Abstract
The intestinal epithelium—which constitutes the interface between the enteric microbiota and host tissues—actively contributes to the maintenance of mucosal homeostasis and defends against pathogenic microbes. The recognition of conserved microbial products by cytosolic or transmembrane pattern recognition receptors in epithelial cells initiates signal transduction and influences effector cell function. However, the signalling pathways, effector molecules and regulatory mechanisms involved are not yet fully understood, and the functional outcome is poorly defined. This review analyses the complex and dynamic role of intestinal epithelial innate immune recognition and signalling, on the basis of results in intestinal epithelial cell-specific transgene or gene-deficient animals. This approach identifies specific epithelial cell functions within the diverse cellular composition of the mucosal tissue, in the presence of the complex and dynamic gut microbiota. These insights have thus provided a more comprehensive understanding of the role of the intestinal epithelium in innate immunity during homeostasis and disease.
Keywords: intestinal epithelium, innate immunity, mucosal immunity, host–microbial homeostasis
See Glossary for abbreviations used in this article.
Glossary.
- Alpi
intestinal alkaline lipopolysaccharide phosphatase
- AOM
azoxymethane
- April
a proliferation inducing ligand
- ATG5/7
autophagy 5/7
- CAC
colitis associated cancer
- CCL20/28
chemokine, CC-motif, ligand 20/28
- CFU
coloy forming units
- CX3CR1+
chemokine, CX3C motif, receptor 1
- DAP
diaminopimelic acid
- DLG5
homologue of drosophila disc large 5
- DSS
dextran sulphate sodium
- ERT2
tamoxifen-sensitive estradiol receptor
- Fadd
Fas-associated death domain protein
- FAE
follicle associated epithelial
- hPepT1
intestinal hydrogen ion/peptide cotransporter
- ICAM1
intracellular adhesion molecule 1
- IEC
intestinal epithelial cell
- IEL
intraepithelial lymphocyte
- I-Fabp/Fabpi/Fabp2
intestinal fatty acid-binding protein
- IκB-α
inhibitor of kappa light chain gene enhancer in B cells alpha
- Ikk
IκB kinase
- IPAF/ Nlrc4
Ice protease-activating factor
- IRAK
IL-1 receptor-associated kinase
- LPS
lipopolysaccharide
- LRR
leucine-rich repeat
- Mda5
melanoma differentiation-associated gene 5
- MDR1
multidrug resistance 1
- Mip
macrophage inflammatory protein
- muc2
intestinal mucin 2
- MyD88
myeloid differentiation primary response gene 88
- NF-κB
nuclear factor kappa B
- Nemo
nuclear factor κ-B essential modulator
- NLR
nucleotide-binding domain and leucin-rich repeat-containing
- NLRC
NACHT, LRR and CARD domains-containing protein
- NLRP
NACHT, LRR and PYD domains-containing protein
- NOD2
nucleotide-binding oligomerization domain-containing protein 2 organic cation transporter 1/2
- OCTN1/2
organic cation transporter 1/2
- pIgR
polymeric immunglobulin receptor
- Poly(I:C)
polyinosinic:polycytidylic acid
- Pparγ
peroxisome proliferator-activated receptor-gamma
- PTEN
phosphatase and tensin homolog
- PYD
pyrin domain
- RBP-J
recombination signal-binding protein for immunoglobulin kappa J region
- RegIIIα/γ
regenerating islet-derived III alpha/gamma
- Rig-I
retinoic acid-inducible gene I
- Rip3
receptor interacting protein 3
- RLR
Rig-I-like receptor
- Sigirr
single immunoglobulin domain-containing IL-1R-related protein
- Tak1
TGF-β-activated kinase 1
- TCR
T-cell receptor
- TFF
trefoil factor
- TLR
Toll-like receptor
- TNBS
trinitrobenzene suflonic acid
- Tnfaip
TNF-α induced protein
- Trif
TIR-domain-containing adaptor inducing interferon-β
- Tslp
thymic stromal lymophopoietin
- Xbp1
X box-binding protein 1
Introduction
The intestinal lumen harbours trillions of microbes and represents one of the most densely populated habitats [1]. Major advances in microbiological research have made possible the analysis of human microbiota composition, and the intimate interplay between the host and its microbial inhabitants is increasingly recognized [2]. Commensal bacteria provide a large repertoire of enzymes not encoded by the host genome, thereby contributing to the digestion of dietary substances and the synthesis of essential food supplements such as vitamins [1,3]. The microbiota also provides protection from infection with enteropathogenic bacteria through competition for space and nutrients [4,5]. Finally, the constant presence of commensal bacteria crucially contributes to the differentiation and maturation of the mucosal immune system [6,7,8,9].
A monolayer of polarized epithelial cells covers the intestinal surface and provides the only cellular border between the microbially colonized lumen and the largely sterile subepithelial tissue (Fig 1). The mucosal surface is significantly enlarged by the formation of small intestinal finger-like protrusions called villi and gland-like invaginations known as crypts, which provide efficient nutrient absorption and generate a protected stem cell niche, respectively. The epithelium is composed of different specialized cell types: stem cells, goblet cells, neuroendocrine cells, Paneth cells and enteroabsorptive cells. Notch-mediated epithelial cell differentiation into the various cell lineages is required to maintain mucosal homeostasis [10]. Goblet cells produce heavily glycosylated mucins that form a mucus matrix covering the epithelium in the small and large bowel [11]. Recent results suggest that they might also contribute to the delivery of luminal antigens to subepithelial antigen-presenting cells [12]. The semisolid mucus barrier matrix produced by goblet cells is reinforced by the local secretion of antimicrobial peptides from crypt-based secretory Paneth cells. These cells produce large amounts of α-defensins—also called cryptdins in mice—cryptdin-related sequence (CRS) peptides, so far only described in mice, RegIIIα in humans and RegIIIγ in mice [2,13,14]. RegIIIα and RegIIIγ are also expressed in IECs [14]. In addition, apical shedding of microvillus-derived vesicles by IECs could have an antimicrobial effector function. These vesicles were shown to contain catalytically active alkaline phosphatase able to detoxify bacterial endotoxin, prevent adherence and restrict the proliferation of enteropathogenic bacteria [15]. Enteroabsorptive cells differ in their gene expression and phenotype depending on their position along the crypt villus axis, their localization along the proximal–distal axis of the intestinal tract and their anatomical relation to other cell types or subepithelial secondary lymphoid structures, such as Peyer's patches, isolated lymphoid follicles—FAE cells and M cells—and CX3CR1+ phagocytes.
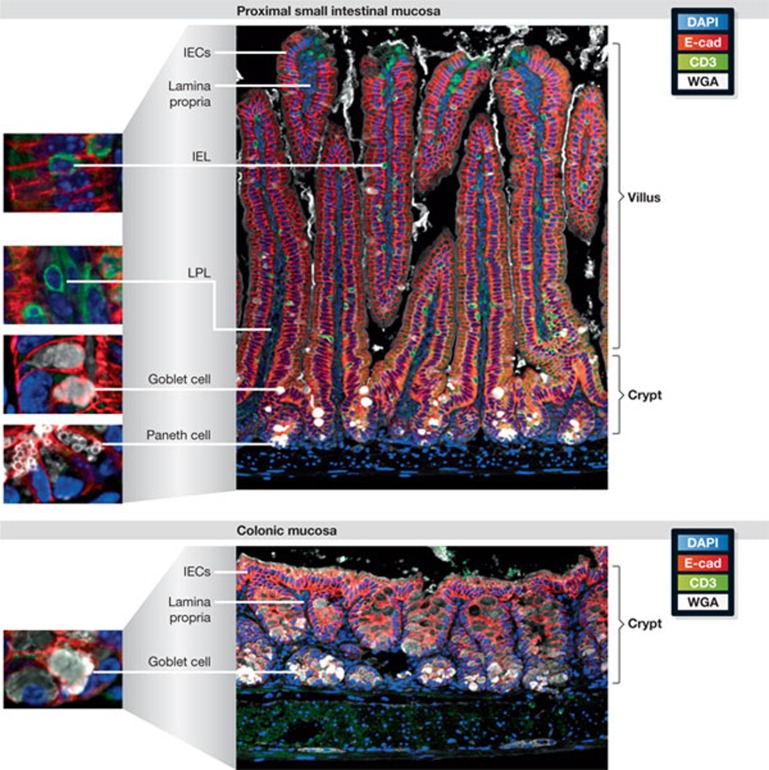
Figure 1.
Structural features of the intestinal mucosa. Paraffin-embedded, formalin-fixed sections of small intestinal (upper panel) and colon tissue (lower panel) from a C57Bl/6 mouse were stained as described elsewhere [49]. E-cadherin (E-cad) is shown in red and CD3 in green. The highly polarized epithelium of the small intestine is organized in a crypt–villus structure. Crypts provide a protective niche for proliferating stem cells (not depicted), and continuous migration along the crypt–villi axis is accompanied by IEC differentiation. The Paneth cells (small white vesicles) are next to the stem cells at the bottom of the crypts, and goblet cells (large white vesicles) are dispersed along the crypt and lower villus region. These two cell types generate the mucus barrier. T cells are shown as an example of intestinal immune cells; lymphocytes can be seen in between or adjacent to the epithelium (IELs) or within the lamina propria (LPL). Epithelium organization in the colon: the crypts end in a flat surface without villi, generating a smoother mucosal tissue. A large number of goblet cells produce a dense mucus layer that covers the epithelium (not depicted). There are no Paneth cells in the healthy colon and the number of immune cells in the lamina propria is much lower than in the small intestine. IEC, intestinal epithelial cell; IEL, intraepithelial lymphocytes; LPL, lamina propria lymphocytes, WGA, wheat germ agglutinin.
