Abstract
Kindlin 2, as a focal adhesion protein, controls integrin activation. However, the association of Kindlin 2 with cancer-related signalling pathways is unknown. Here we identified a new direct interaction between Kindlin 2 and the active β-catenin. Importantly, Kindlin 2 forms a tripartite complex with β-catenin and TCF4. Mechanistically, Kindlin 2 selectively strengthens the occupancy of β-catenin on the Wnt target gene Axin2 and enhances Axin2 gene expression. Functionally, the β-catenin–Axin2–Snail cascade is required for Kindlin 2-induced tumour cell invasion. Our data indicate that Kindlin 2 is a new regulator of Wnt signalling, providing a mechanistic insight into the role of Kindlin 2 in cancer progression.
Keywords: β-catenin, protein complex, Kindlins, Wnt signalling
Introduction
Wnt signalling has critical roles in regulating various cellular processes, including proliferation, self-renewal, and angiogenesis. Hyperactive Wnt signalling is closely associated with tumour development and progression [1, 2]. Mutations in the key regulatory genes of Wnt signalling pathway are reported responsible for their aberrant activation [3, 4]. However, the frequency of such kinds of mutations is low in breast cancer [5]. Although alternative interpretations have been proposed, the mechanism of hyperactive Wnt signalling in breast cancer is not completely understood.
The Fermitin family (also called Kindlin family) comprises structurally similar and evolutionarily conserved proteins that activate integrins and are involved in the regulation of cell–matrix adhesion. Kindlins have been related to some human diseases, including cancer. Fermitin family homologue 1 (FERMT1, Kindlin 1) is highly expressed in colorectal and lung cancers, and has roles in breast cancer lung metastasis [6]. Fermitin family homologue 2 (FERMT2, Kindlin 2) also shows increased expression in human uterine leiomyomas [7]. However, little is known about the correlation between Kindlin 2 and human tumours. Kindlin 2 was found highly expressed in the tumour invasion front, and showed significant nuclear localization in human malignant mesothelioma [8]. These findings suggest that high levels of Kindlin 2 might contribute to tumour invasion by triggering the transcription of some critical genes. Here we report that Kindlin 2 directly interacts with active but not phosphorylated β-catenin, and this interaction allows the recruitment of Kindlin 2 by β-catenin to form a transcriptional complex with T-cell factor 4 (TCF4). The Kindlin 2–β-catenin–TCF4 tripartite complex promotes Wnt target gene expression and, in turn, regulates tumour cell invasion.
Results and discussion
Kindlin 2 interacts with β-catenin in vivo and in vitro
β-Catenin is the central molecule in Wnt signalling. Intriguingly, Kindlin 2 was found coexpressed with β-catenin in the tumour invasive front and in tumour cells (supplementary Fig S1A–B online). Therefore, the physical association between Kindlin 2 and β-catenin warranted further investigation. The interaction between endogenous Kindlin 2 and β-catenin was identified by co-immunoprecipitation (Co-IP) in breast cancer cells (MCF7–Flag–Kindlin 2, MDA-MB-231, and Hs578T), non-cancer-derived cells (HEK293T), and normal cells (human proximal tubular epithelial HKC cells, Fig 1A; supplementary Fig S2A–D online). Kindlin 2 is known to bind to integrin β1 cytoplasmic tail, and mainly localized at focal adhesions [9], which raises a question that whether the integrin-binding capacity of Kindlin 2 is required for its interaction with β-catenin. To answer this question, an integrin-binding deficient mutant Kindlin 2 Q614A/W615A (QW mutant) was generated accordingly [9], and found that it associates with β-catenin as well (Fig 1B). This finding indicates that the interaction between Kindlin 2 and β-catenin is independent of Kindlin 2 binding to integrin β1 cytoplasmic tail, suggesting that Kindlin 2 and β-catenin interaction can be outside of focal adhesions (supplementary Fig S3 online).
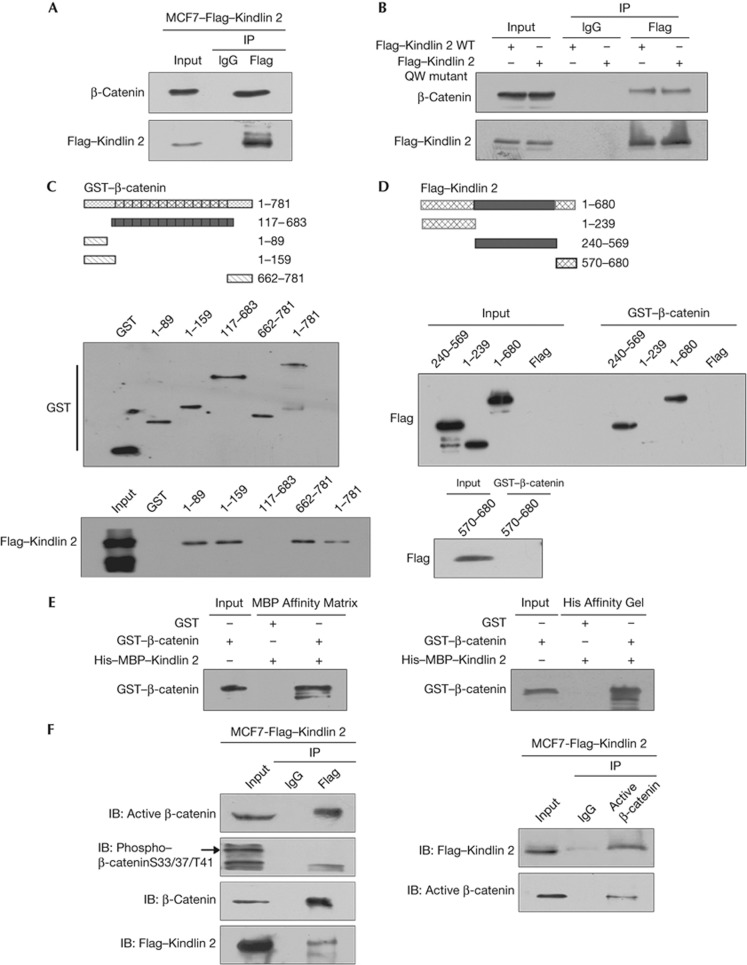
Figure 1.
