Abstract
Aims
Alternative transcription and splicing of the allograft inflammatory factor-1 (AIF-1) gene results in the expression of two different proteins: AIF-1 and interferon responsive transcript-1 (IRT-1). Here, we explore the impact of AIF-1 and IRT-1 on vascular smooth muscle cell (VSMC) activation and neointima formation, the mechanisms underlying their alternative splicing, and associations of AIF-1 and IRT-1 mRNA with parameters defining human atherosclerotic plaque phenotype.
Methods and results
Translation of AIF-1 and IRT-1 results in different products with contrasting cellular distribution and functions. Overexpression of AIF-1 stimulates migration and proliferation of human VSMCs, whereas IRT-1 exerts opposite effects. Adenoviral infection of angioplasty-injured rat carotid arteries with AdAIF-1 exacerbates intima hyperplasia, whereas infection with AdIRT-1 reduces neointima. Expression of these variants is modulated by changes in nuclear factor of activated T-cells (NFAT) activity. Pharmacological inhibition of NFAT or targeting of NFATc3 with small interfering RNA (siRNA) lowers the AIF-1/IRT-1 ratio and favours an anti-proliferative outcome. NFAT acts as a repressor on the IRT-1 transcriptional start site, which is also sensitive to interferon-γ stimulation. Expression of AIF-1 mRNA in human carotid plaques associates with less extracellular matrix and a more pro-inflammatory plaque and plasma profile, features that may predispose to plaque rupture. In contrast, expression of IRT-1 mRNA associates with a less aggressive phenotype and less VSMCs at the most stenotic region of the plaque.
Conclusion
Inhibition of NFAT signalling, by shifting the AIF-1/IRT-1 ratio, may be an attractive target to regulate the VSMC response to injury and manipulate plaque stability in atherosclerosis.
Keywords: AIF-1, NFAT, Restenosis, Atherosclerosis, Vascular smooth muscle
1. Introduction
Vascular smooth muscle cell (VSMC) proliferation and migration after arterial injury results in occlusive neointima formation and plays a key role in the pathogenesis of restenosis after angioplasty, in-stent restenosis, allograft arteriopathy, and vascular bypass graft occlusion.1 Neointima hyperplasia remains the major clinical limitation to vascular interventions and to long-term graft and recipient survival after organ transplantation.2,3 A common sequence of events, involving production of growth factors and cytokines by injured endothelium and inflammatory cells, activates the normally quiescent VSMCs. Despite increased understanding of the pathogenesis of neointima formation, identification of novel molecular mechanisms that regulate VSMC migration and proliferation in response to injury and inflammation is necessary.
Allograft inflammatory factor-1 (AIF-1) and interferon responsive transcript-1 (IRT-1) are alternatively transcribed and spliced mRNAs encoded from the AIF-1 gene within the MHC class III region of chromosome 6, a region densely populated with immune and inflammatory response genes (i.e. tumour necrosis factor α/β, NF-κB). AIF-1 was originally cloned from activated macrophages in cardiac allografts undergoing transplant rejection.4 In the vasculature, AIF-1 protein increases after angioplasty and cytokine stimulation.5 Expression levels are predictive of transplant vasculopathy and lowered after immunosuppressive therapy.6 Overexpression of AIF-1 increases VSMC migration and proliferation, whereas inhibition of AIF-1 exerts the opposite effect.7,8 Recent work using AIF-1 transgenic mice demonstrated that enhanced AIF-1 expression also increases atherosclerotic lesions.9 Less is known regarding the role of the IRT-1 transcript, originally identified as an aberrant PCR product using AIF-1-specific primers and RNA from interferon-γ (IFNγ)-stimulated VSMCs.10 Overexpression of IRT-1 in human VSMCs seems to have anti-proliferative effects.10
The nuclear factor of activated T-cell (NFAT) family of transcription factors consists of four members (NFATc1–c4), originally described as activators of cytokine and immunoregulatory genes in T-cells.11 NFAT has been often implicated in the regulation of VSMC migration and proliferation,12 but the underlying mechanisms are far from clear. NFAT is readily activated by tyrosine kinase and G-protein-coupled receptor stimulation, which is known to promote cell growth.12 In a rat carotid artery injury model, blockade of NFAT signalling reduced neointima formation.13 Recent work also shows that NFATc3 promotes VSMC proliferation and the expression of the pro-inflammatory and atherogenic cytokines interleukin-6 (IL-6)14 and osteopontin.15
In this study, we describe differences in the genomic organization, in the 5′-untranslated region (UTR) and in the cellular distribution of AIF-1 and IRT-1. We compare the impact of AIF-1 and IRT-1 overexpression on neointima hyperplasia in vivo and provide evidence for the involvement of NFATc3 in the regulation of AIF-1 alternative splicing. Finally, we measure the expression levels of these transcripts in carotid plaques from patients undergoing endarterectomies to assess potential associations with plaque and clinical characteristics.