Large structural and functional differences are found between the small and large intestine—divided in the duodenum, jejunum, ileum, caecum and colon. These include variations in: luminal water, ion and nutrient concentration; pH level; oxygen tension; retention time of luminal material; microbiota composition and bacterial density; concentration of immunostimulatory microbial ligands; thickness and composition of the mucus layer; spectrum and regulation of enteric antimicrobial peptides [16]; anatomical surface structure—for example the presence and length of villi; number of epithelial cell subtypes; enzymatic and metabolic features of the epithelium; transepithelial transport capacities and underlying subepithelial immune cells.
Numerous professional immune cells, lymphocytes, dendritic cells and macrophages are in close proximity to the mucosal surface. Intraepithelial lymphocytes (IELs) are found between epithelial cells, and CX3CR1+ phagocytes penetrate the epithelium and extend dendrites to sample luminal antigens. Other cells are situated in organized lymphoid structures such as Peyer's patches and cryptopatches, or are scattered throughout the lamina propria. Studies indicate that the epithelium functions as a communicator between the luminal flora and the subepithelial immune system (Fig 2; [6,17]). For example, the regulatory T lymphocyte (TReg)-promoting effect of certain Clostridium spp. at the colon mucosa is mediated by epithelium-derived TGF-β [6]. The epithelium also reacts through metabolic changes to alterations of the immune system and host microbial homeostasis. The gene expression profile of intestinal epithelial cells shifts from metabolic activity to epithelial host defence in the absence of IgA, possibly to compensate for the loss of adaptive immunity [18].
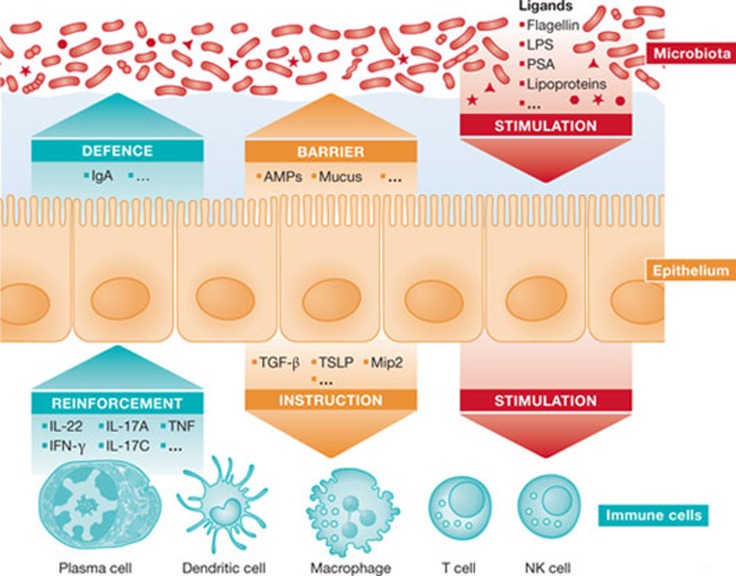
Figure 2.
Intestinal epithelial cells in microbial homeostasis. The microbiota and microbiota-derived immune stimuli are shown in red. IECs, which are the communicators between microbiota and professional immune cells, and IEC-mediated effects are shown in yellow. Immune cells and their influence on epithelial barrier formation and IgA-mediated mucosal host protection are shown in blue. Please refer to the text for details. AMP, antimicrobial peptide; IEC, intestinal epithelial cell,; LPS, lipopolysaccharide; PSA, polysaccharide A; TGF-β, transforming growth factor beta; TNF, tumour necrosis factor; TSLP, thymic stromal lymophopoietin; MIP2, macrophage inflammatory protein 2.
The differences between the small and large intestine seem to contribute to the organ-specific manifestation observed in several human mucosal diseases and animal models, although the mechanisms are not entirely clear. The two best-known human inflammatory diseases of the gastrointestinal tract are ulcerative colitis, which is restricted to the colonic mucosa and cured by colectomy, and Crohn's disease, which can affect the whole gastrointestinal tract and even other body sites. Similarly, certain pathogens predominantly affect specific parts of the intestine. For example, Citrobacter rodentium—a frequently used mouse pathogen—is restricted to colonizing the large intestine and caecum, whereas rotavirus-infected cells are only observed in the small intestine. Non-infectious animal models also present strict organ specificity: inflammatory changes after oral application of DSS are almost completely restricted to the colon, whereas epithelial damage as a consequence of intraperitoneal administration of poly(I:C) or TNF occurs primarily in the small intestine [19,20,21].
Thus, the intestinal epithelium, which is exposed to the microbiota and supported by the underlying immune cells, forms the mucosal barrier. It has a complex anatomical and functional diversity with significant influence on innate immune recognition, host defence and mucosal homeostasis, as discussed in this review.
Innate immune receptor expression in IECs
Similarly to professional immune cells—such as macrophages and dendritic cells—IECs express innate immune receptors, although the set of receptor molecules is less diverse. The best-studied innate immune receptors are those of the TLR family. They are transmembrane receptors that recognize bacterial, viral, parasite or self-derived ligands, and initiate several signalling cascades, leading to transcriptional responses. Tlr2 recognizes di- and tri-acylated lipoproteins, Tlr3 double-stranded RNA, TLR4 lipopolysaccharide, Tlr5 flagellin and TLR9 hypomethylated double-stranded DNA. TLR1–5/9 transcription has been detected in human small intestinal tissue, and Tlr1–9 was detected in murine small intestinal tissue. TLR1–9 mRNA has also been detected in human colon tissue and Tlr1–4/9 in mice [22]. Epithelial TLR expression has been reviewed in depth elsewhere [22].
IECs also express the cytosolic helicases Rig-I and Mda5, which sense the presence of RNA, and the Nod-like receptors (NLRs) Nod1, which detects the DAP-type tri-peptide motif of peptidoglycan, and Nod2, which recognizes the muramyl-di-peptide motif of peptidoglycan. Activation of these receptors is followed by a transcriptional response. Nod2 expression is predominantly found in the crypt region, and immunohistological stainings in human samples have suggested that Paneth cells express Nod2, in agreement with data showing reduced cryptdin expression in Nod2-deficient murine Paneth cells [23,24]. Nod1 and Nod2 seem to act synergistically to protect the colon epithelium during bacterial infection [25,26].
On the other hand, activation of the second group of NLRs leads to a non-transcriptional response. This group of cytosolic NLRs mediates activation of the inflammasome, thereby facilitating the maturation of pro-IL-1β and pro-IL-18 into mature, secreted interleukins. The inflammasome has received significant attention due to the high expression level and functional significance of IL-1β/IL-18 in mucosal host defence. Human small intestinal tissue expresses high levels of NLRP10, whereas there is high expression of Nlrp6 and Nlrp1, 2, 6, 10 and 12 in mouse small intestinal and colon tissue, respectively [27]. Epithelial expression of Nlrp3 (also known as Nalp3), Nlrc4 (also known as IPAF; activated by flagellin and bacterial type III secretion apparatus components) and Nlrp6, of unknown ligand, has been suggested, but their functional role is only beginning to be examined [28]. Although epithelial-specific expression of the inflammasome-activating NLRs has not formally been proven, processed IL-18 is present in colon enterocytes after DSS treatment, suggesting inflammasome activation and therefore the expression of NLR family members in epithelial cells [29]. A summary of intestinal epithelial NLR expression is shown in Table 1.
Table 1. NLR expression and function in the intestinal epithelium.
| NLR | Involved in colitis |
Expressed by IECs | Functional in IECs | |
|---|---|---|---|---|
| Human | Mouse | |||
| CIITA | Colorectal cancer in UC patients: methylation status of CIITA correlates with certain risk alleles [118] | Overexpression in T cellsDSS: no altered pathology,oxazolone-induced TH2-driven model: more severe [119] | Yes [120] | Yes [120,121] |
| NAIP | ND | ND | Yes [122] | ND |
| NOD1 | SNP association with early onset IBD [123] | Nod1/2 double knockout: attenuated Salmonella-colitis model [25], increased susceptibility to DSS [124]; protective in Clostridium difficile colitis [125] | Yes | Yes |
| NOD2 | SNPs linked to cluster of differentiation [126] | Nod1/2 double knockout, see NOD1;decreased clearance of C. rodentium [127];increased susceptibility to TNBS colitis [128] | IEC [129], Paneth cells [23,24] | Yes [24,130] |
| NLRC3 | ND | ND | ND | ND |
| NLRC4/Ipaf | ND | Controversial phenotype in DSS colitis: no effect in knockout [111] compared with increased susceptibility in DSS and Salmonella colitis [131] | RNA [110] | ND [132] |
| NLRC5 | ND | ND | High RNA in human; low in mouse [133] | ND |
| NLRP1/2 | ND | ND | ND | ND |
| NLRP3 | SNPs and decreased expression linked to cluster of differentiation [134] | Controversial:NLRP3 expressed by colonic epithelial cells crucial for protection [29],NLRP3 signalling of haematopoetic cells protective in DSS colitis [111],NLRP3 protective in DSS colitis [112],NLRP3 signalling exacerbates inflammation in DSS colitis [135] | Yes [29] | Yes [29] |
| NLRP4/5 | ND | ND | ND | ND |
| NLRP6 | ND | NLRP6 deficiency leads to colitogenic microflora [109] and increased susceptibility to DSS colits [108,109,136] | RNA, protein [108,109,136] | Yes [109] |
| NLRP7–14 | ND | ND | ND | ND |
| NLRX1 | ND | ND | ND | ND |
CIITA, class II transactivator; DSS, dextran sulphate sodium; IBD, inflammatory bowel disease; IEC, intestinal epithelial cell; Ipaf, ICE protease-activating factor; NAIP, neuronal apoptosis inhibitory protein; ND, not determined; NLR, Nod-like receptor; NLRC3/4/5, NACHT, LRR and CARD domains-containing protein 3/4/5; NLRP1–14, NACHT, LRR and PYD domains-containing protein 1–14; NLRX1, NLR family member X1; NOD1/2, nucleotide-binding oligomerization domain-containing protein 1/2; SNP, single nucleotide polymorphism; TNBS, 2,4,6-trinitrobenzene sulphonic acid; UC, ulcerative colitis.