Kindlin 2 directly interacts with β-catenin. (A) Cell lysates were prepared and Flag antibody was then used in co-immunoprecipitation (Co-IP), followed by western blot (WB) analysis using indicated antibodies. (B) A Flag–Kindlin 2 QW mutant deficient in binding to integrin β1 was generated. Both the wild-type (WT) and mutant Flag–Kindlin 2 were transfected into 293T cells, protein was then extracted for Co-IP. (C) Glutathione S-transferase (GST)-fusion proteins for different regions of β-catenin were designed as in the upper panel. Purified GST or GST–β-catenin fragments were evaluated by WB (middle). Different fusion proteins were incubated with total lysates of MCF7–Flag–Kindlin 2 stable cells for GST-pulldown assay, followed by WB (bottom). (D) Different fragments of Flag–Kindlin 2 were listed as in the upper panel. The Kindlin 2 fragments were expressed in 293T cells and evaluated by WB (input). GST-pulldown assays were performed by incubating the GST–β-catenin with the Kindlin 2 fragments. (E) Fusion protein His–MBP–Kindlin 2 was incubated with GST or GST–β-catenin in vitro for His/MBP-pulldown assays. Affinity matrices for MBP or His-tags were used separately. (F) Lysates from MCF7–Flag–Kindlin 2 were extracted, and then Flag or active β-catenin antibodies were used for Co-IP, followed by WB. IB, immunoblot; IgG, immunoglobin-G.
To map the binding regions between Kindlin 2 and β-catenin, β-catenin was divided into four fragments and fused with glutathione S-transferase (GST). Both the N and C termini of β-catenin were capable of binding to Kindlin 2 in a GST-pulldown assay (Fig 1C). Similarly, Kindlin 2 was divided into three fragments and the central region was found to bind to β-catenin (Fig 1D). Furthermore, both full-length GST–β-catenin and His–MBP–Kindlin 2 were expressed and purified, and a direct interaction between Kindlin 2 and β-catenin was identified (Fig 1E). As both the N and C termini of β-catenin can interact with Kindlin 2, a competition for Kindlin 2 binding may exist between β-catenin N and C termini. Interestingly, we did identify a competition between the N and C termini of β-catenin for interaction with Kindlin 2 in vitro (supplementary Fig S2E online).
Kindlin 2 selectively interacts with active β-catenin
Phosphorylation of β-catenin is related to its stability. It was known that the phosphorylated β-catenin undergoes protein degradation [2], and the unphosphorylated β-catenin is the active β-catenin that functions[10]. This raises an important question as to which form of β-catenin is involved in the interaction with Kindlin 2. We found that unphosphorylated but not phosphorylated β-catenin interacted with Kindlin 2 (Fig 1F). Similarly, in MDA-MB-231, Hs578T, HEK293T, and HKC cells we also confirmed that Kindlin 2 specifically associates with active β-catenin (supplementary Fig S4A–D online). To determine where the two molecules are complexed together, we prepared both cytoplasmic and nuclear extracts. Our results showed that Kindlin 2 specifically interacts with only active β-catenin in both cytoplasm and nucleus (supplementary Fig S4E online).
