Frischknecht R, Beique JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D (2008) Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science 320: 201–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino H, Malinow R (2009) AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron 64: 381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Zhabotinsky AM (2001) A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron 31: 191–201 [DOI] [PubMed] [Google Scholar]
- Kato AS, Gill MB, Yu H, Nisenbaum ES, Bredt DS (2010) TARPs differentially decorate AMPA receptors to specify neuropharmacology. Trends Neurosci 33: 241–248 [DOI] [PubMed] [Google Scholar]
- Emptage N, Bliss TV, Fine A (1999) Single synaptic events evoke NMDA receptor-mediated release of calcium from internal stores in hippocampal dendritic spines. Neuron 22: 115–124 [DOI] [PubMed] [Google Scholar]
- Finch EA, Augustine GJ (1998) Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature 396: 753–756 [DOI] [PubMed] [Google Scholar]
- Takechi H, Eilers J, Konnerth A (1998) A new class of synaptic response involving calcium release in dendritic spines. Nature 396: 757–760 [DOI] [PubMed] [Google Scholar]
- Wang SS, Denk W, Häusser M (2000) Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci 3: 1266–1273 [DOI] [PubMed] [Google Scholar]
- Miyata M et al. (2000) Local calcium release in dendritic spines required for long-term synaptic depression. Neuron 28: 233–244 [DOI] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P (1996) Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci 8: 1488–1500 [DOI] [PubMed] [Google Scholar]
- Kelly PT, Cotman CW (1978) Synaptic proteins. Characterization of tubulin and actin and identification of a distinct postsynaptic density polypeptide. J Cell Biol 79: 173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MB, Bennett MK, Erondu NE (1983) Biochemical and immunochemical evidence that the “major postsynaptic density protein” is a subunit of a calmodulin-dependent protein kinase. Proc Natl Acad Sci USA 80: 7357–7361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H (2002) The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci 3: 175–190 [DOI] [PubMed] [Google Scholar]
- Taha S, Hanover JL, Silva AJ, Stryker MP (2002) Autophosphorylation of alphaCaMKII is required for ocular dominance plasticity. Neuron 36: 483–491 [DOI] [PubMed] [Google Scholar]
- Gordon JA, Cioffi D, Silva AJ, Stryker MP (1996) Deficient plasticity in the primary visual cortex of alpha-calcium/calmodulin-dependent protein kinase II mutant mice. Neuron 17: 491–499 [DOI] [PubMed] [Google Scholar]
- Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R (2009) Activation of CaMKII in single dendritic spines during long-term potentiation. Nature 458: 299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Meyer T (1999) Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science 284: 162–166 [DOI] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Subramanian K, Meyer T (1998) CaMKIIbeta functions as an F-actin targeting module that localizes CaMKIIalpha/beta heterooligomers to dendritic spines. Neuron 21: 593–606 [DOI] [PubMed] [Google Scholar]
- Rose J, Jin SX, Craig AM (2009) Heterosynaptic molecular dynamics: locally induced propagating synaptic accumulation of CaM kinase II. Neuron 61: 351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R (2002) Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell 110: 443–455 [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T (2001) Small GTP-binding proteins. Physiol Rev 81: 153–208 [DOI] [PubMed] [Google Scholar]
- Nakayama AY, Harms MB, Luo L (2000) Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci 20: 5329–5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Minden A, Yuste R (2000) Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex 10: 927–938 [DOI] [PubMed] [Google Scholar]
- Luo L (2000) Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci 1: 173–180 [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Wang H, Yasuda R (2011) Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature 472: 100–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L, Banker GA, Steward O (1987) Selective dendritic transport of RNA in hippocampal neurons in culture. Nature 330: 477–479 [DOI] [PubMed] [Google Scholar]
