Abstract
Purpose
Previous research has demonstrated relationships of social support with disease-related biomarkers in patients with ovarian cancer. However, the clinical relevance of these findings to patient outcomes has not been established. This prospective study examined how social support relates to long-term survival among consecutive patients with ovarian cancer. We focused on two types of social support: social attachment, a type of emotional social support reflecting connections with others, and instrumental social support reflecting the availability of tangible assistance.
Patients and Methods
Patients were prospectively recruited during a presurgical clinic visit and completed surveys before surgery. One hundred sixty-eight patients with histologically confirmed epithelial ovarian cancer were observed from the date of surgery until death or December 2010. Clinical information was obtained from medical records.
Results
In a Cox regression model, adjusting for disease stage, grade, histology, residual disease, and age, greater social attachment was associated with a lower likelihood of death (hazard ratio [HR], 0.87; 95% CI, 0.77 to 0.98; P = .018). The median survival time for patients with low social attachment categorized on a median split of 15 was 3.35 years (95% CI, 2.56 to 4.15 years). In contrast, by study completion, 59% of patients with high social attachment were still alive after 4.70 years. No significant association was found between instrumental social support and survival, even after adjustment for covariates.
Conclusion
Social attachment is associated with a survival advantage for patients with ovarian cancer. Clinical implications include the importance of screening for deficits in the social environment and consideration of support activities during adjuvant treatment.
INTRODUCTION
Epithelial ovarian cancers continue to have a high mortality risk despite advances in treatment regimens, with overall 5-year survival rates of 46% and rates of 28% for patients with metastatic disease.1 Despite growing recognition of molecular alterations in ovarian cancers, it is becoming clear that other considerations such as biobehavioral factors may also affect patient outcome.2 Research on cancer survivorship has highlighted the importance of the social environment in contributing to quality of life as well as morbidity and mortality.3,4 Social support, often defined as the degree of perceived satisfaction with social relationships, has been shown to have both direct effects on health outcomes and indirect effects that protect individuals from the negative influence of stress-related biologic processes.5–8 Several studies9–12 have demonstrated the importance of social networks and cancer survival, although not all findings have been consistent.13,14 Less is known about quality of perceived social support and cancer survival, particularly in ovarian cancer.
In previous work,15–20 we have found that patients with ovarian cancer with low levels of perceived social support show alterations in several disease-related biomarkers, including markers of inflammation, angiogenesis, invasion, and innate immunity, as well as cellular markers of gene expression. Specifically, patients with ovarian cancer with higher levels of perceived social support demonstrated lower levels of vascular endothelial growth factor,19 interleukin-6,20 and matrix metalloproteinase-9,18 particularly in the tumor microenvironment, and higher levels of natural killer cell activity in both peripheral blood mononuclear cells and in tumor-infiltrating lymphocytes.15 Conversely, low social support accompanied by a high level of depressive symptoms was characterized by transcriptional changes in the tumor that were suggestive of a proinflammatory fingerprint.17 Consistent with the hypothesis that beta-adrenergic signaling mediated these effects, intratumor norepinephrine levels were also found to be increased in tissues from patients with low levels of social support.16
The facet of social support most consistently linked to biologic markers in our previous work has been social attachment, a subtype of emotional support reflecting an individual's experience of emotional connection to others that provides a sense of well-being, intimacy, or security. Although social support has been associated with these intermediate markers of ovarian cancer progression, the clinical relevance of these findings has not yet been established by linking social environmental factors with patient outcomes. To define the clinical significance of previously observed links between social support and disease-related biomarkers, this prospective study examined the relationship of social attachment to long-term survival among consecutive patients with ovarian cancer. We hypothesized that patients reporting higher levels of social attachment at the time of diagnosis would have a survival advantage compared with patients with lower levels of social attachment. Secondary analyses examined the hypothesis that these effects would be independent of other social factors such as instrumental social support (availability of help, information, and advice from other people). Instrumental social support is thought to be valuable because it can increase feelings of control by providing information and practical assistance that may help patients cope more effectively with treatment and other cancer-related life stressors.3,21
PATIENTS AND METHODS
Patients
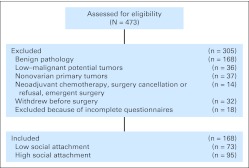
Women older than age 18 years with a newly diagnosed pelvic or abdominal mass suspected for ovarian cancer were potentially eligible for this research. Participation was confirmed following histologic diagnosis of a primary invasive epithelial ovarian, primary peritoneal, or fallopian tube carcinoma. Patients with previous cancer history, primary cancer of another organ, nonepithelial ovarian malignant tumors, systemic steroid medication in the last 4 months, or comorbidities known to alter the immune response (eg, autoimmune disorders) were excluded. This study was approved by institutional review boards of all participating institutions. The final sample included 168 women with epithelial ovarian cancer recruited between November 2003 and December 2009 from the University of Iowa, the University of Miami, and Mercy Medical Center in Miami, FL (Fig 1).
Fig 1.
CONSORT diagram showing patient recruitment.
