Abstract
We have recently described that autophagic targeting of Src maintains cancer cell viability when FAK signalling is defective. Here, we show that the Ret tyrosine kinase is also degraded by autophagy in cancer cells with altered/reduced FAK signalling, preventing its binding to FAK at integrin adhesions. Inhibition of autophagy restores Ret localization to focal adhesions. Importantly, Src kinase activity is required to target Ret to autophagosomes and enhance Ret degradation. Src is thus a general mediator of selective autophagic targeting of adhesion-linked kinases, and Ret a second FAK-binding tyrosine kinase degraded through autophagy in cancer cells under adhesion stress. Src—by controlling not only its own degradation but also that of other FAK-binding partners—allows cancer cell survival, suggesting a new therapeutic strategy.
Keywords: Ret, tyrosine kianse, FAK, autophagy
Introduction
Focal adhesion kinase (FAK) is an essential signal integrator localizing to focal adhesions, where it regulates cell spreading, migration and integrin signalling pathways that promote proliferation and survival [1–5].
This protein consists of a four-point-one, ezrin, radixin, moesin domain at the amino-terminal, a catalytic domain, a proline-rich region and a focal adhesion targeting sequence at the carboxy-terminal. FAK is autophosphorylated on tyrosine (Y) 397 upon integrin engagement, creating a binding site for active Src, a non-receptor tyrosine kinase [6], resulting in the phosphorylation of FAK on multiple tyrosine residues, an increase in kinase activity and initiation of important downstream signalling pathways [7–9].
We have recently described a novel mechanism for the regulation of active Src in murine squamous cell carcinoma (SCC) cells [10]. Detachment of FAK-expressing cells, complete deletion of FAK or expression of non-phosphorylatable FAK mutants results in the targeting of active Src away from peripheral adhesions and into cytoplasmic puncta containing regulators of the autophagy pathway. Inhibition of general autophagy or of the specific targeting of active Src to these puncta restores active Src levels and localization to adhesions, causing loss of cancer cell viability. Thus, when FAK is absent, or when integrin signalling through the Src/FAK pathway is perturbed, specific autophagic targeting/degradation of active Src enables the cells to cope with the stress of excessive ‘un-tethered’ Src and allows them to maintain viability. This work raised the question of whether this autophagy is specific for Src kinase or if it may represent a more general mechanism that cancer cells use to deal with other activated tyrosine kinases in the absence of their key adaptor protein tethering partners.
Rearranged during transfection (Ret) is a receptor tyrosine kinase with numerous functions during development and in disease [11]. It has been detected in human neuroblastomas, pheochromocytomas and medullary thyroid carcinomas [12, 13], and in breast cancer cells it has been shown to interact with the oestrogen receptorα pathway [14]. Ret forms a complex with its ligands, a family of glial-derived neurotrophic factors, and with glycosyl phosphatidylinositol-linked co-receptors, resulting in dimerization, activation of the kinase domain and autophosphorylation of tyrosine residues [15, 16]. These residues act as docking sites for SH2 and PTB domain-containing proteins, such as Src, leading to activation of downstream signalling pathways [17, 18]. Recently, Ret has been shown to bind to the four-point-one, ezrin, radixin, moesin domain of FAK, an interaction that results in transactivation of both proteins [19]. Hence, we asked whether active Ret tyrosine kinase was targeted for autophagic targeting/degradation in cancer cells.
Results and Discussion
Ret localization to autophagic puncta
We have previously described the generation of FAK-proficient (FAK+/+) and FAK-deficient (FAK−/−) murine SCC cells [10]. In both of these cell lines, we identified transcripts for two different Ret isoforms containing either 9 or 51 amino acids in the C-terminal tail (supplementary Fig S1a online). Upon immunoblotting with a Ret-specific antibody (H300), which recognizes both isoforms, we were able to detect some 150–170-kDa Ret protein (supplementary Fig S1b online), but were unable to show that this protein formed a complex with FAK (supplementary Fig S1b online). However, when we carried out both FAK and Ret immunoprecipitation experiments using an antibody that detects a 124-kDa form of Ret (which we assume to be the non-glycosylated form), we found that a complex between FAK and Ret was present; of course this was not present in FAK−/− cells (Fig 1A, left panels). Furthermore, the steady-state levels of Ret were reduced in the absence of FAK and a lower-molecular-weight species of ∼80 kDa was also visible (Fig 1A, right panels). These data implied that there was enhanced degradation of Ret when FAK was absent consistent with increased turnover. Co-immunoprecipitation experiments also showed that there was interaction between Ret and Src in SCC cells, and that this was independent of FAK (Fig 1A, middle panels).