- Schuman EM, Dynes JL, Steward O (2006) Synaptic regulation of translation of dendritic mRNAs. J Neurosci 26: 7143–7146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestman JE, Cline HT (2008) The RNA binding protein CPEB regulates dendrite morphogenesis and neuronal circuit assembly in vivo. Proc Natl Acad Sci USA 105: 20494–20499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestman JE, Cline HT (2009) The relationship between dendritic branch dynamics and CPEB-labeled RNP granules captured in vivo. Front Neural Circuits 3: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG, Guillery RW (1963) A note on the dendritic spine apparatus. J Anat 97: 389–392 [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Andersen P (1994) An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci USA 91: 12673–12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa M, Bundman MC, Greenberger V, Segal M (1995) Morphological analysis of dendritic spine development in primary cultures of hippocampal neurons. J Neurosci 15: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire M, Boyde A (1990) Study of Golgi-impregnated material using the confocal tandem scanning reflected light microscope. J Microsc 158: 285–290 [DOI] [PubMed] [Google Scholar]
- Denk W, Strickler JH, Webb WW (1990) Two-photon laser scanning fluorescence microscopy. Science 248: 73–76 [DOI] [PubMed] [Google Scholar]
- Garaschuk O, Milos RI, Grienberger C, Marandi N, Adelsberger H, Konnerth A (2006) Optical monitoring of brain function in vivo: from neurons to networks. Pflugers Arch 453: 385–396 [DOI] [PubMed] [Google Scholar]
- Helmchen F, Denk W (2005) Deep tissue two-photon microscopy. Nat Methods 2: 932–940 [DOI] [PubMed] [Google Scholar]
- Svoboda K, Yasuda R (2006) Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron 50: 823–839 [DOI] [PubMed] [Google Scholar]
- Mittmann W, Wallace DJ, Czubayko U, Herb JT, Schaefer AT, Looger LL, Denk W, Kerr JN (2011) Two-photon calcium imaging of evoked activity from L5 somatosensory neurons in vivo. Nat Neurosci 14: 1089–1093 [DOI] [PubMed] [Google Scholar]
- Steiner P, Higley MJ, Xu W, Czervionke BL, Malenka RC, Sabatini BL (2008) Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron 60: 788–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NW, Weimer RM, Bureau I, Svoboda K (2006) Rapid redistribution of synaptic PSD-95 in the neocortex in vivo. PLoS Biol 4: e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco T, Clark BA, Häusser M (2010) Dendritic discrimination of temporal input sequences in cortical neurons. Science 329: 1671–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco T, Häusser M (2011) Synaptic integration gradients in single cortical pyramidal cell dendrites. Neuron 69: 885–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A, Israely I, Huang SY, Tonegawa S (2011) The dendritic branch is the preferred integrative unit for protein synthesis-dependent LTP. Neuron 69: 132–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi J, Nagaoka A, Watanabe S, Ellis-Davies GC, Kitamura K, Kano M, Matsuzaki M, Kasai H (2011) In vivo two-photon uncaging of glutamate revealing the structure–function relationships of dendritic spines in the neocortex of adult mice. J Physiol 589: 2447–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell SW (2007) Far-field optical nanoscopy. Science 316: 1153–1158 [DOI] [PubMed] [Google Scholar]
- Berning S, Willig KI, Steffens H, Dibaj P, Hell SW (2012) Nanoscopy in a living mouse brain. Science 335: 551. [DOI] [PubMed] [Google Scholar]
- Nägerl UV, Willig KI, Hein B, Hell SW, Bonhoeffer T (2008) Live-cell imaging of dendritic spines by STED microscopy. Proc Natl Acad Sci USA 105: 18982–18987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Babcock H, Zhuang X (2010) Breaking the diffraction barrier: super-resolution imaging of cells. Cell 143: 1047–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF (2006) Imaging intracellular fluorescent proteins at nanometer resolution. Science 313: 1642–1645 [DOI] [PubMed] [Google Scholar]
- Rust MJ, Bates M, Zhuang X (2006) Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat Methods 3: 793–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grienberger C, Konnerth A (2012) Imaging calcium in neurons. Neuron 73: 862–885 [DOI] [PubMed] [Google Scholar]
- Guthrie PB, Segal M, Kater SB (1991) Independent regulation of calcium revealed by imaging dendritic spines. Nature 354: 76–80 [DOI] [PubMed] [Google Scholar]
- Müller W, Connor JA (1991) Dendritic spines as individual neuronal compartments for synaptic Ca2+ responses. Nature 354: 73–76 [DOI] [PubMed] [Google Scholar]