Kindlin 2 stabilizes the active form of β-catenin
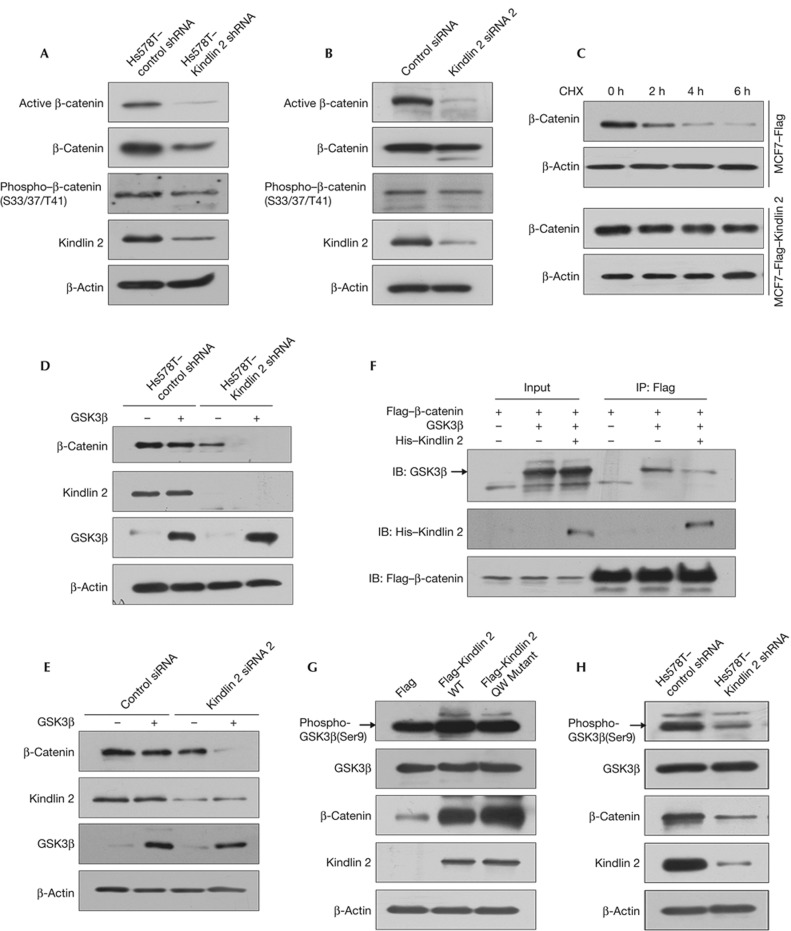
To our surprise, disruption of Kindlin 2 interaction with β-catenin by Kindlin 2 small interfering RNA (siRNA) knockdown decreased the β-catenin level, especially the active β-catenin (Fig 2A–B; supplementary Fig S5A–C online). Similarly, immunofluorescence staining showed that the fluorescent intensity of β-catenin was clearly weakened in Kindlin 2-depletion cells, compared with that of the control cells (supplementary Fig S6 online). Moreover, overexpression of Kindlin 2 enhanced the stability of β-catenin (Fig 2C), raising an important question that if the interaction between Kindlin 2 and β-catenin stabilizes β-catenin. It is known that glycogen synthase kinase 3 beta (GSK3β), as a negative regulator of Wnt signalling, initiates the degradation of β-catenin. To uncover the role of GSK3β in the interaction between Kindlin 2 and β-catenin, we overexpressed GSK3β in control or Kindlin 2-depletion cells. Results showed that a significant decrease of β-catenin was found in Kindlin 2-depletion cells, whereas no obvious changes were observed in the control cells (Fig 2D–E). These data indicated that endogenous Kindlin 2 not only increases the stability of β-catenin, but also inhibits the degradation of β-catenin mediated by GSK3β. Next, to answer how Kindlin 2 influences GSK3β function, we examined the binding capability of GSK3β with β-catenin upon Kindlin 2 overexpression, and found that Kindlin 2 weakened the interaction between GSK3β and β-catenin (Fig 2F). These findings indicated that the interaction between Kindlin 2 and β-catenin blocks the binding of GSK3β with β-catenin and maintains the active status of β-catenin. Furthermore, the effect of Kindlin 2 on GSK3β activity was also tested. Our data showed that overexpression of Kindlin 2 inhibited GSK3β activity by enhancing GSK3β–Ser9 phosphorylation, which is independent of Kindlin 2 binding to integrin β1 (Fig 2G; supplementary Fig S7 online). Conversely, Kindlin 2 knockdown activated GSK3β (Fig 2H). These data indicated that Kindlin 2 regulates β-catenin through modifying GSK3β activity. Given the important role of atypical protein kinase C (aPKC) in the regulation of GSK3β [11], we examined the role of Kindlin 2 on PKCζ activation. We found that Kindlin 2 regulates PKCζ activation in both gain- and loss-of-function experiments (supplementary Fig S8 online), indicating that PKCζ functions between Kindlin 2 and GSK3β. Collectively, our data indicated that Kindlin 2 has a critical role in stabilizing β-catenin by preventing GSK3β binding to β-catenin, and inhibiting GSK3β activity by activation of PKCζ.
Figure 2.
Kindlin 2 is required for maintaining the active form of β-catenin. (A) Stably expressed control or Kindlin 2 short hairpin RNA (shRNA) Hs578T cells were generated. Proteins were extracted for western blot (WB). (B) Control or Kindlin 2 small interfering RNA (siRNA)2 was transfected into 293T cells followed by WB. (C) MCF7–Flag or MCF7–Flag–Kindlin 2 stable cells were treated with cycloheximide (CHX, 25 μg/ml) for different time points as indicated, then proteins were extracted for WB. (D) GSK3β was transfected into the indicated stable cells, and then protein was extracted for WB. (E) GSK3β and control or Kindlin 2 siRNA2 were co-transfected into 293T cells followed by WB. (F) Cells (293T) were transfected with the indicated constructs, later protein was extracted for co-immunoprecipitation. (G) Flag, Flag–Kindlin 2 WT, or QW mutant was transfected into 293T cells, followed by WB. (H) WB analysis was performed for the indicated stable cells. IB, immunoblot; WT, wild-type.