2. Methods
See Supplementary material online for an expanded version of this section.
2.1. Human resistance arteries
Intramyometrial arteries were used either directly after dissection or after organ culture.14 This study conforms to the principles outlined in the Declaration of Helsinki and was approved by Lund University ethics review board (#LU39-02). All women gave their informed consent to participate.
2.2. Cells
Human myometrial VSMCs were obtained from explants of myometrial arteries as described previously.14 Human coronary artery smooth muscle cells (HCASMC) were from Cascade Biologics. Rat arterial VSMCs were prepared from explants of thoracic aorta as described previously.16
2.3. Human carotid plaque biopsies
One hundred and fifty-eight plaques collected at carotid endarterectomies and corresponding plasma samples were analysed (see Supplementary material online, Table S1). Indications for surgery were plaques associated with ipsilateral symptoms [transient ischaemic attacks (TIA), stroke, or amaurosis fugax] and stenosis >70%, or plaques not associated with symptoms but stenosis >80%. Plaques were snap-frozen and weighed; cross-sectional fragments from the most stenotic region were taken for histology, adjacent fragments for RNA isolation, and the remaining plaque was homogenized as before17 for protein and cytokine analyses. The study conforms to the principles outlined in the Declaration of Helsinki and was approved by Lund University ethics review board (#472/2005-LUND). All patients gave their informed consent to participate.
2.4. In vitro transcription and translation
Expression of AIF-1 and IRT-1 proteins was performed by coupled transcription (T3 polymerase) and translation using [35S]Methionine in the TNT reticulocyte lysate system (Promega, Madison, WI, USA). cDNA samples were cloned into pBlueScript vector (Stratagene, La Jolla, CA, USA). Reactions were analysed on a 16.5% Tris-tricinepolyacrylamide gel (Biorad) and labelled proteins detected by autoradiography.
2.5. Confocal microscopy
Human VSMCs and myometrial artery sections were stained for AIF-1 and IRT-1 as previously described14 and samples examined on a Zeiss LSM 5 laser scanning confocal microscope.
2.6. Adenovirus construction
Adenoviral HA-tagged AIF-1 and green fluorescent protein (GFP) have been described previously.8 Recombinant adenovirus containing the IRT-1 gene was prepared using the AdEasy system in an identical way, with the IRT-1 coding region cloned into the shuttle vector pAdTrack-CMV.
2.7. In vivo gene transfer and vessel morphometry
Left common carotid artery balloon angioplasty was performed on male Sprague–Dawley rats as previously described,8 under sodium-pentobarbital anaesthesia [65 mg/kg, intraperitoneal (ip), Steris Laboratories, Phoenix, AZ, USA]. The depth of anaesthesia was assessed by the toe-pinch reflex procedure and absence of muscular tone. After injury, arteries were infected with adenoviral GFP, AIF-1 or IRT-1, or phosphate-buffered saline (PBS) for 15 min. Buprenorphine (0.02 mg/kg, subcutaneous) was given as analgesic pre- and 12 h post-surgery. Fourteen days later, rats were exsanguinated via the vena cava under sodium-pentobarbital anaesthesia (120 mg/kg, ip) and arteries harvested for morphometry. This study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and was approved by the ethics board at Temple University (#3374).
2.8. Proliferation and migration
Rat VSMCs were infected with adenoviral GFP, AIF-1, or IRT-1 genes and proliferation and migration performed as described previously.8 Cells were infected at 30 MOI for 2 h for all experiments as we have described.8 For human VSMCs, proliferation was measured using MTS-solution (CellTiter 96 AQueousOne Solution assay, Promega).