Of note, innate immune receptor expression in IECs is regulated by both endogenous and exogenous stimuli [30,31]. For example, murine Tlr2 and Nod2 expression is upregulated after microbial stimulation [32,33], but species-specific differences in receptor regulation clearly exist [27]. In addition, developmentally regulated alterations in IEC immune receptor level have been reported for Tlr3, Tlr4 and Tlr9 during the pre-and postnatal period, and contribute to age-dependent disease susceptibility in neonates [34,35].
Cell polarization and receptor compartmentalization
Epithelial cells in the intestine—similarly to the epithelium at other body sites—have a polarized phenotype. The expression of lateral cell–cell contacts divides the plasma membrane into apical and basolateral domains, with a distinct lipid and protein composition, facilitating site-specific exo- and endocytic processes and targeted membrane trafficking. The apical plasma membrane is exposed to the enteric microbiota, as well as to environmental and nutrient-derived stimuli, whereas the basolateral side—at least under physiological conditions—is largely protected from direct microbial encounter. Not surprisingly, the cellular localization of innate immune receptors is also influenced by the polarized epithelial cell organization, possibly reflecting the exposure to microbial and environmental stimuli. For example, IEC Tlr5 expression is restricted to the basolateral side of polarized human colon epithelial cells, and murine colon mucosa is only stimulated by luminal flagellin exposure ex vivo after disruption of the epithelial barrier [36,37]. Such subcellular localization might prevent inappropriate stimulation by flagellin from commensal bacteria, but allow recognition after invasive infection. However, Tlr5 is expressed at the apical membrane of murine small intestine FAE cells and, thus, differences might exist between small intestinal and colon epithelium [38]. Tlr9 is found on both the apical and the basolateral membranes [38], but ligand binding initiates different cellular signalling in each compartment. Stimulation at the apical plasma membrane dampens epithelial cell activation and thus supports mucosal tolerance towards microbial exposure, whereas basolateral ligand exposure—and therefore the presence of bacterial DNA underneath the epithelial barrier—strongly stimulates proinflammatory chemokine secretion [39]. Of note, Tlr9 localization in macrophages is restricted to intracellular endosomal compartments and receptor stimulation requires ligand internalization, indicating that significant differences might exist between receptor stimulation and downstream signalling in macrophages and IECs. TLR2 and TLR4 have been detected at the apical epithelial plasma membrane of a human colon epithelial cell line [38,40]. Both receptors were shown to change cellular distribution on ligand stimulation, which might reflect their signalling from intracellular compartments [41,42]. In the small intestine, epithelial expression of TLR4 is restricted to an intracellular compartment and could predominate in the crypt region [32,43]. Stimulation requires ligand internalization, allowing differentiation between different kinds of LPS [44,45] and facilitating a sustained epithelial response [46]. However, it is important to keep in mind that immunohistological studies in primary tissue sections and functional assays have been hampered by the lack of sensitive and specific Tlr antibodies, as well as the extremely limited survival of isolated primary intestinal epithelial cells under in vitro culture conditions. Nevertheless, the evidence suggests that cellular localization of TLRs significantly contributes to their functional role. Other functionally relevant innate immune receptors expressed by IECs, such as NLRs and helicases, are cytosolic molecules. Ligand interaction might occur after cellular invasion by microorganisms, or through so far ill-defined cellular or microbial mechanisms of ligand translocation [47,48].
Epithelial polarization and compartmentalized receptor expression also contribute to restrict the recognition of endogenous soluble mediators. IECs in vivo are readily stimulated by type III IFN produced during viral infection, but fail to respond to type I IFN, despite expression of both the type III and type I IFN receptors. Lack of an epithelial type I IFN response seems to be caused by restriction of the responsive type I IFN receptor to the apical surface [49]. The close proximity and direct intercellular connections through gap junctions might allow yet another unique feature of the epithelial innate immune response. Epithelial cells communicate horizontally to forward the information of immune-receptor-mediated stimulation through the secretion and lateral diffusion of oxygen radicals, or the lateral spread of so far unidentified messenger molecules through gap junctions [50,51]. Interestingly, gap-junctional intercellular communication (GJIC) is promoted by Tlr2 through enhanced connexin-43 expression [52]. In addition, IL-17C secretion has been shown to forward epithelial innate immune stimulation in an autocrine manner to neighbouring cells [53,54]. Cell-to-cell communication thus allows a coordinated antimicrobial response on infection. It also opens up the possibility that immune recognition by individual ‘sensor cells’ might influence total epithelial gene expression.
Technical and methodological considerations
Significant differences in innate immune recognition have been noted between professional immune cells and IECs. A more in-depth analysis is needed to understand the biological features and functional significance of epithelial innate immune recognition (Sidebars A,B; [32,36,39,55,56]). Several cell lines from mouse, rat and human small and large intestine have been established, and their analysis has helped to reveal important aspects of epithelial cell biology. Their clonal character, however, does not reflect the complex composition of the intestinal epithelium, which contains distinct cell types and differentiation stages. On the other hand, the functional analysis of isolated primary IECs has been hampered by their rapid decrease in viability on in vitro culture. Promising new techniques have been reported that allow long-term culture of isolated epithelial cells forming crypt-like structures, including the generation of Paneth cells [57,58]. In vitro epithelial culture conditions, however, do not allow sustained exposure to commensal bacteria and fail to reflect the interaction with underlying immune cells. The use of chimeric mice generated by bone marrow transplantation has revealed the crucial role of innate immune signalling in tissue-resident stromal cells. However, these mice could contain radioresistant myeloid cells, and the complex composition of the so-called ‘non-haematopoietic’ or ‘stromal’ cell compartment precludes final conclusions on epithelial-cell-specific functions. Gene deletion and transgene expression under control of an epithelial-cell-specific promoter allow a more specific analysis under in vivo conditions (Fig 3). The results of in vivo studies on epithelial innate immune function using epithelial-specific gene-deficient or transgene mice are summarized in Table 2 and discussed below.
Sidebar A | In need of answers.
What is the functional outcome of epithelial innate immune stimulation under homeostatic conditions and after challenge?
Which mechanisms allow the discrimination between commensal and enteropathogenic bacteria by IECs?
How does the intestinal epithelium contribute to the establishment and maintenance of the enteric microbiota?
How do alterations of the microbiota affect epithelial homeostasis?
Sidebar B | Tools needed.
Improved breeding conditions to restrict uncontrolled microbiota-mediated effects
The development and improvement of ex vivo models of primary IEC culture
The use of cell-type-specific/conditional gene-deficient and transgene animal models
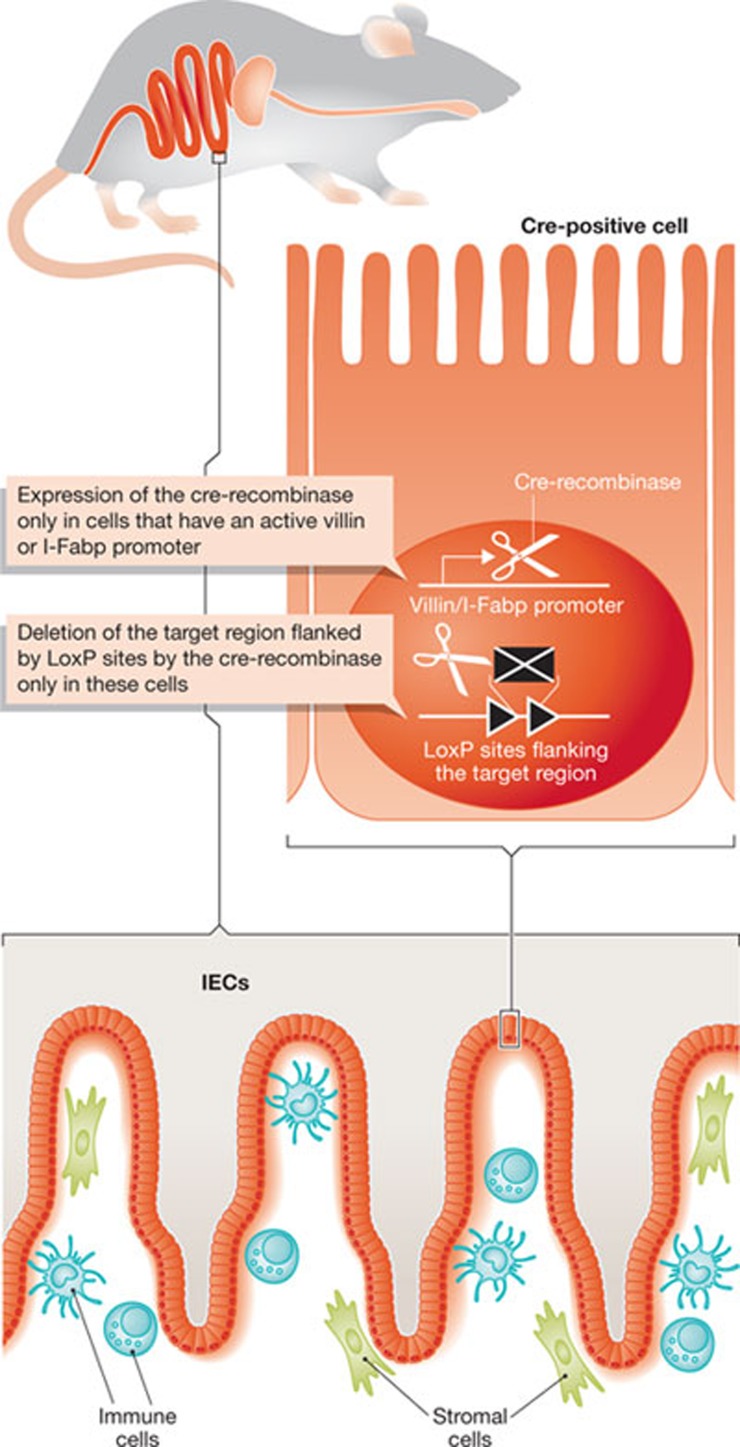
Figure 3.