Figure 1.
Intracellular localization of Ret is altered in the absence of FAK in SCC cells. (A) FAK+/+ and FAK−/− murine SCC cells were lysed and FAK, Ret or Src immunoprecipitated and immunoblotting carried out using anti-FAK, anti-Ret, anti-Src and anti-actin. (B) Cells were fixed and stained with either anti-Ret (green in the upper left panels) or anti-Ret PY1062 (green in the lower left panels) and DAPI (blue). Merged and zoomed images are shown. Solid arrows indicate localization at adhesions while broken arrows show localization in puncta. Quantification of percentage of cells that contained Ret in intracellular puncta is shown. Data are presented as mean±s.d. and the significance calculated using a Student’s t-test (n=3). Lysates were immunoblotted with anti-Ret PY1062 and anti-actin (lower right panels). (C) Cells were fixed and stained with anti-Ret (green) and anti-FAK (red). Merged images are also shown. Solid arrows indicate co-localization between Ret and FAK at adhesions, while broken arrows indicate its absence in puncta. Scale bars, 20 μM. DAPI, 4,6-diamidino-2-phenylindole; FAK, focal adhesion kinase; Ret, rearranged during transfection; SCC, squamous cell carcinoma.
We then examined Ret intracellular localization. In 100% of FAK+/+ cells, Ret was present at peripheral adhesions (Fig 1B, solid arrows in the upper left panels, quantified in the upper right panel), where it co-localized with FAK (Fig 1C, upper panels, solid arrows). In the absence of FAK, however, Ret was clearly visible in distinctive cytoplasmic puncta in 94% of cells (Fig 1B, broken arrows in the upper left panels, quantified in the upper right panel and shown in Fig 1C, lower panels, broken arrows).
To examine whether Ret was tyrosine phosphorylated, we immunoprecipitated phospho-tyrosine (PY) from FAK+/+ and FAK−/− cell lysates and detected a 124-kDa Ret species and another 80-kDa Ret band when FAK was absent (supplementary Fig S1c online). We observed a similar pattern of species when we immunoblotted using an antibody that detects Ret that is phosphorylated on tyrosine 1062 (Ret PY1062) (Fig 1B, lower right panel). Treatment with a Ret inhibitor, RPI-1, reduced Ret PY1062 signal (supplementary Fig S1d online). Furthermore, immunofluorescence revealed that while Ret PY1062 localized to adhesions in FAK+/+ cells (Fig 1B, solid arrows in the lower left panels), it was found to be associated with intracellular puncta in FAK-deficient cells (Fig 1B, broken arrows in the lower left panels). Finally, stimulation with the Ret ligand glial-derived neurotrophic factors failed to further increase Ret PY1062 levels (data not shown) in SCC cells. These data imply that Ret tyrosine kinase in these cells is active, and, in keeping with this, we found a modest increase in total Ret protein levels (1.34-fold), but a significant increase (52.9-fold) in Ret PY1062 when we compared SCC cells with normal keratinocytes (supplementary Fig S1e online).
Furthermore, detachment-induced adhesion stress in FAK-positive MCF7 breast cancer cells resulted in relocation of Ret from adhesions to cytoplasmic puncta (supplementary Fig S1f online) similar to that observed with active Src when FAK-positive SCC cells were subjected to the same type of stress [10].
Inhibition of autophagy restores Ret to adhesions
No co-localization was detected between Ret-positive puncta and endogenous paxillin or cortactin (supplementary Fig S2 online), nor with overexpressed endosomal markers such as Rab11 or EEA1 (supplementary Fig S2 online), confirming that these structures were not focal adhesions, podosomes or endosomes. However, we did observe a striking co-localization of Ret with actin-rich patches that appeared to be aligned along bundled actin filaments that are tethered into focal adhesions (supplementary Fig S2 online).