2.9. Western blot
Protein was extracted from VSMCs and human myometrial arteries as described previously.14 Primary antibodies against AIF-1, IRT-1, NFATc3 (sc-8321, Santa Cruz Biotechnology), GAPDH (Chemicon), and β-actin (GenScript Corporation, Piscataway, NJ, USA) and HRP-conjugated secondary antibodies were used and proteins visualized by chemiluminescence.
2.10. RNA isolation and RT–PCR
RNA was isolated from myometrial arteries, VSMCs, and carotid plaques followed by reverse transcription. cDNA was amplified using primers spanning regions common to both AIF-1 and IRT-1 splice variants and amplicons separated by agarose gel electrophoresis. For quantification, real-time PCR using TaqMan Gene Expression Assays (Applied Biosystems) and the comparative threshold method (delta Ct method) were used.
2.11. siRNA transfection
Human VSMCs were transfected with NFATc3 ON-TARGET plus SMART pool siRNA or siCONTROL Non-Targeting siRNA pool (Dharmacon), complexed with Lipofectamine 2000 (Invitrogen) as described previously.14
2.12. DNA constructs, transfections, and luciferase reporter assay
A region of the human AIF-1 gene 5′-flanking region spanning from −1529 to +231 relative to the AIF-1 translation start site was cloned into the pGL3 Basic Vector (Promega). Mutations were introduced using the QuikChange Site-Directed Mutagenesis Kit (Stratagene). HCASMC were transfected with plasmid DNA (wild-type or mutated AIF-1) or empty pGL3 basic vector (Promega Corporation) complexed with Lipofectamine 2000 (Invitrogen). Luminescence was measured as before.14
2.13. Cytokines and plaque immunohistochemistry
Cytokines were measured in aliquots of human carotid plaque homogenates or plasma (Milliplex Kit-Human Cytokine/Chemokine Immunoassay, Millipore, Electrabox Diagnostica AB, USA). Extracellular matrix components were determined as before.17 Human carotid plaque sections were stained for macrophages (CD68), smooth muscle cells (α-actin), lipids (Oil Red O), and collagen (Masson's trichrome).
2.14. Statistics
Results are expressed as means ± SD. Statistical analysis was performed using GraphPad (Prism4.0) or SPSS version 17.0. Statistical significance was determined using two-tailed Student's t-test or the Mann–Whitney non-parametric test, one-way analysis of variance followed by the Bonferroni test and bivariate correlation analysis (*P< 0.05, **P< 0.01, and ***P< 0.001).
3. Results
3.1. AIF-1 and IRT-1 are differentially spliced products of the AIF-1 gene
Several isoforms of the AIF-1 gene have been reported,18 the two most characterized being AIF-1 and IRT-1. A graphic organization of the gene is represented in Supplementary material online, Figure S1. AIF-1 is composed of six exons and five introns spanning a genomic region of ∼1800 nucleotides. The AIF-1 protein is translated from all six exons and results in a 143-amino acid protein of ∼16.6 kDa. In contrast, IRT-1 is composed of only two exons and one intron. The entire IRT-1 protein is derived from exon 2, which contains an open reading frame (ORF) of 399 nucleotides encoding a deduced 132-amino acid protein with a mass of ∼14.5 kDa.
3.2. IRT-1 mRNA contains an atypical 5′-UTR
To confirm translation of the ORF into the deduced gene products, AIF-1 and IRT-1 were expressed in vitro; either from cDNA containing only the ORF or from full-length cDNA containing both the ORF and UTRs (Figure 1A). For AIF-1, when the input sequence was from full-length 639 nt cDNA, expression levels were nearly identical to those obtained from the 429 nt ORF. In contrast, for IRT-1, using full-length cDNA yielded lower protein expression than when using the ORF alone. This suggests that at least in vitro, the 5′- and/or 3′-UTRs of IRT-1 decrease the efficiency of protein production. The secondary structure of the 74 nt long 5′-UTRs for AIF-1 had a predicted change in free energy of −26.3 kcal/mol, while for IRT-1, the 453 nt long 5′-UTR formed an extensive stem loop structure with a predicted change in free energy of −161.6 kcal/mol (see Supplementary material online, Figure S2). The IRT-1 5′-UTR further contained two short upstream ORFs (8 and 18 amino acids), which together with the longer more complex secondary structure may contribute to lower translation efficiency.19 Ninety per cent of vertebrate mRNAs contain 5′-UTRs that are 10–200 nt long, whereas mRNAs encoding growth factors, transcription factors, or proto-oncogenes often have longer 5′-UTRs.19
Figure 1.