Procedure
Patients were prospectively recruited during a presurgical clinic visit, and they completed questionnaires between the initial visit and surgery. Patients were observed from the time of surgery until death or December 2010. All surgeries were completed at least 1 year before the censor date. Patients were surgically staged according to the International Federation of Gynecologists and Obstetricians (FIGO) guidelines (stages I to IV). Tumor grade was assessed by pathology (low v high grade). Cytoreduction resulting in residual tumor less than 1 cm was considered optimal, and residual disease ≥ 1 cm was considered suboptimal. Following surgery, the majority of patients began adjuvant treatment with platinum and taxane combination chemotherapy and received six or more cycles of therapy. Psychosocial assessments were completed before surgery, at 6 months, and at 1 year.
Psychosocial Assessments
Social support/isolation.
The Social Provisions Scale (SPS) is a 24-item self-report scale measuring the extent to which social relationships are perceived as supportive.22 Items are rated on a 4-point response from “strongly agree” to “strongly disagree.” Six subscales represent facets of social support conceptualized as representative of distress-buffering features of social relationships. The two subscales included in this research were attachment, which assesses emotional closeness, and reliable alliance, which describes perception of relationships as being able to provide concrete assistance. Attachment was measured by items such as “I feel a strong emotional bond with at least one person” and “I have close relationships which give me a sense of emotional security and well-being.” The reliable alliance subscale was used as a measure of instrumental social support with items including “There are people I can count on in an emergency” and “If something went wrong, no one would come to my assistance” (reverse scored). The SPS has demonstrated adequate reliability and validity in several populations.22–24 Primary analyses examined social support factors as continuous variables since there are no externally validated cut points for these variables and because social support as a continuous variable has been associated with biologic variables in our previous work.15,16,18,19 For purposes of illustration and for defining cutoff points with potential clinical relevance, categorical analyses used a median split to define groups as high versus low in attachment. This dichotomy has been associated with biologic differences (eg, gene expression fingerprint associated with metastatic and proinflammatory transcriptional activity in primary ovarian cancers, and levels of the proinflammatory and proangiogenic cytokine interleukin-6 in previous work).17,20
Depression.
The Structured Clinical Interview for DSM-IV [Diagnostic and Statistical Manual of Mental Disorders, 4th Edition] Disorders (SCID-I)25 is a semistructured diagnostic interview used to assess patients for DSM-IV Axis I diagnostic disorders. Screening questions and the SCID interview were used to assess presence/absence of major depressive disorder (MDD) at the time of diagnostic surgery as well as history of previous MDD.26 Depression was examined as a covariate to determine whether social support effects could be accounted for by depression.
Demographic and Clinical Information
Demographic information was provided by self-report. Marital status was categorized as single; divorced, widowed, separated; or married/living with partner. Smoking was categorized as never versus ever smoker. Alcohol use was assessed as drinks per week. Clinical and histopathologic information was obtained from medical records. For analyses, stage was dichotomized into early (I to II) versus advanced (III to IV) stage. Date and cause of death were ascertained from patient medical records. Information for seven patients was ascertained through state death records. Survival time was calculated as the number of days between date of tumor resection and date of death or censoring.
Statistical Methods
Distributions were examined for outliers and non-normality. Examination of whether potential covariates differed between social attachment groups was performed by using independent sample t tests, Pearson χ2 tests, and Mantel-Haenszel χ2 tests. The univariate association of survival time with each potential clinical covariate and with social attachment as a continuous variable was examined by using Cox proportional hazards regression analysis.27 From the fitted Cox models, the unadjusted hazard ratio with 95% CIs was obtained, and significance of the association was tested by using the Wald χ2 statistic. The covariates that were tested included stage (advanced v early), grade (high v low), residual disease (suboptimal v optimal cytoreduction), body mass index (< 20, 20-< 25, 25-< 30, 30-< 40, ≥ 40); smoking (never smoker v ever smoker), education, histology (serous v nonserous), current and past MDD, and age. The covariates found to have a significant association (P < .05) with survival time (age, stage, grade, residual disease, and serous histology) were all included, along with social attachment, as independent variables in a multifactor Cox proportional hazards model to examine the effect of social attachment on time to death after adjusting for these covariates. Standard diagnostics were used to evaluate model adequacy.28 Similar models were examined for instrumental social support. To illustrate the effect of social attachment on survival time by a Kaplan-Meier curve, social attachment was dichotomized at the median of 15.29 Instrumental support and social attachment were entered together in a Cox model to examine the independent contribution of social attachment to survival rate. Ancillary analyses tested whether the effects of social attachment were independent of current depression and history of depression. Social attachment as a time-dependent covariate was used to test the ancillary hypotheses that postsurgery social support levels would be related to survival. Analyses were performed by using SPSS, version 19.0 (SPSS, Chicago, IL) and SAS, version 9.2 (SAS Institute, Cary, NC). The level of significance for all analyses was P < .05.