Kindlin 2 complexes with active β-catenin and TCF4
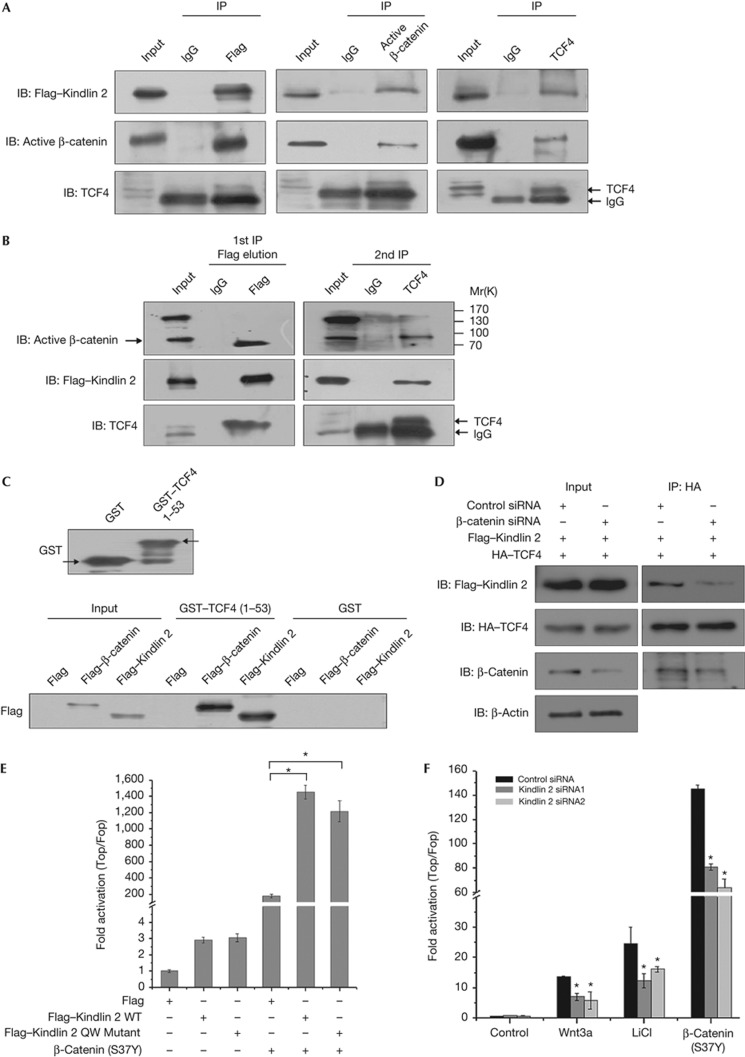
Activation of Wnt signalling requires β-catenin translocation to the nucleus and to activate target genes by forming a transcriptional complex with TCF. Given that the Kindlin 2–β-catenin complex is present in the nucleus as indicated above, Kindlin 2 might participate in β-catenin/TCF4-mediated transcription. To test this idea, we first examined if Kindlin 2 is able to form a tripartite complex with active β-catenin and TCF4. To this end, reciprocal Co-IP and two-step Co-IP assays were performed, identifying that the three molecules do form a complex (Fig 3A–B; supplementary Fig S9 online). Second, we determined the binding region of Kindlin 2 with TCF4, and found that the N terminus of TCF4 that binds to β-catenin was also responsible for the interaction with Kindlin 2 (Fig 3C), indicating that the Kindlin 2–β-catenin complex binds to the N terminus of TCF4. Furthermore, to clarify if β-catenin is required for Kindlin 2 association with TCF4, we examined the interaction between Kindlin 2 and TCF4 in cells with endogenous β-catenin knockdown. Results showed that depletion of β-catenin diminishes the association between Kindlin 2 and TCF4 (Fig 3D), indicating that β-catenin is required for the association of Kindlin 2 with TCF4.
Figure 3.
Kindlin 2 forms a complex with active β-catenin and TCF4 to enhance Wnt signalling. (A) Nuclear extracts were prepared from MCF7–Flag–Kindlin 2 stable cells and co-immunoprecipitation (Co-IP) was performed. (B) Total lysates were extracted from MCF7–Flag–Kindlin 2 stable cells for two-step Co-IP. The first IP was performed with FLAG-M2 beads or mouse immunoglobin (Ig)G. The eluate from the first IP was then used for the second IP with anti-TCF4 antibody or mouse IgG. The immunoprecipitates from each step were detected by western blot (WB). (C) Glutathione S-transferase (GST) or GST–TCF4 1–53 was evaluated by WB (upper panel). Flag, Flag–β-catenin, or Flag–Kindlin 2 were separately transfected into 293T cells. Total protein was extracted and incubated with GST or GST–TCF4 for GST pulldown. (D) Flag–Kindlin 2 and HA–TCF4 were co-transfected into 293T cells, in the presence of control or β-catenin siRNA. Protein was extracted for co-IP. (E) Cells (293T) were transfected as the indicated plasmids plus the SuperTop/Fopflash plasmids for 24 h. (F) After 24 h transfection with control or Kindlin 2 siRNAs, 293T cells were transfected again with SuperTop/Fopflash for 24 h and then treated with Wnt3a (200 ng, 6 h), LiCl (20 mM, 6 h), or 293T cells were co-transfected again with β-catenin (S37Y) and SuperTop/Fopflash for 24 h. Error bars indicate s.d. values, n=3; *indicates P<0.05 by Student's t-test. HA, haemagglutinin; LiCl, lithium chloride; siRNA, small interfering RNA; TCF4, T-cell factor 4; WT, wild-type.