- Chen X, Leischner U, Rochefort NL, Nelken I, Konnerth A (2011) Functional mapping of single spines in cortical neurons in vivo. Nature 475: 501–505 [DOI] [PubMed] [Google Scholar]
- Varga Z, Jia H, Sakmann B, Konnerth A (2011) Dendritic coding of multiple sensory inputs in single cortical neurons in vivo. Proc Natl Acad Sci USA 108: 15420–15425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- London M, Häusser M (2005) Dendritic computation. Annu Rev Neurosci 28: 503–532 [DOI] [PubMed] [Google Scholar]
- Branco T, Häusser M (2010) The single dendritic branch as a fundamental functional unit in the nervous system. Curr Opin Neurobiol 20: 494–502 [DOI] [PubMed] [Google Scholar]
- Govindarajan A, Kelleher RJ, Tonegawa S (2006) A clustered plasticity model of long-term memory engrams. Nat Rev Neurosci 7: 575–583 [DOI] [PubMed] [Google Scholar]
- Cash S, Yuste R (1999) Linear summation of excitatory inputs by CA1 pyramidal neurons. Neuron 22: 383–394 [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K (2002) Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420: 788–794 [DOI] [PubMed] [Google Scholar]
- Grutzendler J, Kasthuri N, Gan WB (2002) Long-term dendritic spine stability in the adult cortex. Nature 420: 812–816 [DOI] [PubMed] [Google Scholar]
- Lendvai B, Stern EA, Chen B, Svoboda K (2000) Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature 404: 876–881 [DOI] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan WB (2005) Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron 46: 181–189 [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y (2009) Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462: 915–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Yang G, Kwon E, Gan WB (2005) Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature 436: 261–265 [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M (2009) Experience leaves a lasting structural trace in cortical circuits. Nature 457: 313–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K (2006) Experience-dependent and cell-type-specific spine growth in the neocortex. Nature 441: 979–983 [DOI] [PubMed] [Google Scholar]
- Wilbrecht L, Holtmaat A, Wright N, Fox K, Svoboda K (2010) Structural plasticity underlies experience-dependent functional plasticity of cortical circuits. J Neurosci 30: 4927–4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska AK, Sur M (2006) Plasticity and specificity of cortical processing networks. Trends Neurosci 29: 323–329 [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M (2006) Prior experience enhances plasticity in adult visual cortex. Nat Neurosci 9: 127–132 [DOI] [PubMed] [Google Scholar]
- Tagawa Y, Kanold PO, Majdan M, Shatz CJ (2005) Multiple periods of functional ocular dominance plasticity in mouse visual cortex. Nat Neurosci 8: 380–388 [DOI] [PubMed] [Google Scholar]
- Fischer QS, Graves A, Evans S, Lickey ME, Pham TA (2007) Monocular deprivation in adult mice alters visual acuity and single-unit activity. Learn Mem 14: 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataga N, Mizuguchi Y, Hensch TK (2004) Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron 44: 1031–1041 [DOI] [PubMed] [Google Scholar]
- Chen JL, Villa KL, Cha JW, So PT, Kubota Y, Nedivi E (2012) Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron 74: 361–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambino F, Holtmaat A (2012) Synapses let loose for a change: inhibitory synapse pruning throughout experience-dependent cortical plasticity. Neuron 74: 214–217 [DOI] [PubMed] [Google Scholar]
- van Versendaal D et al. (2012) Elimination of inhibitory synapses is a major component of adult ocular dominance plasticity. Neuron 74: 374–383 [DOI] [PubMed] [Google Scholar]
- Yu X, Zuo Y (2011) Spine plasticity in the motor cortex. Curr Opin Neurobiol 21: 169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB (2009) Stably maintained dendritic spines are associated with lifelong memories. Nature 462: 920–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Yu X, Lu J, Zuo Y (2012) Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature 483: 92–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TF, Tschida KA, Klein ME, Mooney R (2010) Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature 463: 948–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CS, Franke TF, Gan WB (2012) Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature 483: 87–91 [DOI] [PubMed] [Google Scholar]