RESULTS
Participant Characteristics
Among the 168 patients enrolled onto this study, the median follow-up time was 2.72 years (range, 1 day to 6.88 years). At the time of diagnosis, 71% of patients had advanced-stage disease, and 86% had high-grade tumor (Table 1). Cause of death for the 63 patients who had died was persistent or recurrent ovarian cancer or complications associated with cancer disease and treatment (eg, bowel obstruction, sepsis, pulmonary emboli). One hundred three patients (61.3%) were still alive at the end of the observation period and were censored on December 15, 2010, for survival analyses. Patients who were low versus high in social attachment did not significantly differ with respect to marital status (P = .12), participation in support groups (P > .54 at any time-point), or on demographic characteristics (all P > .27; Appendix Table A1, online only).
Table 1.
Participant Characteristics
| Characteristic | No. of Patients | % | Mean | SD |
|---|---|---|---|---|
| Race | ||||
| American Indian/Alaska Native | 2 | 1.19 | ||
| Asian | 1 | 0.60 | ||
| Black | 4 | 2.38 | ||
| Pacific Islander | 0 | 0.00 | ||
| White | 161 | 95.83 | ||
| Ethnicity | ||||
| Non-Hispanic | 157 | 93.45 | ||
| Hispanic | 11 | 6.55 | ||
| Education | ||||
| High school or less | 65 | 39.16 | ||
| Some college or trade school | 57 | 34.34 | ||
| College degree | 30 | 18.07 | ||
| Advanced degree | 14 | 8.43 | ||
| Relationship status | ||||
| Separated/divorced/widowed | 40 | 23.81 | ||
| Single | 17 | 10.11 | ||
| Married/living with partner | 111 | 66.07 | ||
| Stage | ||||
| I | 36 | 21.69 | ||
| II | 12 | 7.23 | ||
| III | 102 | 61.45 | ||
| IV | 16 | 9.64 | ||
| Grade | ||||
| Low | 24 | 14.37 | ||
| High | 143 | 85.63 | ||
| Histology | ||||
| Nonserous | 42 | 25.15 | ||
| Serous | 125 | 74.85 | ||
| Residual disease | ||||
| Optimal (< 1 cm) | 44 | 73.81 | ||
| Suboptimal (≥ 1 cm) | 124 | 26.19 | ||
| Smoker | ||||
| Never | 111 | 66.07 | ||
| Ever | 57 | 33.93 | ||
| BMI category at diagnosis | ||||
| Underweight | 9 | 5.36 | ||
| Normal | 35 | 20.83 | ||
| Overweight | 65 | 38.69 | ||
| Obese | 52 | 30.95 | ||
| Morbidly obese | 7 | 4.17 | ||
| Social attachment | 168 | |||
| Median | 15.00 | |||
| Interquartile range | 13-16 | |||
| Instrumental social support | 168 | |||
| Median | 16.00 | |||
| Interquartile range | 14-16 | |||
| No. of chemotherapy cycles | 168 | 5.45 | 2.57 | |
| Age, years | 168 | 59.4 | 12.7 | |
Abbreviations: BMI, body mass index; SD, standard deviation.
Univariate Cox regression analyses indicated that advanced-stage disease (P < .001), high grade (P = .003), residual disease (P = .001), and age (P < .001) were significantly associated with shorter survival time (Table 2). Serous histology was marginally associated with shorter survival time (P = .056). Smoking history (P = .85), alcohol (P = .33), body mass index (P = .70), education (P = .25), current depression (P = .42), and past depression (P = .97) were not significantly associated with survival time. Higher levels of social attachment as a continuous variable were significantly associated with longer survival time (P = .002).
Table 2.
Unadjusted Cox Proportional HRs for Overall Survival in Patients With Ovarian Cancer (N = 168)
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Stage (advanced v early) | 11.66 | 3.64 to 37.35 | < .001 |
| Grade (high relative to low) | 8.37 | 2.04 to 34.30 | .003 |
| Histology (serous v nonserous) | 1.99 | 0.98 to 4.03 | .056 |
| Residual disease (suboptimal v optimal) | 2.42 | 1.47 to 3.97 | .001 |
| Education | |||
| Overall (referent: college/advanced degree) | .25 | ||
| High school or less | 1.40 | 0.75 to 2.61 | .29 |
| Some college or technical school | 0.87 | 0.44 to 1.72 | .69 |
| BMI at cancer diagnosis | |||
| Overall (referent: normal 20-< 25) | .70 | ||
| < 20 (underweight) | 0.98 | 0.32 to 3.02 | .98 |
| 25-< 30 (overweight) | 1.06 | 0.54 to 2.06 | .86 |
| 30-< 40 (obese) | 1.27 | 0.59 to 2.39 | .63 |
| ≥ 40 (morbidly obese) | 0.21 | 0.04 to 2.05 | .27 |
| Smoker (ever v never) | 1.05 | 0.62 to 1.77 | .85 |
| Current depression (present v absent) | 1.21 | 0.49 to 3.02 | .68 |
| Previous depression (present v absent) | 0.91 | 0.48 to 1.75 | .79 |
| Age (per 5 years) | 1.20 | 1.07 to 1.35 | < .001 |
| Alcohol use (per one drink) | 1.30 | 0.76 to 2.20 | .34 |
| Social attachment (per one unit) | 0.84 | 0.75 to 0.93 | .002 |
| Instrumental social support (per one unit) | 0.93 | 0.82 to 1.05 | .22 |
Abbreviations: BMI, body mass index; HR, hazard ratio.