Interestingly, we detected almost complete co-localization of Ret with activated Src tyrosine kinase (PY416) in adhesions in FAK+/+ cells (Fig 2A, upper panels, solid arrow) and in puncta in FAK−/− cells (Fig 2A, lower panels, broken arrows). We have previously identified indistinguishable Src-containing puncta as autophagosomes [10], using specific antibodies against autophagy-related proteins (Atg) such as LC3B, which co-localizes with Src in adhesions (Fig 2B, upper panel, solid arrow) and in autophagosomes (Fig 2B, lower panel, broken arrows) in the presence and absence of FAK, respectively. Immune-gold staining also showed that both Src and Ret were sequestered in vesicles with a single membrane similar to those formed during the intermediate stages of the autophagy pathway (not shown).
Figure 2.
Ret localizes to Src-positive autophagosomes in FAK-deficient SCC cells. Cells were fixed and stained with either (A) anti-Ret (green) and anti-PY416 Src (red) or (B) anti-PY416 Src (red) and anti-LC3B (green). Merged and zoomed images are shown. Solid arrows indicate co-localization at adhesions, while broken arrows show co-localization in intracellular puncta. (C) Cells were transfected with scrambled, Atg5 or Atg7 siRNA, trypsinized and allowed to readhere before immunoblotting with anti-Atg5, anti-Atg7 and anti-actin (right panels). Cells were fixed and stained with anti-Ret (green), anti-paxillin (red) and DAPI (blue). Merged images are shown. Solid arrows indicate co-localization between Ret and paxillin at adhesions, while broken arrows indicate its absence in puncta. (D) Quantification of percentage of cells that contained Ret in intracellular puncta is shown. Data are presented as mean±s.d. and the significance calculated using a Student’s t-test (n=3). (E) Cells, either untreated or treated with chloroquine for 24 h, were fixed and stained with anti-Ret (green) and DAPI (blue). Scale bars, 20 μM. DAPI, 4,6-diamidino-2-phenylindole; FAK, focal adhesion kinase; Ret, rearranged during transfection; SCC, squamous cell carcinoma; siRNA, short interfering RNA.
To confirm that these Ret-positive structures were autophagosomes, we treated the cells with 3-methyladenine (3MA), an inhibitor that prevents the formation of autophagosomes [20]. In FAK+/+ cells 3MA had no effect on the localization of Ret (supplementary Fig S3a online), but in FAK−/− cells Ret was restored to adhesions containing paxillin (supplementary Fig S3a online, quantified in supplementary Fig S3b online), and the lower molecular weight 80-kDa Ret species was diminished (supplementary Fig S3c online).
We also used short interfering RNA (siRNA) against two different components of the autophagy machinery. In FAK+/+ cells, knockdown of Atg5 or Atg7 (Fig 2C, right panels) had little effect on Ret localization (Fig 2C, upper panels, solid arrows, quantified in Fig 2D). However, in the absence of FAK, Ret localization to puncta was impaired and its targeting to focal adhesions partially restored when Atg5 or Atg7 siRNA was expressed (Fig 2C, lower panels, solid arrows, quantified in Fig 2D). These data suggest that a general mechanism exists in SCC cells whereby overactive tyrosine kinases, such as Src and Ret, are selectively targeted from focal adhesions to autophagic puncta in the absence of their binding partner FAK. In keeping with a key role for the autophagy/lysosomal pathway, chloroquine treatment of FAK−/− cells also led to an accumulation of Ret-positive puncta (Fig 2E).