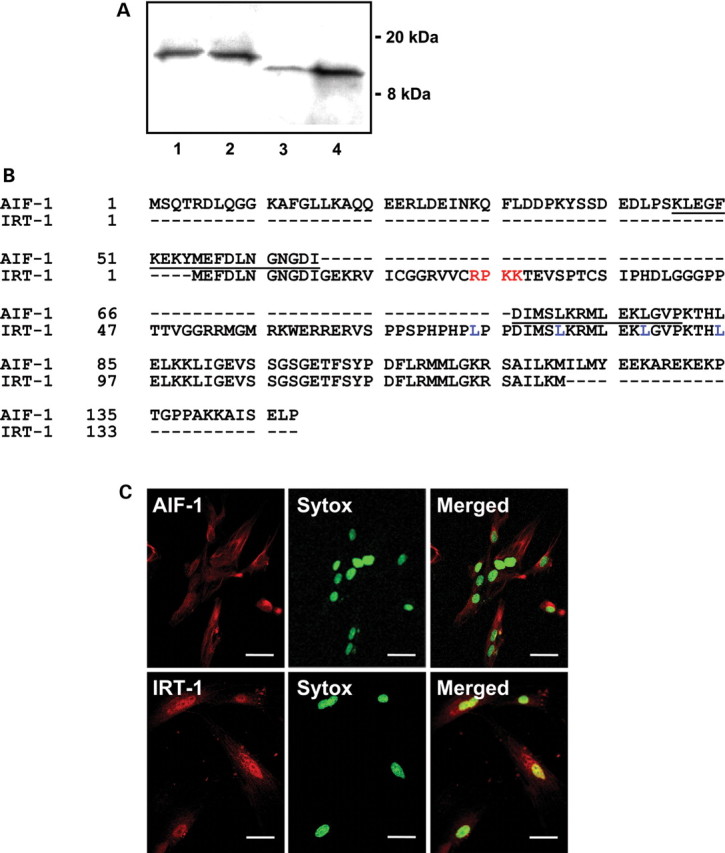
The IRT-1 protein contains a nuclear localization signal. (A) Coupled in vitro transcription and translation showing gene products expressed from 1 μg of AIF-1 full-length cDNA (lane 1) or ORF (lane 2) and IRT-1 full-length cDNA (lane 3) or ORF (lane 4). (B) Protein sequences for AIF-1 (upper line) and IRT-1 (lower line): calcium-binding EF-hand (underlined); core nuclear localization sequence (red) and leucine residues (blue) are shown. (C) Confocal immunofluorescence images of human VSMCs stained for AIF-1 (upper left panel, red), IRT-1 (lower left panel, red), and SYTOX Green (middle panels, green). Right panels show merged images and yellow indicates co-localization of IRT-1 with nuclear staining. Bars: 50 μm.
3.3. Differential cellular localization of AIF-1 and IRT-1
AIF-1 contains a functional EF-hand calcium-binding domain required for inducing VSMC proliferation and migration.5,20 This domain is disrupted in the IRT-1 protein (Figure 1B). Instead, IRT-1 contains a basic region immediately followed by a consensus leucine zipper motif, a four-amino acid core nuclear localization sequence, and a single, strongly hydrophobic region,10 all characteristics of transcription factors.21 Using confocal immunofluorescence microscopy, we found that IRT-1 is predominantly nuclear, whereas AIF-1 is mainly cytosolic (Figure 1C).