Given that Kindlin 2 forms a complex with active β-catenin and TCF4, Kindlin 2 may participate in β-catenin/TCF-mediated transcription. Indeed, Kindlin 2 overexpression significantly enhanced a non-degradable β-catenin (S37Y)-stimulated transcription in a SuperTopflash reporter assay (Fig 3E; supplementary Fig S10 online), indicating that Kindlin 2 is involved in the activation of β-catenin/TCF4-mediated transcription. Similarly, the Kindlin 2 integrin-binding deficient mutant also activates β-catenin/TCF4-mediated transcription. To uncover if Kindlin 2 has a critical role in Wnt/β-catenin signalling, two siRNAs were used to knockdown endogenous Kindlin 2. Fig 3F shows that Kindlin 2 knockdown partially abolishes the activation of β-catenin/TCF4-mediated transcription in the presence of Wnt3a, lithium chloride (LiCl), or non-degradable β-catenin mutant separately. These data indicate that Kindlin 2, as a new regulator, is required for the activation of Wnt signalling.
Kindlin 2 promotes Axin2 transcription
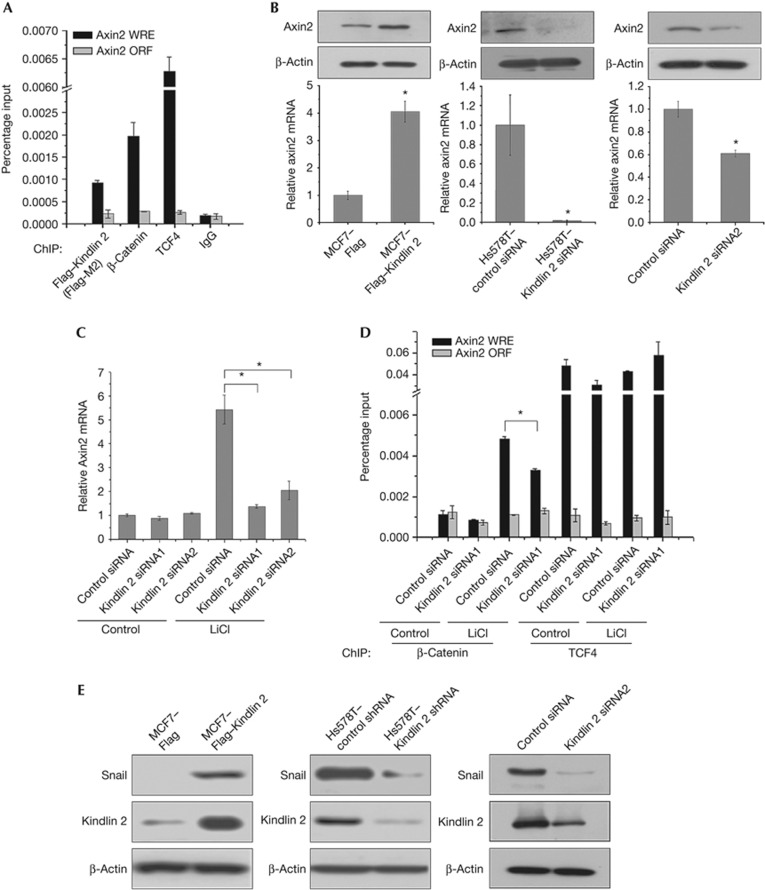
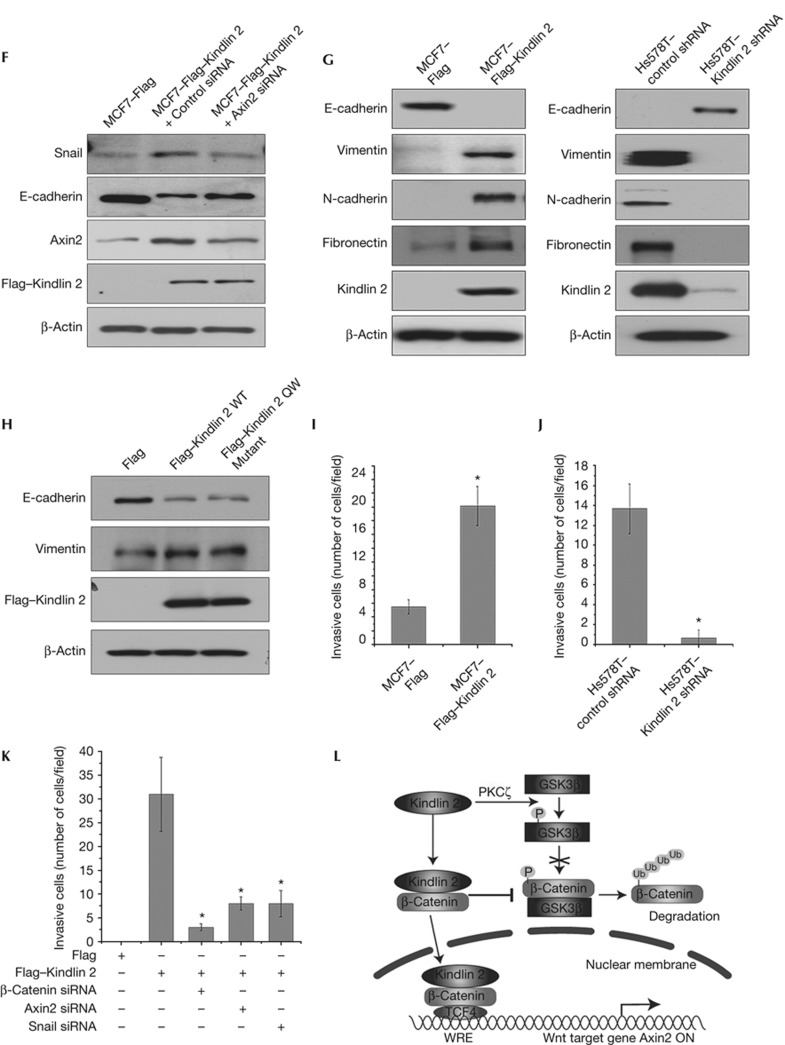
Kindlin 2 interacts with active β-catenin and TCF4 to activate Wnt signalling. However, the transcriptional targets of this complex remain elusive. β-Catenin and TCF4 are known to co-occupy the promoter of Axin2—a genuine Wnt target gene containing TCF-binding elements or Wnt responsive elements [12]. A chromatin immunoprecipitation assay was performed to test the occupancy of Kindlin 2 on the Axin2 promoter, and we found that Kindlin 2 do occupy Axin2 promoter (Fig 4A; supplementary Fig S11 online). Furthermore, Axin2 regulation by Kindlin 2 was examined at both mRNA and protein levels. Not surprised, Kindlin 2 was found required for Axin2 expression in gain- and loss-of-function experiments (Fig 4B; supplementary Fig S5D online). The expression of Axin2 was significantly inhibited by Kindlin 2 knockdown even in the presence of GSK3β inhibitor LiCl (Fig 4C), suggesting that Kindlin 2 promotes Axin2 expression and is mainly mediated by Kindlin 2 formation with