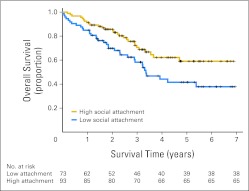
For purposes of illustration, Kaplan-Meier estimates based on a median split of social attachment showed that at the date of longest follow-up of 6.88 years, 59.1% (95% CI, 58.9% to 59.23%) of patients with high social attachment were still alive. In contrast, only 37.8% (95% CI, 37.66% to 37.94%) of patients with low social attachment were still alive (Fig 2). The median survival time for patients with low social attachment was 3.35 years (95% CI, 2.55 to 4.15 years). Because more than 50% of patients in the high social attachment group were still alive at the end of the study, with the last recorded death at 4.7 years, median survival time could not be calculated for this group.
Fig 2.
Survival time for patients with high social attachment (Social Provisions Scale attachment subscale ≥ 15) versus patients with low social attachment (score < 15). Cox regression adjusted for covariates indicates that patients with higher social attachment had longer survival times (P = .018). Numbers of at-risk (still alive) patients in the low versus high attachment groups are indicated below the x-axis.
In the Cox multivariate model, adjusting for disease stage, grade, histology, volume of residual disease, and age, greater social attachment was associated with a lower likelihood of death (adjusted hazard ratio [HR], 0.87; 95% CI, 0.77 to 0.98; P = .018; Table 3). Thus, for example, the adjusted HR of death for a patient whose score was at the 75th percentile of social attachment (score = 16) relative to a patient whose social support was at the 25th percentile (score = 13) was 0.66 (95% CI, 0.46 to 0.93) or a 34% lower hazard of death. To address the question of whether current or past MDD would affect the social attachment results, multivariate models were examined that included either current or past MDD in the model along with the other covariates. Neither past (P = .998) nor current (P = .43) MDD significantly predicted survival in the multivariate models, whereas social attachment remained significantly associated with survival in both models (past MDD model: P = .023; current MDD model: P = .037).
Table 3.
Cox Proportional Hazards Model for Overall Survival in Patients With Ovarian Cancer With Social Attachment Adjusted for Covariates
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Age at cancer diagnosis | 1.04 | 1.01 to 1.06 | .002 |
| High grade v low grade | 2.06 | 0.44 to 9.65 | .360 |
| Advanced stage v early stage | 7.02 | 1.92 to 25.58 | .003 |
| Serous histology v nonserous | 0.70 | 0.33 to 1.50 | .840 |
| Suboptimal residual disease v optimal | 1.76 | 1.04 to 2.97 | .035 |
| Social attachment at cancer diagnosis* | 0.87 | 0.77 to 0.98 | .018 |
Social attachment score is used as a continuous variable; thus hazard ratio (HR) is expressed per one unit increase in social attachment score. In multivariate analyses, n = 166 because of two patients who could not be staged.
Instrumental Social Support and Survival
Secondary analyses assessed whether attachment-related differences in survival might be attributable to differences in instrumental social support (Table 4 ). Instrumental social support, however, was not significantly associated with survival time by itself (P = .22) or with adjustment for covariates (P = .31; Tables 2 and 3). When both instrumental social support and social attachment were included in a Cox model, along with clinical covariates, social attachment remained significantly related to survival (adjusted HR, 0.85; 95% CI, 0.74 to 0.98; P = .025) but instrumental social support was not significant (adjusted HR, 1.03; 95% CI, 0.87 to 1.22; P = .73).
Table4.
Cox Proportional Hazards Model for Overall Survival in Patients With Ovarian Cancer With Instrumental Social Attachment Adjusted for Covariates
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Age at cancer diagnosis | 1.04 | 1.02 to 1.07 | .001 |
| High grade v low grade | 1.31 | 0.34 to 5.01 | .760 |
| Advanced stage v early stage | 8.88 | 2.08 to 37.88 | .003 |
| Serous histology v nonserous | 0.67 | 0.32 to 1.41 | .290 |
| Suboptimal residual disease v optimal | 1.68 | 0.99 to 2.84 | .053 |
| Instrumental social support at cancer diagnosis* | 0.94 | 0.83 to 1.06 | .310 |
Instrumental social support score is used as a continuous variable; thus, hazard ratio (HR) is expressed per one unit increase in instrumental social support score. In multivariate analyses, n = 166 because of two patients who could not be staged.
Social Support Over Time and Survival
To address the question of whether ongoing social support was related to study outcomes, we examined the continuous score of social attachment at the time of surgery, at 6 months, and at 1 year as a time-varying covariate. In this analysis, adjusting for the same covariates as in Table 3, social attachment remained a significant predictor of survival (P = .049).
DISCUSSION
The key finding of this prospective study was that social attachment is associated with a survival advantage for patients with ovarian cancer. This effect was significant when adjusted for disease-related covariates and predominately involved the subjective experience of supportive relationships as opposed to specific functional or instrumental social support. Furthermore, analyses examining changes in social attachment over the first year after diagnosis indicated that social attachment over time was related to survival as well. Instrumental social support was not significantly associated with survival time, either in univariate or multivariate analyses. These findings are the first to link a facet of the social environment with survival in a homogeneous ovarian cancer population and establish the clinical relevance of our previous reports linking social environmental factors with intermediate biomarkers in ovarian cancer.