Generation of IEC-specific gene-deficient animals. Transgenic mice carrying cre-recombinase (cre) under the control of the IEC-specific villin or I-Fabp promoter are cross-bred with mice that encode the target gene flanked by loxP sites. Expression of the cre-recombinase in IECs leads to excision of the region flanked by loxP sites in the target gene and thereby the generation of an IEC-specific gene-deficient mouse. IEC, intestinal epithelial cell; I-Fabp, intestinal fatty acid-binding protein.
Table 2. Intestinal epithelium-specific transgene mice.
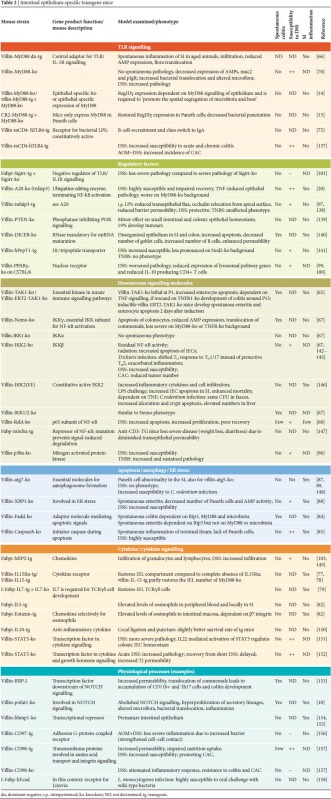
|
The most widely used promoter to target intestinal epithelial gene expression is that of the villin gene. Villin modulates the structure and assembly of actin filaments and contributes to the reorganization of the actin cytoskeleton elicited by stress conditions. Villin is a major structural component of the brush border of specialized absorptive cells and is mainly expressed in IECs. It is found in intestinal crypts and reaches its maximum level at the base of the villus, in which the cells become fully differentiated. Of note, expression is also found in the urogenital and respiratory tracts [59,60] and in cells derived from the embryonic gut—such as duct cells of the exocrine pancreas and biliary cells of the liver [61].
The villin promoter induces gene expression within the crypt epithelium and is maximal at the villus base, whereas expression under control of the second, frequently used, epithelial promoter, that of the intestinal fatty-acid-binding protein known as I-Fabp/Fabpi/Fabp2, is not observed in intestinal crypts and reaches its maximum at the villus tip. I-Fabp is involved in the cytoplasmic transport and metabolism of long-chain fatty acids, and belongs to a group of tissue-specific proteins involved in the uptake and transport of ligands to the site of metabolic processing. I-Fabp is highly expressed and represents 2–3% of the cytoplasmic protein content of the enterocyte [62]. Increasing expression levels are found along the proximal–distal axis of the small intestine, whereas low levels are observed in the caecum and even lower levels in murine colon epithelium [63]. Both I-Fabp and villin are expressed during fetal gut development, and transgene expression is already expected at birth. In addition to the villin and I-Fabp promoters, Paneth-cell-specific gene expression under control of a human and mouse α-defensin promoter has been reported [13,64].
Maintenance of homeostasis and barrier integrity
What is the evidence for epithelial innate immune signalling and its biological significance (Sidebar A)? In contrast to professional immune cells, innate immune receptor stimulation occurs under homeostatic conditions in IECs but does not seem to mount a proinflammatory response, although the epithelium is constantly exposed to a certain amount of microbial innate immune stimuli. These are released from viable and dead members of the microbiota within the intestinal lumen, and are also present in food and drinking water, although the concentration of stimuli in the intestinal epithelium is unknown. Fig 4A provides an overview of the mechanisms and functional significance of epithelial innate immune signalling.
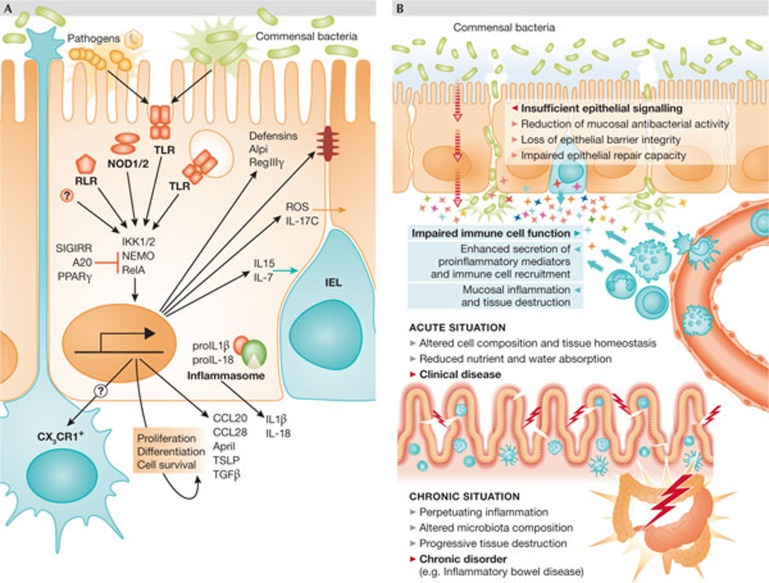
Figure 4.
Innate immune signalling in IECs. (A) Both pathogenic and commensal microorganisms stimulate innate immune receptors in IECs, such as TLRs, NLRs and RLRs. Gene expression is induced among other pathways through IKK1 and 2, NEMO and RelA/p65. Inflammasome activation leads to pro-IL-1β and pro-IL-18 cleavage by caspase 1, resulting in IL-1β and IL-18 secretion. The functional relevance of the inflammasome in IECs has not formally been shown. Innate immune signalling in IECs is also controlled by negative regulatory factors, such as SIGIRR/TIR8, A20 and PPARγ. IEC stimulation leads to the secretion of antimicrobial effectors, such as defensins and RegIIIγ, as well as the immunomodulatory Alpi, reinforces tight junctions and drives intraepithelial communication through the production of ROS and IL-17C. In addition, it facilitates cross-talk to professional immune cells of the lamina propria, leading to the recruitment of IELs through the secretion of IL-15 and IL-7, and stimulating CX3CR1+ non-migratory phagocytes to sample the luminal content. Finally, epithelial cell differentiation, proliferation and survival is influenced by the secretion of a variety of soluble mediators, such as CCL20, CCL28, April, TSLP and TGF-β is stimulated. (B) When the intestinal epithelial barrier loses integrity, commensal bacteria can translocate to the subepithelial tissue, inducing the secretion of proinflammatory mediators and leukocyte recruitment. This, in turn, induces organ dysfunction, reduced nutrient and water absorption, and can lead to mucosal inflammation and clinical disease. If the situation becomes chronic, it can impair wound healing and contribute to the development of disorders such as inflammatory bowel disease.
Lack of innate immune signal transduction in mice with an epithelial-specific deficiency in central signalling molecules such as Nemo, Ikk1/2, Tak1 and p65/RelA, and overexpression of a dominant-negative form of MyD88 in the epithelium, leads to spontaneous barrier dysfunction associated with mucosal damage and inflammation [65,66,67,68]. These results argue in favour of the functional relevance of homeostatic epithelial signalling. However, significant differences in manifestation frequency, organ tropism and disease severity were reported, indicating that homeostatic epithelial cell function is regulated by a complex signalling network. Spontaneous inflammation and Tnf-dependent epithelial apoptosis were observed in the small and large bowel of epithelial-specific Tak1-deficient mice shortly after birth. Inducible deletion of epithelial Tak1 expression in adult mice also resulted in mucosal inflammation a few days after induction [65]. Similarly, spontaneous mucosal damage in both the small and large intestine was observed during early postnatal development in 10–15% of animals with epithelial deficiency in RelA, whereas most animals survived to adulthood. The reason for this surprising finding, as well as the characterization of the underlying mechanisms, deserves further investigation. Translocation of commensal bacteria was observed in epithelial-specific Nemo, Ikk1/2 knock-out and MyD88 dominant-negative mice and, therefore, inflammation might occur due to a lack of homeostatic signalling, epithelial barrier dysfunction and bacterial ligand translocation. In accordance, deficiency of epithelial Nemo and Ikk1/2 expression in a MyD88 knockout background does not lead to spontaneous inflammation [67], and MyD88 signalling in subepithelial myeloid immune cells promotes inflammation [56]. MyD88-mediated inflammation might involve the production and secretion of Tnf, because the phenotype of epithelial Nemo-, Tak1- and Ikk1/2-deficient mice was equally ameliorated in mice lacking Tnfr expression [65,67]. Notably, administration of TNF antibodies represents an established therapeutic option in patients with inflammatory bowel disease [69].
Of note, the contribution of individual microbial ligands and innate immune receptors has not been clearly identified, and no spontaneous inflammation has been observed in animals deficient for individual TLRs and other innate immune receptors. This raises the possibility that MyD88-dependent signalling through Il-1β, Il-18 and Il-33 receptors—or others so far unidentified that recognize endogenous or exogenous ligands—contributes to, or is largely responsible for, the phenotype observed in mice lacking central downstream signalling molecules. Interestingly, epithelial expression of the dominant-negative MyD88 isoform induces inflammation in the small intestine, whereas epithelial deficiency of Nemo and Ikk1/2 causes colitis [66,67]. In addition, small intestinal inflammation in MyD88 dominant-negative transgenic mice is only observed when they are older than 24 weeks, whereas Nemo, Ikk1 and Ikk2 IEC-deficient mice exhibit colitis already at 3 weeks. Finally, enhanced Tnfr-mediated epithelial apoptosis occurs in IEC Nemo-deficient animals, whereas in epithelium-specific MyD88 dominant-negative transgenic mice there is increased epithelial proliferation but not apoptosis. Although MyD88 dominant-negative transgene expression is highest in the small intestine, and each model uses a different way of impairing signalling, this striking difference could indicate that homeostatic epithelial signalling is elicited by different upstream events in the small intestine and colon epithelium. A report using murine epithelial-specific MyD88 knockout did not find spontaneous colitis, further complicating the issue. Nevertheless, these mice have reduced AMP and pIgR expression and a higher incidence of bacterial translocation, which is a similar—but milder—phenotype as compared with the MyD88 dominant-negative transgenic mice [70].