Loss of FAK phosphorylation drives Ret targeting
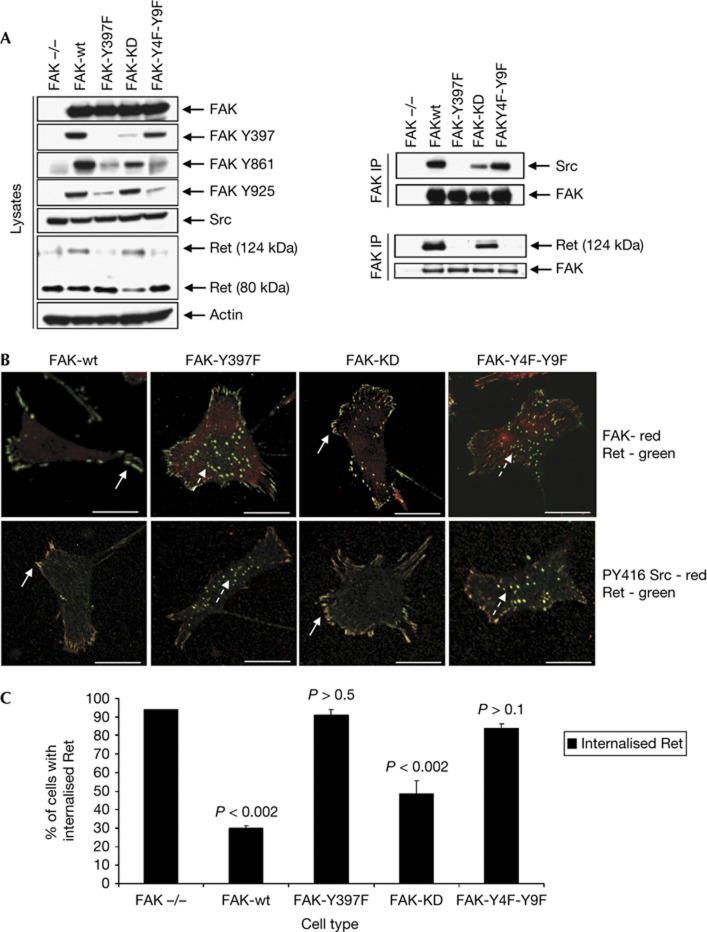
To ascertain whether Ret targeting was affected by FAK phosphorylation, we expressed wild-type FAK (FAK-wt), an autophosphorylation mutant that cannot bind to Src or respond to integrin signalling (FAK-Y397F), a kinase-defective mutant (FAK-KD) or a mutant in which all of the Src-dependent phospho-acceptor sites have been mutated (FAK-Y4F-Y9F) (Fig 3A; [21]). We first confirmed that Y397, Y861 and Y925 were all phosphorylated in FAK-wt and that there was no phosphorylation of these sites in FAK-Y397F. We also demonstrated that FAK-Y4F-Y9F exhibited near-wild-type levels of Src binding, whereas FAK-KD showed much reduced binding, while, as expected, FAK-Y397F did not bind Src (Fig 3A, upper right panels). Interestingly, co-immunoprecipitation of FAK and immunoblotting with anti-Ret showed that while expression of FAK-wt or FAK-KD permitted the interaction between FAK and Ret, expression of either FAK-Y397F or FAK-Y4F-Y9F proteins did not (Fig 3A, lower right panels). Furthermore, expression of FAK-Y397F and FAK-Y4F-Y9F did not rescue the FAK-deficiency phenotype as Ret was clearly still present in puncta (Fig 3B, upper panels, broken arrows, quantified in Fig 3C), where it also co-localized with active Src (Fig 3B, lower panels, broken arrows). This was in contrast to FAK-wt or FAK-KD, which could still bind to Ret and partially restore localization of both FAK and Ret to peripheral adhesions (Fig 3B, upper panels, solid arrows, quantified in Fig 3C). Collectively, these data imply that FAK binding to Ret is necessary to prevent Ret translocation to autophagosomes and that Src-dependent phosphorylation of FAK is essential for this interaction to occur. These conclusions are in keeping with the findings that FAK-Y397F and FAK-Y4F-Y9F do not bind to Ret, which is then targeted for autophagic degradation.
Figure 3.
Src-dependent phosphorylation of FAK is required for Ret/FAK binding. (A) FAK−/− cells stably re-expressing FAK-wt, FAK-Y397F, FAK-KD or FAK-Y4F-Y9F were immunoblotted using anti-FAK, anti-FAK Y397, anti-FAK Y861, anti-FAK Y925, anti-Src, anti-Ret and anti-actin. FAK and Ret were immunoprecipitated from these cells and then samples were immunoblotted with anti-Src, anti-FAK or anti-Ret. (B) Cells were fixed and stained with anti-Ret (green) and either anti-FAK (red in the upper panels) or anti-PY416 Src (red in the lower panels). Merged images are shown. Solid arrows indicate Ret in adhesions, while broken arrows show Ret in puncta. Scale bars, 20 μM. (C) Quantification of percentage of cells that contained Ret in intracellular puncta is shown. Data are presented as mean±s.d. and the significance calculated using a Student’s t-test (n=3). FAK, focal adhesion kinase; Ret, rearranged during transfection.