the β-catenin/TCF4 transcriptional complex, but not by inhibition of GSK3β. This finding indicates the importance of Kindlin 2 in the tripartite complex. To test this idea, we depleted Kindlin 2 and detected the occupancy of β-catenin on the Axin2 promoter with or without inhibition of GSK3β by LiCl. As shown in Fig 4D, on addition of LiCl a great increase in the binding of β-catenin on Axin2 promoter was observed, suggesting the involvement of GSK3β in the regulation of Axin2 expression. However, when Kindlin 2 was depleted the β-catenin occupancy on the Axin2 promoter is decreased, suggesting that Kindlin 2 is required for β-catenin binding onto the Axin2 promoter. In a sharp contrast, Kindlin 2 had no effect on the binding of TCF4 onto the Axin2 promoter. Altogether, these findings indicate a new role of Kindlin 2 in the regulation of Wnt signalling, in which Kindlin 2 enhances β-catenin occupancy on the Axin2 promoter and promotes Axin2 transcription. In addition, Kindlin 2 was also found to regulate other Wnt targets, including CyclinD1, LEF1, Twist, MMP2, sFRP1, and Versican (Supplementary Fig S12 online), again strengthening that Kindlin 2 is an important Wnt signalling regulator.
Figure 4.
Kindlin 2 promotes tumour cell invasion by enhancing Axin2 expression. (A) Lysates from MCF7–Flag–Kindlin 2 stable cells were used for chromatin immunoprecipitation (ChIP) with antibodies as indicated. Quantitative PCR (qPCR) was used to quantify the ChIP-enriched DNAs. The Axin2 open reading frame (ORF) region was used as a negative control. (B) The levels of Axin2 were detected by western blot (WB) and qPCR in indicated stable cells, and Kindlin 2 small interfering RNA (siRNA)2-treated 293T cells. (C) Cells (293T) were transfected with control or Kindlin 2 siRNAs plus LiCl treatment (20 mM, 6 h). Total RNAs were extracted for qPCR. (D) ChIP were performed using the indicated antibodies. (E,F) The levels of Snail were analysed by WB in indicated stable cells, Kindlin 2 siRNA2-treated 293T cells and Axin2 siRNA-treated MCF7–Flag–Kindlin 2 stable cells.