In contrast to the MyD88-induced proinflammatory signalling in subepithelial immune cells, MyD88-dependent signalling inthe non-haematopoietic cell compartment seems to provide protection from inflammation and drive tissue homeostasis [55,56]. Importantly, the homeostatic effect of epithelial MyD88-dependent signalling in adult chimeric mice might also involve other stromal cells, such as fibroblasts or endothelial cells. Nevertheless, IECs seem to have a significant role and a variety of epithelial factors could be implicated. For example, Tlr2-induced secretion of TFF3 by goblet cells promotes mucosal repair after transient damage [71]. Another example is the production of antimicrobial molecules—such as RegIIIγ—that contribute to the compartmentalized bacterial colonization of the intestinal lumen [14]. By using genetic means, Paneth cells and IECs were shown to express RegIIIγ in response to MyD88-mediated stimuli [13]. Paneth-cell-derived α-defensins are also reduced in MyD88 dominant-negative epithelial-specific transgene animals [66], and luminal administration of Tlr3 and Tlr9 ligands induces rapid Paneth cell degranulation and antimicrobial peptide secretion [22,72,73]. Similarly, Tlr4, Tlr5 and Tlr9 stimulation induces hormonal release by neuroendocrine cells [72]. In addition, Tlr2 stimulation of FAE cells was suggested to enhance cellular translocation of luminal antigen and recruitment of antigen-presenting cells [74]. Expression of a constitutively active Tlr4 mutant in the mouse intestinal epithelium increases B-cell recruitment and IgA synthesis, which is a well-established mechanism of mucosal barrier maintenance [75]. In addition, Tlr stimulation in the villus of the small intestine induces luminal sampling of subepithelial phagocytes [76]. IEC and microbiota-induced innate immune signalling might also influence the intestinal T-lymphocyte compartment. MyD88-dependent epithelial IL-15 secretion and expression of the IL-15 transpresenting receptor IL-15Rα contributes to the maintenance of the IEL compartment [77,78]. Similarly, transgene epithelial Il-7 expression restores extrathymic development of TCRγδ+ IELs in the intestine of Il-7-deficient mice [77,79]. Both Il-7 and Il-15 expression have previously been associated with microbial exposure [80,81]. Also, IEC-derived Il-5 or eotaxin enhance eosinophils resident in the gut, as shown in transgene animals [82], but the endogenous regulation of intestinal epithelial Il-5 and eotaxin expression under homeostatic conditions is ill-defined. Finally, NLRs seem to also significantly influence intestinal lymphoid tissue development, as microbial recognition by Nod1 in stromal cells—such as epithelial cells—crucially stimulates the formation of isolated lymphoid follicles in the intestinal mucosa [83].
In addition, epithelial signalling—potentially induced and modified by innate immune receptor stimulation—significantly contributes to cellular differentiation and survival. Epithelial-specific deletion of Fadd or caspase 8 leads to spontaneous inflammation in the small and large intestine [84,85]. Fadd/caspase 8-mediated proapoptotic signalling occurs downstream from Trif, an adaptor of Tlr3 and Tlr4 [86]. Interestingly, inflammation in the colon is dependent on MyD88, Rip3 and the presence of the microbiota and relies at least partly on Tnf, whereas small intestinal inflammation is MyD88 and flora-independent but requires Rip3 expression [84]. In addition, an absence of caspase 8 induces abnormal Paneth cell granule structures, as occurs in epithelial-specific Xbp1- and Atg5/7-deficient mice [85,87,88,89].
Together, these results suggest that microbiota-mediated sustained activation of epithelial innate immune signalling—through the production of antibacterial (such as RegIIIγ, α- and β-defensins and CRS peptides), anti-inflammatory (Tgf-β, Tslp and Alpi), and chemoattractive and immunomodulatory (Il-5/7/15, Ccl20/28 and April) mediators—might promote cell proliferation, maintenance of epithelial barrier integrity, IgA transcytosis and immune cell recruitment [22,66,90,91]. This would contribute to intestinal homoeostasis and tissue maturation.
Failure to maintain the complex functional and anatomical features of the intestinal epithelium reduces the antimicrobial, immunoregulatory and regenerative ability of the epithelial barrier and might allow translocation of commensal bacteria from the intestinal lumen to the subepithelial tissue (Fig 4B; [66,67,70]). Microbial stimulation of subepithelial immune cells promotes the secretion of proinflammatory mediators, inducing the recruitment of leukocytes [56]. Functional dysregulation and leukocyte infiltration in turn lead to organ dysfunction, reduced nutrient and water absorption and thereby the clinical signs of enterocolitis—watery or bloody diarrhoea and malabsorption. The permanent exposure of the inflamed mucosa to highly immunostimulatory ligands might impair wound healing and contribute to chronic mucosal inflammation, as seen for example in patients with chronic inflammatory bowel disease.
Epithelial barrier integrity after mucosal challenge
Not surprisingly, the lack in IECs of several genes involved in immune recognition, downstream signal transduction, cytokine signalling and cell physiology induces phenotypic alterations after challenge (Table 2). Therefore, more complex signalling is clearly required for epithelial repair and restitution of barrier integrity, as compared with maintenance of an established mucosa. In addition, a redundancy of mechanisms might exist to maintain epithelial barrier integrity. However, these experiments provide information of what occurs to mice bred under microbially restricted, so-called ‘specific pathogen free’ conditions, which might not be extensive to natural situations, such as that of humans intermittently exposed to enteropathogenic microorganisms.
The most frequently used model of experimental mucosal damage is the oral administration of DSS, a chemical agent that impairs the stability of the mucus layer and facilitates penetration of commensal bacteria. In addition, it damages the epithelial barrier directly [92,93]. Mice deficient in the prominent mucus constituent Muc2 suffer spontaneous colitis, emphasizing the adverse effect of mucus layer impairment [94]. DSS almost exclusively affects colon tissue, but minor changes have also been noted in the small intestine [95]. DSS-induced inflammation is aggravated in mice with epithelial deletion of the NF-κB subunits RelA/p65 or p38 MAPK [68,96], both of which are part of dominant signalling pathways downstream from Tlr/Il-1r stimulation. DSS also evidences the mucosal impairment in mice deficient in epithelial Ikk2, which—in contrast to epithelial Ikk1/Ikk2 double knockout animals—do not manifest a spontaneous phenotype [67]. Thus, epithelial NF-κB and MAPK signalling confer resistance to DSS-induced mucosal damage. These signalling pathways, however, require tight regulation, as the NF-κB inhibitor A20 (Tnfaip3) mediates protection during DSS-induced colitis; mice with an epithelial-specific deletion show enhanced susceptibility to, and delayed recovery from, DSS-mediated mucosal damage, and mice with epithelial-specific transgene expression exhibit increased protection from DSS-induced pathology [20,97]. Epithelial deficiency in Pparγ—which suppresses epithelial chemokine secretion in response to Tlr stimulation [98]—also aggravates mucosal inflammation after DSS administration [99,100]. In turn, specific expression of the Il-1r/Tlr antagonist Sigirr in the epithelium induces a less severe phenotype after DSS treatment compared with Sigirr-deficient animals [101]. Finally, mice overexpressing Tlr4 or the proinflammatory chemokine Mip2 (Cxcl2) in the epithelium are affected more significantly by DSS compared with wild-type littermates, although epithelial gene overexpression does not have a spontaneous phenotype [102,103]. In all, inappropriate or dysregulated epithelial Il-1r or TLR signalling worsens mucosal tissue inflammation and damage after DSS administration, adding support for a mainly beneficial outcome of epithelial MyD88-dependent cell signalling [55,56,104].
No epithelial-specific deletion of NLRs has been reported. Increasing evidence indicates, however, that NLRs significantly contribute to mucosal host defence. For example, Nod2 influences the expression of CRS antimicrobial peptides after Listeria monocytogenes infection, consistent with a stimulatory activity of the Nod2 ligand muramyl di-peptide on Paneth cell degranulation [24,105]. By using bone marrow transplanted chimeric mice, stromal Nod1 and Nod2 were shown to have a protective role after infection with enteropathogenic Citrobacter rodentium and Salmonella, and to contribute to CD4+ T lymphocyte activation and T helper 2 cell responses after vaccination [26,106]. These results are consistent with the significant contribution of non-haematopoietic cell signalling to protection after oral infection of chimeric bone marrow transplanted mice with Listeria monocytogenes and Citrobacter rodentium [55,107]. Nlrp6—which is expressed by IECs and colonic myofibroblasts [108]—is reported to be involved in shaping the microbiota and influences the susceptibility towards chemically induced mucosal inflammation [109]. The role of Nlrp3 during colitis is controversial: most groups have observed an increased susceptibility towards chemically induced colitis in Nlrp3-deficient mice, but there is one report of a major contribution of colonic epithelial Nlrp3 signalling, and another that ascribes protective Nlrp3 signalling to the haematopoietic cell compartment [110,111,112].