Src activity is required for Ret targeting
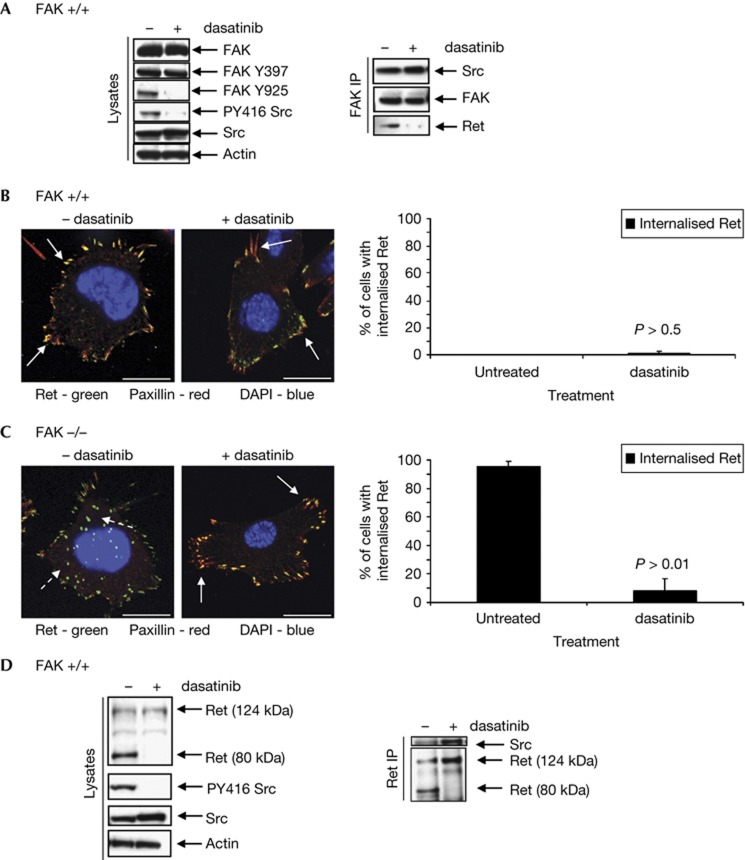
When FAK+/+ cells were treated with dasatinib, an inhibitor of Src kinase activity, (Fig 4A, left panels), we observed a pattern of FAK phosphorylation and binding similar to that of FAK-Y4F-Y9F. In both cases, FAK was phosphorylated on Y397 but not on Src phospho-acceptor sites (Fig 4A, left panels) and could bind Src but not Ret (Fig 4A, right panels). As Ret was present in autophagosomes in FAK-Y4F-Y9F cells, we therefore hypothesized that in dasatinib-treated FAK+/+ cells Ret would also be detected in these structures. Surprisingly, this was not so; in 98% of dasatinib-treated FAK+/+ cells Ret clearly remained in adhesions (Fig 4B, solid arrows, quantified in the right panel). This implied that the presence of active Src was essential for cells to traffic Ret to the autophagy pathway, and loss of FAK phosphorylation was not sufficient. Hence, we have shown that Src tyrosine kinase has two functions in this process: (1) normal flux through the pathway (that is, Src-dependent phosphorylation of FAK) is necessary to keep Ret from being sequestered to autophagosomes; and (2) Src kinase activity has a FAK-independent role that orchestrates not only its own sequestration into autophagosomes but also the sequestration of other binding partners, as we show here for Ret.
Figure 4.