These results extend findings from previous reports linking the presence of close emotional bonds with survival among women with breast cancer.30–35 In addition, these data are consistent with the minimal association of instrumental social support with survival that has previously been reported in other cancer populations,3 with a notable exception in acute myeloid leukemia.36 Several potential mechanisms may underlie these findings. The sympathetic nervous system, one of the major stress response systems, has been shown to promote ovarian cancer growth in preclinical models via beta adrenergic stimulation of angiogenesis, invasion, anoikis, and other metastatic processes.37–40 Similar processes have been observed in mammary cancer in vivo as well as in other tumor types in vitro.41–44 Moreover, social isolation has been linked to inflammatory leukocyte gene expression profiles and related upstream transcription control pathways in other populations.45–47 Glucocorticoids, secreted as the end product of the hypothalamic pituitary adrenal axis, a second stress response system, directly mediate processes promoting tumor growth as well,48–51 and may also serve to inhibit chemotherapy-induced apoptosis.52–54 Other neurohormones such as dopamine, epinephrine, prolactin, and so on are released as part of the stress response and may be implicated in these processes, although their effects have been less well characterized.55–57 Social support is thought to be most critical during times of stress, such as receipt of a cancer diagnosis.3 The social bond relationship in women has been described as a stress regulatory system in which attachment and caregiving behaviors downregulate the sympathetic nervous system and the hypothalamic pituitary adrenal axis.58 The proposed role of social support as a stress buffer3,5 would serve to blunt the stimulation of the major stress response systems, thus undercutting processes that would potentially mediate tumor growth. Consistent with the notion of stress buffering, we have found that patients with ovarian cancer with high levels of social attachment have lower levels of norepinephrine in both tumor and ascites at the time of surgery.16 In addition, social attachment behaviors have been shown to activate oxytocin,58 a known inhibitor of tumor growth59 in preclinical models.
Inclusion of depression in analyses did not change the relationship of social support and survival, suggesting that the relationship between social support and survival is not accounted for because of its association with either current or past history of depression.
The sources of social attachment and its survival benefits remain a significant topic for future research. We found no significant difference in marital relationship distributions across social attachment groups, suggesting that other objective social factors may play a more dominant role in shaping social attachment experiences. This likely stems from the highly subjective nature of social attachment, which is less strongly related to objective relationship status (eg, marital status) than are other parameters of social support, and which can also be substantially affected by nonmarital relationships (eg, friends, relatives, community relationships, and so on).58 Assessment of other nonmarital sources of social support is thus a significant topic for future research in ovarian cancer clinical outcomes.
This study is limited by the fact that we have no information regarding who the primary confidant of the patient is or whether the patient's needs for social support are adequately met. Although these findings are correlational, experimental studies of social isolation in a preclinical ovarian cancer model found faster tumor progression,37 suggesting a potential causal basis for these findings. Additional mechanistic work would be informative to shed light on social influences on tumor biology and disease progression.
Patients generally reported high levels of social attachment. Thus, if they indicated a lack of social support on any item, they would have fallen into the low social support group category. Clinically, this might suggest that if patients indicate moderate concern about an area of emotional social support, they might be considered to be at risk.
In conclusion, this study demonstrates that social attachment appears to confer a survival advantage to patients with ovarian cancer. Clinical implications of this finding include the importance of screening for deficits in the social environment in patients with ovarian cancer and consideration of support activities during adjuvant cancer care and beyond.
Acknowledgment
We thank Katherine Collins, Lauren Clevenger, Heena Maiseri, Madeline Godar, and David Villanueva for their assistance; we also thank the gynecologic oncology faculty and staff at all institutions and are grateful for the time and efforts of the patients who participated in this study.
Appendix
TableA1.