Innate immunity in the human intestinal epithelium
Little is known about intestinal epithelial gene expression in response to innate immune stimulation in humans. One study examined small intestinal biopsies from volunteers after oral ingestion of the commensal Lactobacillus plantarum and suggested a significant influence on mucosal homeostatic gene expression [113]. Individuals with genetic deficiency in NEMO and IκB-α suffer from recurrent diarrhoea and colitis, which was not alleviated after bone marrow transplantation in at least two NEMO-deficient patients, suggesting a contribution of non-haematopoietic cells to the clinical outcome [114]. By contrast, patients with MyD88 or IRAK4 deficiencies typically have upper respiratory infections, but no enteric symptoms [114]. In addition, humans homozygous for the ATG16L1 Crohn's disease risk allele have Paneth cell abnormalities similar to norovirus-positive, Atg16l1-hypomorph mice [115]. The expression of other human polymorphisms in genes such as NOD2 (CARD15), TLR4, MDR1, OCTN1/2, DLG5 and ICAM1—which are associated with an enhanced risk of inflammatory bowel disease—have also been suggested to cause an impairment of the epithelial barrier, but a detailed cell-type-specific analysis of the functional consequences of the identified risk alleles requires further investigation. For example, patients with Crohn's disease—including those with the associated polymorphism in the CARD15 locus—seem to benefit from haematopoietic cell transplantation [116]. Nevertheless, impairment of epithelial function, including dysregulated or abrogated innate immune signalling, might significantly affect intestinal mucosal homeostasis and promote an inflammatory response—as occurs in mice.
Outlook
Although much is known about the active role of epithelial innate immune stimulation in antimicrobial host defence and host–microbial homeostasis, many questions remain unresolved (Sidebar A). The impact of the enteric microbiota composition on host immune homeostasis and mucosal innate immune response requires careful control of the experimental conditions. Variations in the microbiota composition and other unknown factors between animal facilities could explain the discrepancies in the field. Efforts to establish a defined minimal but sufficient microbiota might help to improve the reproducibility and comparability, but will on the other hand reduce the physiological relevance of the results obtained.
In addition, communication between epithelial cells [50,51], synergistic action of epithelial cells with other cell types and the influence of regulatory circuits—such as the enteric nervous system and hormonal influences—require further attention. In vivo imaging techniques now allow high resolution visualization and will reveal unexpected features of the host–microbial interaction [117]. Finally, the use of advanced tissue culture techniques and cell-type-specific gene-deficient or transgene animals in combination with pharmacological, immunological and microbial models of mucosal challenge will improve our understanding of epithelial innate immune receptor expression and its functional relevance in antimicrobial host defence and mucosal homeostasis.
Acknowledgments
The work of M.H. and J.P. is supported by the German Research Foundation (Ho2236/8-1), the Federal Ministry of Education and Research (DLR 01GU0825) as well as the Collaborative Research Center SFB 621and SFB 900.
Footnotes
The authors declare that they have no conflict of interest.
References
- Qin J et al. (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464: 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins CL, Salzman NH (2011) Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 9: 356–368 [DOI] [PubMed] [Google Scholar]
- Hooper LV, Midtvedt T, Gordon JI (2002) How host–microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr 22: 283–307 [DOI] [PubMed] [Google Scholar]
- Stecher B et al. (2007) Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol 5: 2177–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endt K et al. (2010) The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog 6: e1001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K et al. (2011) Induction of colonic regulatory T cells by indigenous clostridium species. Science 331: 337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II et al. (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139: 485–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V et al. (2009) The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31: 677–689 [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N, Gaboriau-Routhiau V (2010) The immune system and the gut microbiota: friends or foes? Nat Rev Immunol 10: 724–733 [DOI] [PubMed] [Google Scholar]
- Guilmeau S, Flandez M, Bancroft L, Sellers RS, Tear B, Stanley P, Augenlicht LH (2008) Intestinal deletion of Pofut1 in the mouse inactivates notch signaling and causes enterocolitis. Gastroenterology 135: 849–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GC (2011) Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol 15: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDole JR et al. (2012) Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV (2008) Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host–microbial interface. Proc Natl Acad Sci USA 105: 20858–20863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV (2011) The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 334: 255–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifrin DA, McConnell RE, Nambiar R, Higginbotham JN, Coffey RJ, Tyska MJ (2012) Enterocyte microvillus-derived vesicles detoxify bacterial products and regulate epithelial–microbial interactions. Curr Biol 22: 627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J, Pütsep K, Chu H, Kays RJ, Bevins CL, Andersson M (2008) Regional variations in Paneth cell antimicrobial peptide expression along the mouse intestinal tract. BMC Immunol 9: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B et al. (2007) Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26: 812–826 [DOI] [PubMed] [Google Scholar]
- Shulzhenko N et al. (2011) Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med 17: 1585–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Wei H, Sun R, Tian Z (2007) Recognition of double-stranded RNA by TLR3 induces severe small intestinal injury in mice. J Immunol 178: 4548–4556 [DOI] [PubMed] [Google Scholar]
- Vereecke L, Sze M, McGuire C, Rogiers B, Chu Y, Schmidt-Supprian M, Pasparakis M, Beyaert R, van Loo G (2010) Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J Exp Med 207: 1513–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiando AM et al. (2010) Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol 189: 111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu MT (2010) Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10: 131–144 [DOI] [PubMed] [Google Scholar]
- Ogura Y et al. (2003) Expression of NOD2 in Paneth cells: a possible link to Crohn's ileitis. Gut 52: 1591–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA (2005) Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307: 731–734 [DOI] [PubMed] [Google Scholar]
- Geddes K, Rubino S, Streutker C, Cho JH, Magalhaes JG, Le Bourhis L, Selvanantham T, Girardin SE, Philpott DJ (2010) Nod1 and Nod2 regulation of inflammation in the Salmonella colitis model. Infect Immun 78: 5107–5115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes K et al. (2011) Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat Med 17: 837–844 [DOI] [PubMed] [Google Scholar]
- Lech M, Avila-Ferrufino A, Skuginna V, Susanti HE, Anders H-J (2010) Quantitative expression of RIG-like helicase, NOD-like receptor and inflammasome-related mRNAs in humans and mice. Int Immunol 22: 717–728 [DOI] [PubMed] [Google Scholar]
- Shimada K et al. (2012) Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36: 401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD (2010) The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32: 379–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo JJ, Forster S, Mansell A (2011) Toll-like receptors as interferon-regulated genes and their role in disease. J Interferon Cytokine Res 31: 13–25 [DOI] [PubMed] [Google Scholar]
- Lichtinger M, Ingram R, Hornef M, Bonifer C, Rehli M (2007) Transcription factor PU.1 controls transcription start site positioning and alternative TLR4 promoter usage. J Biol Chem 282: 26874–26883 [DOI] [PubMed] [Google Scholar]
- Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A (2002) Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med 195: 559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez O (2002) Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J Biol Chem 277: 41701–41705 [DOI] [PubMed] [Google Scholar]
- Gribar SC et al. (2009) Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol 182: 636–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott J et al. (2012) Age-dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathog 8: e1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL (2001) Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol 167: 1882–1885 [DOI] [PubMed] [Google Scholar]
- Rhee SH, Im E, Riegler M, Kokkotou E, O'brien M, Pothoulakis C (2005) Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc Natl Acad Sci USA 102: 13610–13615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot S, Wagner JS, Farrant S, Neutra MR (2006) TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J Immunol 176: 4275–4283 [DOI] [PubMed] [Google Scholar]
- Lee J et al. (2006) Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol 8: 1327–1336 [DOI] [PubMed] [Google Scholar]
- Cario E, Brown D, McKee M, Lynch-Devaney K, Gerken G, Podolsky DK (2002) Commensal-associated molecular patterns induce selective toll-like receptor-trafficking from apical membrane to cytoplasmic compartments in polarized intestinal epithelium. Am J Pathol 160: 165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A (2000) The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA 97: 13766–13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R (2008) TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-β. Nat Immunol 9: 361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Cava CF, Ishihara S, Rumi MA, Kawashima K, Ishimura N, Kazumori H, Udagawa J, Kadowaki Y, Kinoshita Y (2003) Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J Immunol 170: 3977–3985 [DOI] [PubMed] [Google Scholar]
- Hornef MW, Normark BH, Vandewalle A, Normark S (2003) Intracellular recognition of lipopolysaccharide by Toll-like receptor 4 in intestinal epithelial cells. J Exp Med 198: 1225–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr CU, Zenk SF, Chassin C, Pott J, Gütle D, Hensel M, Hornef MW (2009) O-antigen delays lipopolysaccharide recognition and impairs antibacterial host defense in murine intestinal epithelial cells. PLoS Pathog 5: e1000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin C, Kocur M, Pott J, Duerr CU, Gütle D, Lotz M, Hornef MW (2010) miR-146a mediates protective innate immune tolerance in the neonate intestine. Cell Host Microbe 8: 358–368 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F (2011) The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477: 1–7 [DOI] [PubMed] [Google Scholar]