In reminiscence of the role of Axin2 in enhancing the stability of Snail in breast cancer [13], Kindlin 2 may also involve in Snail-dependent tumour cell invasion by upregulating Axin2 expression. Indeed, we identified a role of Kindlin 2 in the regulation of Snail stability (Fig 4E). Axin2 knockdown in MCF7–Flag–Kindlin 2 cells inhibited the raise of Snail stability induced by Kindlin 2, and rescued the decrease of E-cadherin, a marker of epithelial-to-mesenchymal transition (EMT), which validates a link between Kindlin 2/Axin2 and Snail. This shows a functional role of Kindlin 2 in EMT (Fig 4F). To uncover the role of Kindlin 2 in EMT, Kindlin 2 regulation on EMT markers was detected in both gain- and loss-of-function experiments. Results showed that Kindlin 2 is required for EMT (Fig 4G), which is independent of Kindlin 2 binding to integrin β1 (Fig 4H). Importantly, Kindlin 2 regulation on tumour cell invasion was found in both gain- and loss-of-function experiments (Fig 4I–J; supplementary Fig S5E online), which links Kindlin 2 with the Snail-mediated EMT. To unravel the possible functional link between Kindlin 2 with β-catenin, Axin2 and Snail, siRNAs for β-catenin, Axin2 and Snail were applied separately. Individual knockdown of β-catenin, Axin2 or Snail all jeopardized Kindlin 2-induced tumour cell invasion (Fig 4K), indicating that β-catenin–Axin2–Snail cascade is required for Kindlin 2-induced tumour cell invasion. Consistent with this, Gozgit et al [14] found that Kindlin 2 promotes the invasion of breast cancer cell TMX2-28 [14]. However, an opposite role of Kindlin 2 in mesenchymal cancer cell invasion was also reported [15], suggesting a complex role of Kindlin 2 in controlling cancer cell invasion that may exist [7, 8, 14]. Besides cell invasion, other cellular effects of Kindlin 2 were also observed. For example, Kindlin 2 promotes cell proliferation (supplementary Fig S13 online) and adhesion [8], inhibits apoptosis and cell death [16].
Figure 4.
(G) WB was performed in indicated stable cells to detect the epithelial-to-mesenchymal transition markers. (H) Flag, Flag–Kindlin 2 WT, or QW mutant was transfected into MCF7 cells, followed by WB. (I–J) Invasion assays were performed using the indicated stable cells. The number of invasive cells was counted from six random fields and averaged. (K) Flag or Flag–Kindlin 2 was transfected into 293T cells in the presence of the indicated siRNAs. Invasion assays were then performed. Error bars indicate s.d. values, n=3; * indicates P<0.05 by Student's t-test. (L) A working model for the role of Kindlin 2 in enhancing Wnt/β-catenin signalling. IgG, immunoglobin-G; LiCl, lithium chloride; PKC, protein kinase C; shRNA, short hairpin RNA; TCF4, T-cell factor 4; WRE, Wnt responsive element; WT, wild-type.
Kindlin 2–β-catenin complex nuclear translocation remains an interesting question. Smad3 was known to protect β-catenin from degradation by interacting with β-catenin and enhancing both β-catenin stability and its nuclear translocation [17]. Although a nuclear localization signal has been found in Kindlin 2, we have not observed a direct effect of Kindlin 2 on β-catenin nuclear translocation, as examined by a Kindlin 2 nuclear localization signal deletion mutant (data not shown). Recently, FoxM1 was found to promote β-catenin nuclear localization in gliomas [18]. Our data from a PCR array indicated that FoxM1 was downregulated by approximately 2.3-fold in Kindlin 2-depleted cells, compared with the control cells (data not shown). This may suggest that FoxM1 is involved in the nuclear translocation of Kindlin 2–β-catenin complex, which leaves an opportunity for future study.
In conclusion, we found that Kindlin 2 is important in enhancing Wnt/β-catenin signalling by selectively binding to the active β-catenin. On one hand, Kindlin 2 stabilizes β-catenin by preventing GSK3β binding to the active β-catenin. On the other hand, Kindlin 2 promotes the transcription of Wnt target gene Axin2, mainly through the formation of a tripartite complex with β-catenin/TCF4, as illustrated in a working model (Fig 4L). Thus, disruption of the interaction between Kindlin 2 and the active β-catenin may provide a new strategy for inhibition of cancer invasion.
Methods
Generation of mutations. Point mutants were generated using the QuickChange XL Site-Directed Mutagenesis Kit (Stratagene). Kindlin 2 QW mutant is an integrin-binding deficient Kindlin 2Q614A/W615A mutant. Kindlin 2 siRNA-resistant mutant is a siRNA1-resistant Kindlin 2 G147C/G150A mutant.
Two-step co-immunoprecipitation. For two-step IP, the first IP was performed with FLAG-M2 beads at 4 °C for 4 h or with mouse immunoglobin-G at 4 °C for 4 h, followed by incubating with protein A/G-Sepharose. After the beads were washed four times, the bound proteins were eluted with 3 × FLAG peptide (250 μg/ml, Sigma). Then, the eluates were processed for the second IP with TCF4 antibody or mouse immunoglobin-G, followed by incubating with protein A/G-Sepharose. After the beads were washed three times, the bound proteins were eluted with 2 × SDS loading buffer. The eluates of protein samples from each step were subjected to western blotting analysis.