Characteristics of High and Low Social Attachment Groups
| Characteristic | Low Social Attachment (< 15)* |
High Social Attachment (≥15)* |
P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | Mean | SD | No. | % | Mean | SD | ||
| Race | .27 | ||||||||
| American Indian/Alaska Native | 2 | 2.74 | 0 | 0.00 | |||||
| Asian | 0 | 0.00 | 1 | 1.05 | |||||
| Black | 1 | 1.37 | 3 | 3.16 | |||||
| Pacific Islander | 0 | 0.00 | 0 | 0.00 | |||||
| White | 70 | 95.89 | 91 | 95.79 | |||||
| Ethnicity | .62 | ||||||||
| Non-Hispanic | 69 | 94.52 | 88 | 92.63 | |||||
| Hispanic | 4 | 5.48 | 7 | 7.37 | |||||
| Education | .87 | ||||||||
| High school or less | 29 | 40.28 | 36 | 38.30 | |||||
| Some college or trade school | 23 | 31.94 | 34 | 36.17 | |||||
| College degree | 15 | 20.83 | 15 | 15.96 | |||||
| Advanced degree | 5 | 6.94 | 9 | 9.57 | |||||
| Relationship status | .12 | ||||||||
| Separated/divorced, widowed | 19 | 26.03 | 21 | 22.10 | |||||
| Single | 11 | 15.07 | 6 | 6.32 | |||||
| Married/living with partner | 43 | 58.90 | 68 | 71.58 | |||||
| Stage | .12 | ||||||||
| I | 15 | 20.55 | 21 | 22.58 | |||||
| II | 3 | 4.11 | 9 | 9.68 | |||||
| III | 44 | 60.27 | 58 | 62.37 | |||||
| IV | 11 | 15.07 | 5 | 5.38 | |||||
| Grade | .11 | ||||||||
| Low | 7 | 9.59 | 17 | 18.09 | |||||
| High | 66 | 90.41 | 77 | 81.91 | |||||
| Histology | .56 | ||||||||
| Nonserous | 20 | 27.40 | 22 | 23.40 | |||||
| Serous | 53 | 72.60 | 72 | 76.60 | |||||
| Residual disease, cm | .27 | ||||||||
| Optimal (< 1) | 16 | 78.08 | 28 | 70.53 | |||||
| Suboptimal (≥ 1) | 57 | 21.92 | 67 | 29.47 | |||||
| Smoker | .65 | ||||||||
| Never | 46 | 63.0 | 65 | 68.42 | |||||
| Ever | 27 | 37.0 | 30 | 31.58 | |||||
| BMI category at diagnosis | .72 | ||||||||
| Underweight | 3 | 4.11 | 6 | 6.32 | |||||
| Normal | 16 | 21.92 | 19 | 20.00 | |||||
| Overweight | 25 | 34.25 | 40 | 42.11 | |||||
| Obese | 25 | 34.25 | 21 | 28.42 | |||||
| Morbidly obese | 4 | 5.48 | 3 | 3.16 | |||||
| Social attachment | < .001 | ||||||||
| Median | 13.00 | 16.00 | |||||||
| Interquartile range | 12-14 | 16-16 | |||||||
| Instrumental social support | < .001 | ||||||||
| Median | 13.00 | 16.00 | |||||||
| Interquartile range | 12-14 | 16-16 | |||||||
| Age, years | 62.7 | 13.3 | 56.9 | 11.6 | .003 | ||||
| No. of chemotherapy cycles | 5.42 | 3.07 | 5.48 | 2.13 | .89 | ||||
Abbreviations: BMI, body mass index; SD, standard deviation.
Patients with high social attachment had scores ≥ 15 on the Social Provisions Scale attachment subscale; patients with low social attachment had scores < 15 on the Social Provisions Scale attachment subscale.
Footnotes
Supported by Grants No. CA88293, CA104825, CA140933, CA109298, CA116778, and P50CA083639 from the National Cancer Institute.
Presented at the Annual Meeting of the Academy for Behavioral Medicine Research, Park City, UT, June 15-18, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Susan K. Lutgendorf, Koen De Geest, Frank J. Penedo, David M. Lubaroff, Steven W. Cole, Anil K. Sood
Financial support: Susan K. Lutgendorf
Administrative support: Susan K. Lutgendorf
Provision of study materials or patients: Susan K. Lutgendorf, Koen De Geest, David Bender, Amina Ahmed, Michael J. Goodheart, Frank J. Penedo, Joseph A. Lucci III, Parvin Ganjei-Azar, Premal H. Thaker, Luis Mendez
Collection and assembly of data: Susan K. Lutgendorf, David Bender, Amina Ahmed, Michael J. Goodheart, Joseph A. Lucci III, Parvin Ganjei-Azar, Premal H. Thaker, Luis Mendez, George M. Slavich
Data analysis and interpretation: Susan K. Lutgendorf, Laila Dahmoush, M. Bridget Zimmerman, Steven W. Cole, Anil K. Sood
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Atlanta, GA: American Cancer Society; 2010. American Cancer Society: Cancer Facts & Figures 2010. [Google Scholar]
- 2.Lutgendorf SK, Sood AK, Antoni MH. Host factors and cancer progression: Biobehavioral signaling pathways and interventions. J Clin Oncol . 2010;28:4094–4099. doi: 10.1200/JCO.2009.26.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helgeson VS, Cohen S. Social support and adjustment to cancer: Reconciling descriptive, correlational, and intervention research. Health Psychol . 1996;15:135–148. doi: 10.1037//0278-6133.15.2.135. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter KM, Fowler JM, Maxwell GL, et al. Direct and buffering effects of social support among gynecologic cancer survivors. Ann Behav Med . 2010;39:79–90. doi: 10.1007/s12160-010-9160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull . 1985;98:310–357. [PubMed] [Google Scholar]
- 6.Cohen S, Underwood LG, Gottlieb BH. New York, NY: Oxford University Press; 2000. Social Support Measurement and Intervention: A Guide for Health and Social Scientists. [Google Scholar]
- 7.Cutrona C, Russell D. Type of social support and specific stress: Toward a theory of optimal matching. In: Sarason BR, Sarason IG, Pierce GR, editors. Social Support: An Interactional View. New York, NY: Wiley; 1990. [Google Scholar]