- Viala J et al. (2004) Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol 5: 1–9 [DOI] [PubMed] [Google Scholar]
- Pott J, Mahlakõiv T, Mordstein M, Duerr CU, Michiels T, Stockinger S, Staeheli P, Hornef MW (2011) IFN-lambda determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci USA 108: 7944–7949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolowschiak T, Chassin C, Ben Mkaddem S, Fuchs TM, Weiss S, Vandewalle A, Hornef MW (2010) Potentiation of epithelial innate host responses by intercellular communication. PLoS Pathog 6: e1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper CA, Sorg I, Schmutz C, Tschon T, Wischnewski H, Kim ML, Arrieumerlou C (2010) Cell–cell propagation of NF-κB transcription factor and MAP kinase activation amplifies innate immunity against bacterial infection. Immunity 33: 804–816 [DOI] [PubMed] [Google Scholar]
- Ey B, Eyking A, Gerken G, Podolsky DK, Cario E (2009) TLR2 mediates gap junctional intercellular communication through connexin-43 in intestinal epithelial barrier injury. J Biol Chem 284: 22332–22343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Carrozzi V et al. (2011) IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol 12: 1159–1166 [DOI] [PubMed] [Google Scholar]
- Song X, Zhu S, Shi P, Liu Y, Shi Y, Levin SD, Qian Y (2011) IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat Immunol 12: 1151–1158 [DOI] [PubMed] [Google Scholar]
- Brandl K et al. (2010) MyD88 signaling in nonhematopoietic cells protects mice against induced colitis by regulating specific EGF receptor ligands. Proc Natl Acad Sci USA 107: 19967–19972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asquith MJ, Boulard O, Powrie F, Maloy KJ (2010) Pathogenic and protective roles of MyD88 in leukocytes and epithelial cells in mouse models of inflammatory bowel disease. Gastroenterology 139: 519–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootani A et al. (2009) Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med 15: 701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T et al. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265 [DOI] [PubMed] [Google Scholar]
- Ongeri EM, Anyanwu O, Reeves WB, Bond JS (2011) Villin and actin in the mouse kidney brush–border membrane bind to and are degraded by meprins, an interaction that contributes to injury in ischemia-reperfusion. Am J Physiol Renal Physiol 301: F871–F882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich E, Pringault E, Arpin M, Louvard D (1990) From the structure to the function of villin, an actin-binding protein of the brush border. Bioessays 12: 403–408 [DOI] [PubMed] [Google Scholar]
- Maunoury R, Robine S, Pringault E, Léonard N, Gaillard JA, Louvard D (1992) Developmental regulation of villin gene expression in the epithelial cell lineages of mouse digestive and urogenital tracts. Development 115: 717–728 [DOI] [PubMed] [Google Scholar]
- Ockner RK, Manning JA, Poppenhausen RB, Ho WK (1972) A binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science 177: 56–58 [DOI] [PubMed] [Google Scholar]
- Cohn SM, Simon TC, Roth KA, Birkenmeier EH, Gordon JI (1992) Use of transgenic mice to map cis-acting elements in the intestinal fatty acid binding protein gene (Fabpi) that control its cell lineage-specific and regional patterns of expression along the duodenal–colonic and crypt–villus axes of the gut epithelium. J Cell Biol 119: 27–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL (2003) Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422: 522–526 [DOI] [PubMed] [Google Scholar]
- Kajino-Sakamoto R, Inagaki M, Lippert E, Akira S, Robine S, Matsumoto K, Jobin C, Ninomiya-Tsuji J (2008) Enterocyte-derived TAK1 signaling prevents epithelium apoptosis and the development of ileitis and colitis. J Immunol 181: 1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J Xu J, Zhu W, Gao X, Li N, Li J (2010) Epithelial-specific blockade of MyD88-dependent pathway causes spontaneous small intestinal inflammation. Clin Immunol 136: 245–256 [DOI] [PubMed] [Google Scholar]
- Nenci A et al. (2007) Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446: 557–561 [DOI] [PubMed] [Google Scholar]
- Steinbrecher KA, Harmel-Laws E, Sitcheran R, Baldwin AS (2008) Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J Immunol 180: 2588–2599 [DOI] [PubMed] [Google Scholar]
- Rutgeerts P, van Assche G, Vermeire S (2004) Optimizing anti-TNF treatment in inflammatory bowel disease. Gastroenterology 126: 1593–1610 [DOI] [PubMed] [Google Scholar]
- Frantz AL, Rogier EW, Weber CR, Shen L, Cohen DA, Fenton LA, Bruno ME, Kaetzel CS (2012) Targeted deletion of MyD88 in intestinal epithelial cells results in compromised antibacterial immunity associated with downregulation of polymeric immunoglobulin receptor, mucin-2, and antibacterial peptides. Mucosal Immunol [Epub ahead of print] doi:; DOI: 10.1038/mi.2012.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky DK, Gerken G, Eyking A, Cario E (2009) Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology 137: 209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo M et al. (2007) Activation of enteroendocrine cells via TLRs induces hormone, chemokine, and defensin secretion. J Immunol 178: 4296–4303 [DOI] [PubMed] [Google Scholar]
- Rumio C, Sommariva M, Sfondrini L, Palazzo M, Morelli D, Viganò L, De Cecco L, Tagliabue E, Balsari A (2011) Induction of Paneth cell degranulation by orally administered toll-like receptor ligands. J Cell Physiol 227: 1107–1113 [DOI] [PubMed] [Google Scholar]
- Chabot SM, Chernin TS, Shawi M, Wagner J, Farrant S, Burt DS, Cyr S, Neutra MR (2007) TLR2 activation by proteosomes promotes uptake of particulate vaccines at mucosal surfaces. Vaccine 25: 5348–5358 [DOI] [PubMed] [Google Scholar]
- Shang L et al. (2008) Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology 135: 529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieppa M, Rescigno M, Huang AYC, Germain RN (2006) Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med 203: 2841–2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Tang C, Xun S, Yajima T, Takeda K, Yoshikai Y (2006) MyD88-dependent signaling for IL-15 production plays an important role in maintenance of CD8 alpha alpha TCR alpha beta and TCR gamma delta intestinal intraepithelial lymphocytes. J Immunol 176: 6180–6185 [DOI] [PubMed] [Google Scholar]
- Ma LJ, Acero LF, Zal T, Schluns KS (2009) Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8 IELs. J Immunol 183: 1044–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laky K, Lefrançois L, Lingenheld EG, Ishikawa H, Lewis JM, Olson S, Suzuki K, Tigelaar RE, Puddington L (2000) Enterocyte expression of interleukin 7 induces development of gammadelta T cells and Peyer's patches. J Exp Med 191: 1569–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalapour S, Deiser K, Sercan O, Tuckermann J, Minnich K et al. (2010) Commensal microflora and interferon-gamma promote steady-state interleukin-7 production in vivo. Eur J Immunol 40: 2391–2400 [DOI] [PubMed] [Google Scholar]
- Kaneko M, Mizunuma T, Takimoto H, Kumazawa Y (2004) Development of TCR alpha beta CD8 alpha alpha intestinal intraepithelial lymphocytes is promoted by interleukin-15-producing epithelial cells constitutively stimulated by gram-negative bacteria via TLR4. Biol Pharm Bull 27: 883–889 [DOI] [PubMed] [Google Scholar]
- Mishra A (2001) Enterocyte expression of the eotaxin and interleukin-5 transgenes induces compartmentalized dysregulation of eosinophil trafficking. J Biol Chem 277: 4406–4412 [DOI] [PubMed] [Google Scholar]
- Bouskra D, Brézillon C, Bérard M, Werts C, Varona R, Boneca IG, Eberl G (2008) Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456: 507–510 [DOI] [PubMed] [Google Scholar]
- Welz P-S et al. (2011) FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477: 330–334 [DOI] [PubMed] [Google Scholar]
- Günther C et al. (2011) Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature 477: 335–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KJ, Su X, Xu LG, Bin LH, Zhang J, Shu HB (2004) Mechanisms of the TRIF-induced interferon-stimulated response element and NF-kappaB activation and apoptosis pathways. J Biol Chem 279: 15652–15661 [DOI] [PubMed] [Google Scholar]
- Cadwell K, Patel KK, Komatsu M, Virgin HW, Stappenbeck TS (2009) A common role for Atg16L1, Atg5 and Atg7 in small intestinal Paneth cells and Crohn disease. Autophagy 5: 250–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser A et al. (2008) XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134: 743–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkopf N, Günther C, Martini E, Waldner M, Amann KU, Neurath MF, Becker C (2012) Lack of intestinal epithelial Atg7 affects Paneth cell granule formation but does not compromise immune homeostasis in the gut. Clin Dev Immunol 2012: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk PG, Hooper LV, Midtvedt T, Gordon JI (1998) Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev 62: 1157–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebel LN, Liberati DM (2011) Disparate effects of bacteria and Toll-like receptor-dependant bacterial ligand stimulation on immunoglobulin A transcytosis. J Trauma 70: 691–700 [DOI] [PubMed] [Google Scholar]
- Melgar S (2005) Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol 288: G1328–G1338 [DOI] [PubMed]
- Johansson MEV, Gustafsson JK, Sjöberg KE, Petersson J, Holm L, Sjövall H, Hansson GC (2010) Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE 5: e12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Sluis M et al. (2006) Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131: 117–129 [DOI] [PubMed] [Google Scholar]
- Yazbeck R, Howarth GS, Butler RN, Geier MS, Abbott CA (2011) Biochemical and histological changes in the small intestine of mice with dextran sulfate sodium colitis. J Cell Physiol 226: 3219–3224 [DOI] [PubMed] [Google Scholar]
- Otsuka M, Kang YJ, Ren J, Jiang H, Wang Y, Omata M, Han J (2010) Distinct effects of p38α deletion in myeloid lineage and gut epithelia in mouse models of inflammatory bowel disease. Gastroenterology 138: 1255–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee L et al. (2012) Expression of TNFAIP3 in intestinal epithelial cells protects from DSS but not TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol [Epub ahead of print] doi:; DOI: 10.1152/ajpgi.00077.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D, Glass C (2007) Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol 28: 1–8 [DOI] [PubMed] [Google Scholar]
- Adachi M (2005) Peroxisome proliferator activated receptor in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut 55: 1104–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra SK, Guri AJ, Climent M, Vives C, Carbo A, Horne WT, Hontecillas R, Bassaganya-Riera J (2010) Immunoregulatory actions of epithelial cell PPARγ at the colonic mucosa of mice with experimental inflammatory bowel disease. PLoS ONE 5: e10215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H et al. (2007) The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity 26: 461–475 [DOI] [PubMed] [Google Scholar]