Src activity is required for targeting of Ret to puncta in both the presence and absence of FAK. (A) FAK+/+ cells were treated with dasatinib, then lysates were immunoblotted with anti-FAK, anti-FAK Y397, anti-FAK Y925, anti-PY416 Src, anti-Src and anti-actin. FAK was immunoprecipitated and then immunoblotting performed using anti-Src, anti-FAK or anti-Ret. (B) Cells were treated with dasatinib, then fixed and stained with anti-Ret (green), anti-paxillin (red) and DAPI (blue). Solid arrows indicate co-localization in adhesions. Quantification of percentage of cells that contained Ret in intracellular puncta is shown. Data are presented as mean±s.d. and the significance calculated using a Student’s t-test (n=3). (C) FAK−/− cells treated with dasatinib were fixed and stained with anti-Ret (green), anti-paxillin (red) and DAPI (blue). Solid arrows indicate co-localization in adhesions, while broken arrows show lack of co-localization in puncta. Scale bars, 20 μM. Quantification of percentage of cells that contained Ret in intracellular puncta is shown. Data are presented as mean±s.d. and the significance calculated using a Student’s t-test (n=3). (D) Lysates from untreated and dasatinib-treated FAK−/− cells were immunoblotted with anti-Ret, anti-PY416 Src, anti-Src and anti-actin. Ret was also immunoprecipitated from cells and then immunoblotted using anti-Src and anti-Ret. DAPI, 4,6-diamidino-2-phenylindole; FAK, focal adhesion kinase; Ret, rearranged during transfection.
We have previously shown that treatment with dasatinib prevents the trafficking of Src to autophagic puncta in FAK−/− cells [10]. When these cells were treated with dasatinib, we also observed a striking relocalization of Ret from autophagosomes to paxillin-containing focal adhesions (Fig 4C, solid arrows, quantified in the right panel). Although Ret and Src could still form a complex in cells lacking FAK expression or Src activity (Fig 4D, right panels), there was loss of the lower-molecular-weight Ret species we previously observed in cell lysate (Fig 4D, left and right panels).
Targeting of Ret is not mediated by c-Cbl
The selective targeting of Src to autophagosomes is dependent on the ability of its binding partner c-Cbl to interact with LC3B via an evolutionary conserved sequence known as an LC3-interacting region [10, 22, 23]. To determine whether Ret also required c-Cbl, we knocked down its expression in FAK-deficient cells (supplementary Fig S4a online). In cells expressing a scrambled siRNA control, we observed a 124- and an 80-kDa Ret band (supplementary Fig S4a online) and both Ret and active Src localized to autophagic puncta (supplementary Fig S4b online, solid arrows in the upper panels) in the majority of cells (supplementary Fig S4c online). As expected, the number of Src-positive puncta was significantly reduced in FAK-deficient cells expressing c-Cbl siRNA, and active Src was restored to peripheral adhesions (supplementary Fig S4b online). However, we did not observe any change in Ret localization (supplementary Fig S4b online, quantified in supplementary Fig S4c online) and both the 124- and 80-kDa bands were detected (supplementary Fig S4a online). These data suggest that while c-Cbl is essential for the trafficking of Src to autophagic puncta, Ret is still targeted to these structures in its absence. It is, however, possible that the presence of active Src in adhesions in c-Cbl siRNA-expressing cells is sufficient to drive this process or that Ret itself can form a complex with LC3B; indeed, when we incubated glutathione S-transferase-tagged LC3B with cell lysate and immunoblotted, we were able to detect Ret (supplementary Fig S4d online).
Overall, these experiments imply that advanced SCC cancer cells have developed a general mechanism to deal with active, potentially oncogenic, tyrosine kinases, such as Src and Ret, when their adhesion-binding partner, namely FAK, is either lost or signalling impaired. This involves the selective sequestration of a highly active kinase away from adhesion complexes into autophagosomes upon ‘inhibition’ of FAK, triggering protein degradation and reduced steady-state levels. Importantly, we have shown that Src has two functions in this system: (1) preventing the targeting of Ret to autophagosomes via phosphorylation of FAK; and (2) a FAK-independent role that requires the catalytic activity of Src to orchestrate the sequestration and destruction of other FAK-binding tyrosine kinases, like Ret. As we showed previously, this permits survival of cancer cells and may provide an opportunity for the use of combinations of FAK and/or Src inhibitors with autophagy inhibitors to reduce the viability of cancer cells that use this mechanism. More work is needed to establish the full range of tyrosine kinases that are targeted to autophagosomes upon adhesion stress (beyond the scope here), which may permit identification of a range of thymidine kinase inhibitors that could be of therapeutic value when used in conjunction with autophagy inhibitors.