Purification of fusion proteins. GST, GST–β-catenin, His–β-catenin, or His–MBP–Kindlin 2 were expressed in E. coli BL21, and purified with Glutathione Sepharose 4B beads (Pharmacia Biotech), HIS-Select HF Nickel Affinity Gel (Sigma) or MBP Affinity Matrix (Amylose Resin, New England Biolabs) separately.
Luciferase reporter assay. HEK293T cells were seeded into 24-well plates the day before transfection. Super 8x TOPFlash/FOPFlash (100 ng) [19] plasmid with 1 ng of pRL were transfected per well with Lipofectamine 2000. Plasmids (0.5 μg) expressing Flag, Flag–Kindlin 2 WT, Flag–Kindlin 2 QW, or 0.2 μg of non-degradable β-catenin were co-transfected as indicated per well. The reporter activity was measured at 24-h post transfection using a Dual-luciferase Reporter Assay System (Promega).
Supplementary Material
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China key project 30830048, the Ministry of Science and Technology of China 2010CB912203 and 2010CB529402, NSFC 31170711 and 81101495, Beijing Natural Science Foundation 7120002, and by grants from the Swedish Cancer Foundation, the Swedish Research Council to H.Z.
Author contributions: Y.Y. designed and performed the experiments, analysed the data and wrote the manuscript; J.W. performed the experiments and analysed the data; Y.W., T.Z., B.M., and Y.L. performed the experiments; W.F. and W.Z. analysed the data; H.Z. designed the experiments, analysed the data and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Lu Z, Ghosh S, Wang Z, Hunter T (2003) Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of β-catenin, and enhanced tumor cell invasion. Cancer Cell 4: 499–515 [DOI] [PubMed] [Google Scholar]
- Clevers H (2006) Wnt/β-catenin signaling in development and disease. Cell 127: 469–480 [DOI] [PubMed] [Google Scholar]
- Damalas A, Kahan S, Shtutman M, Ben-Ze'ev A, Oren M (2001) Deregulated β-catenin induces a p53- and ARF-dependent growth arrest and cooperates with Ras in transformation. EMBO J 20: 4912–4922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A (2004) WNT and β-catenin signalling: diseases and therapies. Nat Rev Genet 5: 691–701 [DOI] [PubMed] [Google Scholar]
- Howe LR, Brown AM (2004) Wnt signaling and breast cancer. Cancer Biol Ther 3: 36–41 [DOI] [PubMed] [Google Scholar]
- Sin S et al. (2011) Role of the focal adhesion protein kindlin-1 in breast cancer growth and lung metastasis. J Natl Cancer Inst 103: 1323–1337 [DOI] [PubMed] [Google Scholar]
- Kato K, Shiozawa T, Mitsushita J, Toda A, Horiuchi A, Nikaido T, Fujii S, Konishi I (2004) Expression of the mitogen-inducible gene-2 (mig-2) is elevated in human uterine leiomyomas but not in leiomyosarcomas. Hum Pathol 35: 55–60 [DOI] [PubMed] [Google Scholar]
- An Z, Dobra K, Lock JG, Stromblad S, Hjerpe A, Zhang H (2010) Kindlin-2 is expressed in malignant mesothelioma and is required for tumor cell adhesion and migration. Int J Cancer 127: 1999–2008 [DOI] [PubMed] [Google Scholar]
- Shi X, Ma YQ, Tu Y, Chen K, Wu S, Fukuda K, Qin J, Plow EF, Wu C (2007) The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J Biol Chem 282: 20455–20466 [DOI] [PubMed] [Google Scholar]
- Staal FJ, Noort Mv M, Strous GJ, Clevers HC (2002) Wnt signals are transmitted through N-terminally dephosphorylated β-catenin. EMBO Rep 3: 63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A (2001) Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCζ. Cell 106: 489–498 [DOI] [PubMed] [Google Scholar]
- Bottomly D, Kyler SL, McWeeney SK, Yochum GS (2010) Identification of {β}-catenin binding regions in colon cancer cells using ChIP-Seq. Nucleic Acids Res 38: 5735–5745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yook JI et al. (2006) A Wnt-Axin2-GSK3β cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol 8: 1398–1406 [DOI] [PubMed] [Google Scholar]
- Gozgit JM, Pentecost BT, Marconi SA, Otis CN, Wu C, Arcaro KF (2006) Use of an aggressive MCF-7 cell line variant, TMX2-28, to study cell invasion in breast cancer. Mol Cancer Res 4: 905–913 [DOI] [PubMed] [Google Scholar]
- Shi X, Wu C (2008) A suppressive role of mitogen inducible gene-2 in mesenchymal cancer cell invasion. Mol Cancer Res 6: 715–724 [DOI] [PubMed] [Google Scholar]
- Gong X, An Z, Wang Y, Guan L, Fang W, Stromblad S, Jiang Y, Zhang H (2010) Kindlin-2 controls sensitivity of prostate cancer cells to cisplatin-induced cell death. Cancer Lett 299: 54–62 [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang M, Tan X, Li TF, Zhang YE, Chen D (2010) Smad3 prevents β-catenin degradation and facilitates β-catenin nuclear translocation in chondrocytes. J Biol Chem 285: 8703–8710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N et al. (2011) FoxM1 promotes β-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell 20: 427–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT (2003) Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol 13: 680–685 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.