- 8.House JS, Landis KR, Umberson D. Social relationships and health. Science . 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 9.Nausheen B, Gidron Y, Peveler R, et al. Social support and cancer progression: A systematic review. J Psychosom Res . 2009;67:403–415. doi: 10.1016/j.jpsychores.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Pinquart M, Duberstein PR. Associations of social networks with cancer mortality: A meta-analysis. Crit Rev Oncol Hematol . 2010;75:122–137. doi: 10.1016/j.critrevonc.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sprehn GC, Chambers JE, Saykin AJ, et al. Decreased cancer survival in individuals separated at time of diagnosis: Critical period for cancer pathophysiology? Cancer . 2009;115:5108–5116. doi: 10.1002/cncr.24547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroenke CH, Kubzansky LD, Schernhammer ES, et al. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol . 2006;24:1105–1111. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- 13.Lehto US, Ojanen M, Dyba T, et al. Baseline psychosocial predictors of survival in localised breast cancer. Br J Cancer . 2006;94:1245–1252. doi: 10.1038/sj.bjc.6603091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villingshøj M, Ross L, Thomsen BL, et al. Does marital status and altered contact with the social network predict colorectal cancer survival? Eur J Cancer . 2006;42:3022–3027. doi: 10.1016/j.ejca.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Lutgendorf SK, Sood AK, Anderson B, et al. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol . 2005;23:7105–7113. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Lutgendorf SK, De Geest K, Dahmoush L, et al. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun . 2010;25:250–255. doi: 10.1016/j.bbi.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutgendorf SK, De Geest K, Sung CY, et al. Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain Behav Immun . 2009;23:176–183. doi: 10.1016/j.bbi.2008.04.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutgendorf SK, Lamkin DM, Jennings NB, et al. Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clin Cancer Res . 2008;14:6839–6846. doi: 10.1158/1078-0432.CCR-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lutgendorf SK, Johnsen EL, Cooper B, et al. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer . 2002;95:808–815. doi: 10.1002/cncr.10739. [DOI] [PubMed] [Google Scholar]
- 20.Costanzo ES, Lutgendorf SK, Sood AK, et al. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer . 2005;104:305–313. doi: 10.1002/cncr.21147. [DOI] [PubMed] [Google Scholar]
- 21.House J, Kahn R, McLeod J, et al. Measures and concepts of social support. In: Cohen S, Syme SL, editors. Social Support and Health. New York, NY: Academic Press; 1985. pp. 83–108. [Google Scholar]
- 22.Cutrona CE. Social support and stress in the transition to parenthood. J Abnorm Psychol . 1984;93:378–390. doi: 10.1037//0021-843x.93.4.378. [DOI] [PubMed] [Google Scholar]
- 23.Cutrona CE, Russell DW. The provisions of social relationships and adaptation to stress. In: Perlman D, Jones WH, editors. Advances in Personal Relationships, Vol. 1. Greenwich, CT: JAI Press; 1987. pp. 37–67. [Google Scholar]
- 24.Russell D, Cutrona CE, Rose J, et al. Social and emotional loneliness: An examination of Weiss's typology of loneliness. J Pers Soc Psychol . 1984;46:1313–1321. doi: 10.1037//0022-3514.46.6.1313. [DOI] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, et al. New York, NY: New York State Psychiatric Institute; 1997. Structured Clinical Interview for DSM-IV Axis I Disorders-Non-patient Edition (SCID-I/NP, version 2.0-4/97 revision) [Google Scholar]
- 26.Williams JB, Gibbon M, First MB, et al. The Structured Clinical Interview for DSM-III-R (SCID): II. Multisite test-retest reliability. Arch Gen Psychiatry . 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 27.Cox DR. Regression models and life tables. J R Stat Soc B . 1972;34:187–220. [Google Scholar]
- 28.Singer JD, Willett JB. New York, NY: Oxford University Press; 2003. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. [Google Scholar]
- 29.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc . 1958;53:457–481. [Google Scholar]
- 30.Ell K, Nishimoto R, Mediansky L, et al. Social relations, social support and survival among patients with cancer. J Psychosom Res . 1992;36:531–541. doi: 10.1016/0022-3999(92)90038-4. [DOI] [PubMed] [Google Scholar]
- 31.Maunsell E, Brisson J, Deschênes L. Social support and survival among women with breast cancer. Cancer . 1995;76:631–637. doi: 10.1002/1097-0142(19950815)76:4<631::aid-cncr2820760414>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Burns CM, Craft PS, Roder DM. Does emotional support influence survival? Findings from a longitudinal study of patients with advanced cancer. Support Care Cancer . 2005;13:295–302. doi: 10.1007/s00520-004-0722-2. [DOI] [PubMed] [Google Scholar]
- 33.Marshall JR, Funch DP. Social environment and breast cancer: A cohort analysis of patient survival. Cancer . 1983;52:1546–1550. doi: 10.1002/1097-0142(19831015)52:8<1546::aid-cncr2820520835>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 34.Waxler-Morrison N, Hislop TG, Mears B, et al. Effects of social relationships on survival for women with breast cancer: A prospective study. Soc Sci Med . 1991;33:177–183. doi: 10.1016/0277-9536(91)90178-f. [DOI] [PubMed] [Google Scholar]