- Fukata M et al. (2011) Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis-associated tumorigenesis. Inflamm Bowel Dis 17: 1464–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka Y, Lee J, Stamm DS, Sanderson IR (2001) MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut 49: 526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R (2004) Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241 [DOI] [PubMed] [Google Scholar]
- Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ (2000) Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 1: 113–118 [DOI] [PubMed] [Google Scholar]
- Magalhães JG et al. (2011) Nucleotide oligomerization domain-containing proteins instruct T cell helper type 2 immunity through stromal activation. Proc Natl Acad Sci USA 108: 14896–14901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeis SL, Bommarius B, Parkos CA, Sherman MA, Kalman D (2007) TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J Immunol 179: 566–577 [DOI] [PubMed] [Google Scholar]
- Normand S, Delanoye-Crespin A, Bressenot A, Huot L, Grandjean T, Peyrin-Biroulet L, Lemoine Y, Hot D, Chamaillard M (2011) Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc Natl Acad Sci USA 108: 9601–9606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav E et al. (2011) NLRP6 Inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145: 745–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Elinav E, Huber S, Booth CJ, Strowig T, Jin C, Eisenbarth SC, Flavell RA (2010) Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci USA 107: 21635–21640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen IC, TeKippe EM, Woodford RMT, Uronis JM, Holl EK, Rogers AB, Herfarth HH, Jobin C, Ting JP (2010) The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med 207: 1045–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota SA et al. (2010) NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis 17: 1359–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Baarlen P, Troost FJ, van Hemert S, van der Meer C, de Vos WM, de Groot PJ, Hooiveld GJ, Brummer RJ, Kleerebezem M (2009) Differential NF-kappaB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc Natl Acad Sci USA 106: 2371–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C et al. (2010) Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine (Baltimore) 89: 403–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell K et al. (2008) A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456: 259–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung Y, Geddes M, Storek J, Panaccione R, Beck PL (2006) Hematopoietic cell transplantation for Crohn's disease; is it time? World J Gastroenterol 12: 6665–6673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller AJ et al. (2012) Salmonella gut invasion involves TTSS-2-dependent epithelial traversal, basolateral exit, and uptake by epithelium-sampling lamina propria phagocytes. Cell Host Microbe 11: 19–32 [DOI] [PubMed] [Google Scholar]
- Garrity-Park MM, Loftus EV, Sandborn WJ, Bryant SC, Smyrk TC (2009) MHC class II alleles in ulcerative colitis-associated colorectal cancer. Gut 58: 1226–1233 [DOI] [PubMed] [Google Scholar]
- Kim TW, Park HJ, Choi EY, Jung KC (2006) Overexpression of CIITA in T cells aggravates Th2-mediated colitis in mice. J Korean Med Sci 21: 877–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson IR, Bustin SA, Dziennis S, Paraszczuk J, Stamm DS (2004) Age and diet act through distinct isoforms of the class II transactivator gene in mouse intestinal epithelium. Gastroenterology 127: 203–212 [DOI] [PubMed] [Google Scholar]
- Hershberg RM, Cho DH, Youakim A, Bradley MB, Lee JS, Framson PE, Nepom GT (1998) Highly polarized HLA class II antigen processing and presentation by human intestinal epithelial cells. J Clin Invest 102: 792–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier JKX, Balabanian S, Coffill CR, Stewart A, Pelletier L, Franks DJ, Gendron NH, MacKenzie AE (2007) Distribution of neuronal apoptosis inhibitory protein in human tissues. J Histochem Cytochem 55: 911–923 [DOI] [PubMed] [Google Scholar]
- Lu WG, Zou YF, Feng XL, Yuan FL, Gu YL, Li X, Li CW, Jin C, Li JP (2010) Association of NOD1 (CARD4) insertion/deletion polymorphism with susceptibility to IBD: a meta-analysis. World J Gastroenterol 16: 4348–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad JM et al. (2011) Commensal and probiotic bacteria influence intestinal barrier function and susceptibility to colitis in Nod1(−/−); Nod2(−/−) mice. Inflamm Bowel Dis [Epub ahead of print] doi:; DOI: 10.1002/ibd.22848 [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Yamazaki T, Kamada N, Tawaratsumida K, Kim YG, Núñez G, Inohara N (2011) Nucleotide-binding oligomerization domain 1 mediates recognition of Clostridium difficile and induces neutrophil recruitment and protection against the pathogen. J Immunol 186: 4872–4880 [DOI] [PubMed] [Google Scholar]
- Ogura Y et al. (2001) A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411: 603–606 [DOI] [PubMed] [Google Scholar]
- Kim Y-G, Kamada N, Shaw MH, Warner N, Chen GY, Franchi L, Núñez G (2011) The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity 34: 769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreau F et al. (2007) CARD15/NOD2 is required for Peyer's patches homeostasis in mice. PLoS ONE 2: e523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstiel P, Fantini M, Bräutigam K, Kühbacher T, Waetzig GH, Seegert D, Schreiber S (2003) TNF-α and IFN-γ regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology 124: 1001–1009 [DOI] [PubMed] [Google Scholar]
- Petnicki-Ocwieja T, Hrncir T, Liu Y-J, Biswas A, Hudcovic T, Tlaskalova-Hogenova H, Kobayashi KS (2009) Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci USA 106: 15813–15818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho FA, Nalbantoglu I, Aitken JD, Uchiyama R, Su Y, Doho GH, Vijay-Kumar M, Gewirtz AT (2012) Cytosolic flagellin receptor NLRC4 protects mice against mucosal and systemic challenges. Mucosal Immunol 5: 288–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho FA, Aitken JD, Gewirtz AT, Vijay-Kumar M (2010) TLR5 activation induces secretory interleukin-1 receptor antagonist (sIL-1Ra) and reduces inflammasome-associated tissue damage. Mucosal Immunol 4: 102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benko S, Magalhães JG, Philpott DJ, Girardin SE (2010) NLRC5 limits the activation of inflammatory pathways. J Immunol 185: 1681–1691 [DOI] [PubMed] [Google Scholar]
- Villani A-C et al. (2009) Common variants in the NLRP3 region contribute to Crohn's disease susceptibility. Nat Genet 41: 71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M (2010) Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 59: 1192–1199 [DOI] [PubMed] [Google Scholar]
- Chen GY, Liu M, Wang F, Bertin J, Núñez G (2011) A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J Immunol 186: 7187–7194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M et al. (2011) Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis-associated tumorigenesis. Inflamm Bowel Dis 17: 1464–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej LE et al. (2011) TNFAIP3 maintains intestinal barrier function and supports epithelial cell tight junctions. PLoS ONE 6: e26352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun DS et al. (2011) Intestinal epithelial-specific PTEN inactivation results in tumor formation. Am J Physiol Gastrointest Liver Physiol 301: G856–G864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna LB, Schug J, Vourekas A, McKenna JB, Bramswig NC, Friedman JR, Kaestner KH (2010) MicroRNAs control intestinal epithelial differentiation, architecture, and barrier function. Gastroenterology 139: 1654–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmasso G, Nguyen HTT, Ingersoll SA, Ayyadurai S, Laroui H, Charania MA, Yan Y, Sitaraman SV, Merlin D (2011) The PepT1–NOD2 signaling pathway aggravates induced colitis in mice. Gastroenterology 141: 1334–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan LJ, Eckmann L, Greten FR, Chae S, Li ZW, Myhre GM, Robine S, Karin M, Kagnoff MF (2004) IkappaB-kinasebeta-dependent NF-kappaB activation provides radioprotection to the intestinal epithelium. Proc Natl Acad Sci USA 101: 2452–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaph C et al. (2007) Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature 446: 552–556 [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M (2004) IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118: 285–296 [DOI] [PubMed] [Google Scholar]
- Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M (2003) The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med 9: 575–581 [DOI] [PubMed] [Google Scholar]
- Guma M, Stepniak D, Shaked H, Spehlmann ME, Shenouda S, Cheroutre H, Vicente-Suarez I, Eckmann L, Kagnoff MF, Karin M (2011) Constitutive intestinal NF-κB does not trigger destructive inflammation unless accompanied by MAPK activation. J Exp Med 208: 1889–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y et al. (2010) Epithelial NF-κB enhances transmucosal fluid movement by altering tight junction protein composition after T cell activation. Am J Pathol 176: 158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J, Nishiumi S, Fujishima Y, Masuda A, Shiomi H, Yamamoto K, Nishida M, Azuma T, Yoshida M (2012) Autophagy in the intestinal epithelium regulates Citrobacter rodentium infection. Arch Biochem Biophys 521: 95–101 [DOI] [PubMed] [Google Scholar]
- Ohtsuka Y (2003) Dextran sulfate sodium—Induced inflammation is enhanced by intestinal epithelial cell chemokine expression in mice. Pediatr Res 53: 143–147 [DOI] [PubMed] [Google Scholar]
- Rajan S et al. (2007) Intestine-specific overexpression of IL-10 improves survival in polymicrobial sepsis. Shock 29: 483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickert G et al. (2009) STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 206: 1465–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S, Zhang R, Denson L, Moriggl R, Steinbrecher K, Shroyer N, Lin J, Han X (2012) Enterocyte STAT5 promotes mucosal wound healing via suppression of myosin light chain kinase-mediated loss of barrier function and inflammation. EMBO Mol Med 4: 109–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata Y et al. (2012) Epithelial cell-intrinsic notch signaling plays an essential role in the maintenance of gut immune homeostasis. J Immunol 188: 2427–2436 [DOI] [PubMed] [Google Scholar]
- Harper J, Mould A, Andrews RM, Bikoff EK, Robertson EJ (2011) The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc Natl Acad Sci USA 108: 10585–10590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncan V et al. (2011) Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat Commun 2: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Wandel E, Wobus M, Schneider R, Amasheh S, Sittig D, Kerner C, Naumann R, Hamann J, Aust G (2010) Overexpression of CD97 in intestinal epithelial cells of transgenic mice attenuates colitis by strengthening adherens junctions. PLoS ONE 5: e8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HTT et al. (2011) CD98 expression modulates intestinal homeostasis, inflammation, and colitis-associated cancer in mice. J Clin Invest 121: 1733–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit M (2001) A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292: 1722–1725 [DOI] [PubMed] [Google Scholar]