Methods
Materials. Antibodies used: anti-Ret and anti-Ret PY1062 (Abcam, Cambridge, UK), anti-FAK, anti-phospho-FAK-Y925, anti-phospho-FAK-Y397, anti-Src 36D10, anti-PY416-Src, anti-Atg7, anti-cortactin, anti-c-Cbl, anti-LC3B, IgG-peroxidase-conjugated secondary antibodies (New England Biolabs, Herts, UK), anti-PY416-Src (Invitrogen, Paisley, UK), anti-paxillin and anti-phospho-tyrosine (BD Transduction Laboratories, Oxford, UK), anti-actin, TRITC phalloidin and anti-tubulin (Sigma, Poole, UK), anti-Atg5 (Novus, Cambridge, UK), anti-phospho-FAK-Y861 (Millipore, Watford, UK) and anti-Ret H300 (Santa Cruz, CA, USA). Vectashield mounting medium, Alexa 488 and Alexa 594-conjugated secondary antibodies were from Invitrogen. Amaxa nucleofection kit V was from Lonza (Valais, Switzerland), Hygromycin B and RPI-1 from Merck Biosciences (Nottingham, UK), dasatinib from BMS (Middlesex, UK), 3MA from Sigma and chloroquine from Invivogen (San Diego, USA). Micro BCA Protein Assay kit was from Pierce Ltd (Northumbria, UK) and siRNA from Dharmacon (Colorado, USA).
Generation of FAK-deficient SCC cell lines. K14CreERT2/FAKflox/flox mice were subjected to chemical carcinogenesis [24] and FAK+/+ cells grown from the resultant tumours as previously described [10]. FAK was deleted using 10 μM 4-hydroxy tamoxifen and stable FAK−/− single-cell clones generated [10].
Cell culture and transfection. SCC cells were cultured in minimum essential medium (10% fetal calf serum, 2 mM L-glutamine, NEAA, sodium pyruvate and MEM vitamins). Generation of FAK mutant-expressing cells has been described previously [10] and these cells were maintained in hygromycin B (1 mg ml−1). MCF7 cells were cultured in DMEM with 10% fetal calf serum and 2 mM L-glutamine. For adhesion stress assay, cells were trypsinized, suspended in PBS at 4 °C for 1 h, then cytospun onto glass slides. Cells were then fixed and stained as outlined below.
siRNA and drug treatment. Atg5, Atg7, c-Cbl or scrambled siRNA of volume 40 nM was transiently transfected into cells using HiPerFect (Qiagen, Sussex, UK). Cells were left for 3 days, trypsinized and replated for 24 h at a lower density than that in the experiments performed. Adherent cells were treated for 24 h with dasatinib (200 nM), chloroquine (10 μM) or RPI-1 (40 μM), or for 48 h with 3MA (10 mM).
Immunoblotting and immunoprecipitation. Cells were washed with PBS, lysed in RIPA (50 mM Tris–HCl at pH 7.4, 150 mM NaCl, 0.1% SDS and 1% deoxycholate) containing a protease and a phosphatase inhibitor tablet (Sigma) and centrifuged for 15 min at high speed (16 000g at 4 °C min). Immune complexes were collected when 1 mg of cell lysate was immunoprecipitated with 2 μg of antibody or with control IgG. Lysis buffer was used to wash the beads three times before a final wash using 0.6 M lithium chloride was performed. These samples or 20 μg lysate was then supplemented with sample buffer (Tris at pH 6.8, 20% glycerol, 5% SDS, β-mercaptoethanol and bromo-phenol blue), separated by SDS–PAGE, transferred to a nitrocellulose membrane and then immunoblotted.
Immunofluorescence. Cells were washed in Tris-buffered saline (TBS), then fixed for 10 min (formaldehyde (3.7%), K-Pipes pH 6.8 (100 mM), EGTA (10 mM), MgCl2 (1 mM) and triton X100 (0.2%)). Cells were then washed in TBS containing 0.1% triton X100 and blocked in TBS containing 0.1% BSA. The primary antibody was incubated overnight and fluorescently labelled secondary antibodies added for 45 min after three washes in TBS with 0.1% triton X100. Imaging was carried out using an FV1000 Olympus Confocal Microscope (Olympus, Essex, UK).