- 35.Hislop TG, Waxler NE, Coldman AJ, et al. The prognostic significance of psychosocial factors in women with breast cancer. J Chronic Dis . 1987;40:729–735. doi: 10.1016/0021-9681(87)90110-x. [DOI] [PubMed] [Google Scholar]
- 36.Pinquart M, Höffken K, Silbereisen RK, et al. Social support and survival in patients with acute myeloid leukaemia. Support Care Cancer . 2007;15:81–87. doi: 10.1007/s00520-006-0114-x. [DOI] [PubMed] [Google Scholar]
- 37.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and metastasis in a mouse model of ovarian carcinoma. Nat Med . 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 38.Landen CN, Jr, Lin YG, Armaiz Pena GN, et al. Neuroendocrine modulation of signal transducer and activator of transcription-3 in ovarian cancer. Cancer Res . 2007;67:10389–10396. doi: 10.1158/0008-5472.CAN-07-0858. [DOI] [PubMed] [Google Scholar]
- 39.Sood AK, Bhatty R, Kamat AA, et al. Stress hormone mediated invasion of ovarian cancer cells. Clin Cancer Res . 2006;12:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sood AK, Armaiz-Pena GN, Halder J, et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest . 2010;120:1515–1523. doi: 10.1172/JCI40802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sloan EK, Priceman SJ, Cox BF, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res . 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang EV, Donovan EL, Benson DM, et al. VEGF is differentially regulated in multiple myeloma-derived cell lines by norepinephrine. Brain Behav Immun . 2008;22:318–323. doi: 10.1016/j.bbi.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang EV, Kim SJ, Donovan EL, et al. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: Implications for stress-related enhancement of tumor progression. Brain Behav Immun . 2009;23:267–275. doi: 10.1016/j.bbi.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang EV, Sood AK, Chen M, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res . 2006;66:10357–10364. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 45.Miller GE, Chen E, Sze J, et al. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry . 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irwin MR, Wang M, Campomayor CO, et al. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med . 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 47.Cole SW, Hawkley LC, Arevalo JM, et al. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao XY, Malloy PJ, Krishnan AV, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med . 2000;6:703–706. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 49.Simon WE, Albrecht M, Trams G, et al. In vitro growth promotion of human mammary carcinoma cells by steroid hormones, tamoxifen, and prolactin. J Natl Cancer Inst . 1984;73:313–321. doi: 10.1093/jnci/73.2.313. [DOI] [PubMed] [Google Scholar]
- 50.Moran TJ, Gray S, Mikosz CA, et al. The glucocorticoid receptor mediates a survival signal in human mammary epithelial cells. Cancer Res . 2000;60:867–872. [PubMed] [Google Scholar]
- 51.Antonova L, Mueller CR. Hydrocortisone down-regulates the tumor suppressor gene BRCA1 in mammary cells: A possible molecular link between stress and breast cancer. Genes Chromosomes Cancer . 2008;47:341–352. doi: 10.1002/gcc.20538. [DOI] [PubMed] [Google Scholar]
- 52.Herr I, Ucur E, Herzer K, et al. Glucocorticoid cotreatment induces apoptosis resistance toward cancer therapy in carcinomas. Cancer Res . 2003;63:3112–3120. [PubMed] [Google Scholar]
- 53.Wu W, Chaudhuri S, Brickley DR. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res . 2004;64:1757–1764. doi: 10.1158/0008-5472.can-03-2546. [DOI] [PubMed] [Google Scholar]
- 54.Pang D, Kocherginsky M, Krausz T, et al. Dexamethasone decreases xenograft response to Paclitaxel through inhibition of tumor cell apoptosis. Cancer Biol Ther . 2006;5:933–940. doi: 10.4161/cbt.5.8.2875. [DOI] [PubMed] [Google Scholar]
- 55.Moreno-Smith M, Lu C, Shahzad MM, et al. Dopamine blocks stress-mediated ovarian carcinoma growth. Clin Cancer Res . 2011;17:3649–3659. doi: 10.1158/1078-0432.CCR-10-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sastry KS, Karpova Y, Prokopovich S, et al. Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. J Biol Chem . 2007;282:14094–14100. doi: 10.1074/jbc.M611370200. [DOI] [PubMed] [Google Scholar]
- 57.Sastry KS, Smith AJ, Karpova Y, et al. Diverse antiapoptotic signaling pathways activated by vasoactive intestinal polypeptide, epidermal growth factor, and phosphatidylinositol 3-kinase in prostate cancer cells converge on BAD. J Biol Chem . 2006;281:20891–20901. doi: 10.1074/jbc.M602928200. [DOI] [PubMed] [Google Scholar]
- 58.Taylor SE, Klein LC, Lewis BP, et al. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychol Rev . 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 59.Morita T, Shibata K, Kikkawa F, et al. Oxytocin inhibits the progression of human ovarian carcinoma cells in vitro and in vivo. Int J Cancer. 2004;109:525–532. doi: 10.1002/ijc.20017. [DOI] [PubMed] [Google Scholar]