- Brown CE, Aminoltejari K, Erb H, Winship IR, Murphy TH (2009) In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J Neurosci 29: 1719–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Li P, Boyd JD, Delaney KR, Murphy TH (2007) Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J Neurosci 27: 4101–4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS (2006) Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp Neurol 198: 401–415 [DOI] [PubMed] [Google Scholar]
- Keck T, Mrsic-Flogel TD, Vaz Afonso M, Eysel UT, Bonhoeffer T, Hubener M (2008) Massive restructuring of neuronal circuits during functional reorganization of adult visual cortex. Nat Neurosci 11: 1162–1167 [DOI] [PubMed] [Google Scholar]
- Perez-Cruz C, Nolte MW, van Gaalen MM, Rustay NR, Termont A, Tanghe A, Kirchhoff F, Ebert U (2011) Reduced spine density in specific regions of CA1 pyramidal neurons in two transgenic mouse models of Alzheimer's disease. J Neurosci 31: 3926–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT (2005) Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci 25: 7278–7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J, Grutzendler J, Duff K, Gan WB (2004) Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci 7: 1181–1183 [DOI] [PubMed] [Google Scholar]
- Cruz-Martin A, Crespo M, Portera-Cailliau C (2010) Delayed stabilization of dendritic spines in fragile X mice. J Neurosci 30: 7793–7803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Aldridge GM, Greenough WT, Gan WB (2010) Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc Natl Acad Sci USA 107: 17768–17773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck T, Scheuss V, Jacobsen RI, Wierenga CJ, Eysel UT, Bonhoeffer T, Hubener M (2011) Loss of sensory input causes rapid structural changes of inhibitory neurons in adult mouse visual cortex. Neuron 71: 869–882 [DOI] [PubMed] [Google Scholar]
- Takao K, Okamoto K, Nakagawa T, Neve RL, Nagai T, Miyawaki A, Hashikawa T, Kobayashi S, Hayashi Y (2005) Visualization of synaptic Ca2+/calmodulin-dependent protein kinase II activity in living neurons. J Neurosci 25: 3107–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Yasuda R, Zhong H, Svoboda K (2008) The spread of Ras activity triggered by activation of a single dendritic spine. Science 321: 136–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mower AF, Kwok S, Yu H, Majewska AK, Okamoto K, Hayashi Y, Sur M (2011) Experience-dependent regulation of CaMKII activity within single visual cortex synapses in vivo. Proc Natl Acad Sci USA 108: 21241–21246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Majewska AK, Sur M (2011) Rapid experience-dependent plasticity of synapse function and structure in ferret visual cortex in vivo. Proc Natl Acad Sci USA 108: 21235–21240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino H, Malinow R (2011) Compartmentalized versus global synaptic plasticity on dendrites controlled by experience. Neuron 72: 1001–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC (2011) Observations on clustered synaptic plasticity and highly structured input patterns. Neuron 72: 887–888 [DOI] [PubMed] [Google Scholar]
- Matsuo N, Reijmers L, Mayford M (2008) Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science 319: 1104–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kitamura K, Matsuo N, Mayford M, Kano M, Matsuki N, Ikegaya Y (2012) Locally synchronized synaptic inputs. Science 335: 353–356 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Cummins GI, Jacobs GA, Oka K (2008) Dendritic design implements algorithm for synaptic extraction of sensory information. J Neurosci 28: 4592–4603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peron SP, Jones PW, Gabbiani F (2009) Precise subcellular input retinotopy and its computational consequences in an identified visual interneuron. Neuron 63: 830–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollmann JH, Engert F (2009) Subcellular topography of visually driven dendritic activity in the vertebrate visual system. Neuron 61: 895–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Rochefort NL, Chen X, Konnerth A (2010) Dendritic organization of sensory input to cortical neurons in vivo. Nature 464: 1307–1312 [DOI] [PubMed] [Google Scholar]
- Jia H, Rochefort NL, Chen X, Konnerth A (2011) In vivo two-photon imaging of sensory-evoked dendritic calcium signals in cortical neurons. Nat Protoc 6: 28–35 [DOI] [PubMed] [Google Scholar]



 Nathalie L Rochefort & Arthur Konnerth
Nathalie L Rochefort & Arthur Konnerth