Supplementary Material
Acknowledgments
This work was supported by Cancer Research UK (Programme Grant to MCF number C157/A11473).
Author contributions: E.S., B.S. and S.W. designed and performed the experiments and analysed the data. E.S. and M.F. wrote the manuscript. M.F. was the grant holder under which this work was performed.
Footnotes
The authors declare that they have no conflict of interest.
References
- Hanks SK, Ryzhova L, Shin NY, Brabek J (2003) Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci 8: d982–d996 [DOI] [PubMed] [Google Scholar]
- Ilic D et al. (1995) Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377: 539–544 [DOI] [PubMed] [Google Scholar]
- Parsons JT (2003) Focal adhesion kinase: the first ten years. J Cell Sci 116: 1409–1416 [DOI] [PubMed] [Google Scholar]
- Schaller MD (2001) Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta 1540: 1–21 [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P (1994) Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature 372: 786–791 [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS (1997) Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13: 513–609 [DOI] [PubMed] [Google Scholar]
- Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT (1994) Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol 14: 1680–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD (2006) Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol 18: 516–523 [DOI] [PubMed] [Google Scholar]
- Calalb MB, Polte TR, Hanks SK (1995) Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol 15: 954–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandilands E et al. (2012) Autophagic targeting of Src promotes cancer cell survival following reduced FAK signalling. Nat Cell Biol 14: 51–60 [DOI] [PubMed] [Google Scholar]
- Manie S, Santoro M, Fusco A, Billaud M (2001) The RET receptor: function in development and dysfunction in congenital malformation. Trends Genet 17: 580–589 [DOI] [PubMed] [Google Scholar]
- Santoro M, Rosati R, Grieco M, Berlingieri MT, D'Amato GL, de Franciscis V, Fusco A (1990) The ret proto-oncogene is consistently expressed in human pheochromocytomas and thyroid medullary carcinomas. Oncogene 5: 1595–1598 [PubMed] [Google Scholar]
- Takahashi M, Buma Y, Taniguchi M (1991) Identification of the ret proto-oncogene products in neuroblastoma and leukemia cells. Oncogene 6: 297–301 [PubMed] [Google Scholar]
- Boulay A et al. (2008) The Ret receptor tyrosine kinase pathway functionally interacts with the ERalpha pathway in breast cancer. Cancer Res 68: 3743–3751 [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Saarma M (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3: 383–394 [DOI] [PubMed] [Google Scholar]
- Jing S et al. (1996) GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell 85: 1113–1124 [DOI] [PubMed] [Google Scholar]
- Encinas M, Crowder RJ, Milbrandt J, Johnson EM Jr (2004) Tyrosine 981, a novel ret autophosphorylation site, binds c-Src to mediate neuronal survival. J Biol Chem 279: 18262–18269 [DOI] [PubMed] [Google Scholar]
- Lundgren TK, Stenqvist A, Scott RP, Pawson T, Ernfors P (2008) Cell migration by a FRS2-adaptor dependent membrane relocation of ret receptors. J Cell Biochem 104: 879–894 [DOI] [PubMed] [Google Scholar]
- Plaza-Menacho I et al. (2011) Focal adhesion kinase (FAK) binds RET kinase via its FERM domain, priming a direct and reciprocal RET-FAK transactivation mechanism. J Biol Chem 286: 17292–17302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ et al. (2008) Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4: 151–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff MA, Serrels B, Fincham VJ, Frame MC, Carragher NO (2004) SRC-mediated phosphorylation of focal adhesion kinase couples actin and adhesion dynamics to survival signaling. Mol Cell Biol 24: 8113–8133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282: 24131–24145 [DOI] [PubMed] [Google Scholar]
- Yokouchi M, Kondo T, Sanjay A, Houghton A, Yoshimura A, Komiya S, Zhang H, Baron R (2001) Src-catalyzed phosphorylation of c-Cbl leads to the interdependent ubiquitination of both proteins. J Biol Chem 276: 35185–35193 [DOI] [PubMed] [Google Scholar]
- McLean GW et al. (2004) Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev 18: 2998–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.