3.4. Opposing effects of AIF-1 and IRT-1 on neointima formation after arterial injury in vivo
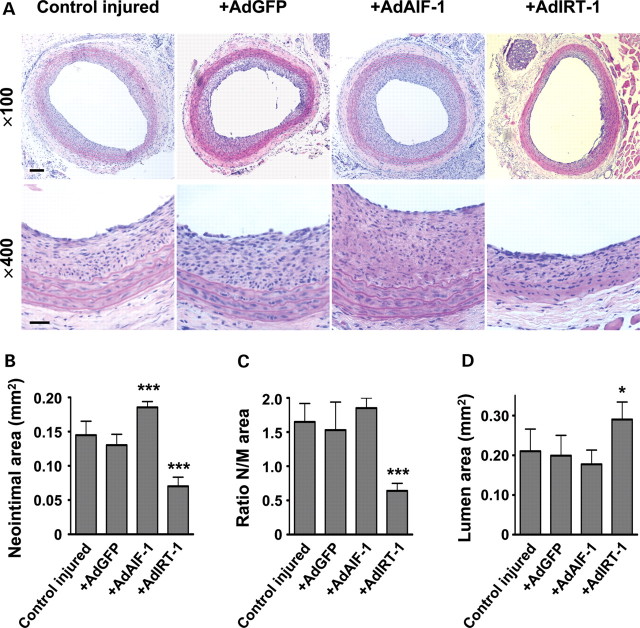
Rat carotid arteries were injured by balloon angioplasty and then infected with recombinant adenovirus encoding AIF-1 (AdAIF-1), IRT-1 (AdIRT-1), or GFP (AdGFP). PBS was used as a vehicle control. A distinct neointima was observed 14 days later in injured control arteries not exposed to adenovirus (Figure 2A). No neointima was formed in contralateral uninjured carotid arteries (data not shown). In arteries infected with AdAIF-1, neointima area was significantly larger when compared with AdGFP or control arteries (Figure 2B). Arteries infected with AdIRT-1 instead displayed reduced neointima area compared with AdGFP or vehicle (Figure 2B). The effect of AdIRT-1 also resulted in a reduced neointima/media ratio (Figure 2C) and larger lumen area (Figure 2A and D).
Figure 2.
AIF-1 exacerbates restenosis after angioplasty in the rat carotid artery, whereas IRT-1 reduces it. (A) The effect of adenoviral delivery of AIF-1, IRT-1, or GFP to balloon angioplasty-injured rat carotid arteries in vivo evaluated 14 days after injury in sections stained with haematoxylin–eosin. Scale bars = 150 and 50 µm, for upper and lower panels, respectively. Summarized data from morphometric analysis for (B) neointimal, (C) neointimal/media ratio, and (D) lumen area. *P< 0.05 and ***P< 0.001 vs. AdGFP, n= 6 rats/group.
To verify successful infections, AIF-1-HA, IRT-1, and GFP expression were examined (see Supplementary material online, Figure S3). HA protein was detected in AdAIF-1-infected arteries, whereas no signal was observed in sections from injured control and AdGFP- or AdIRT-1-infected arteries. HA-tagged AIF-1 was used to specify exogenous expression, since endogenous AIF-1 is expressed in the media and constitutively expressed in leucocytes.8 Despite non-specific binding of anti-GFP antibody to the adventitia of all vessels, only those that had been infected with AdGFP showed intense GFP expression in the neointima. IRT-1 protein was expressed only in vessels that had been infected with AdIRT-1.
3.5. Differential effect of AIF-1 and IRT-1 overexpression on VSMC proliferation and migration
Primary VSMCs were infected with AdAIF-1 or AdIRT-1 and proliferation was compared with cells that had been infected with AdGFP. Overexpression was confirmed by western blotting using antibodies against AIF-1 or IRT-1, respectively (Figure 3A). Equal numbers of cells were seeded into 12-well plates 24 h after transduction and cultured for 4 and 7 days in medium containing 10% foetal bovine serum (FBS). Overexpression of AIF-1 yielded increased cell number at day 7, while overexpression of IRT-1 had the opposite effect (Figure 3B). Infected VSMCs were also seeded into Boyden chambers and allowed to migrate towards a PDGF stimulus. Compared with AdGFP control cells, AIF-1 overexpression resulted in a larger number of migrated cells, whereas IRT-1 had the opposite effect (Figure 3C).
Figure 3.
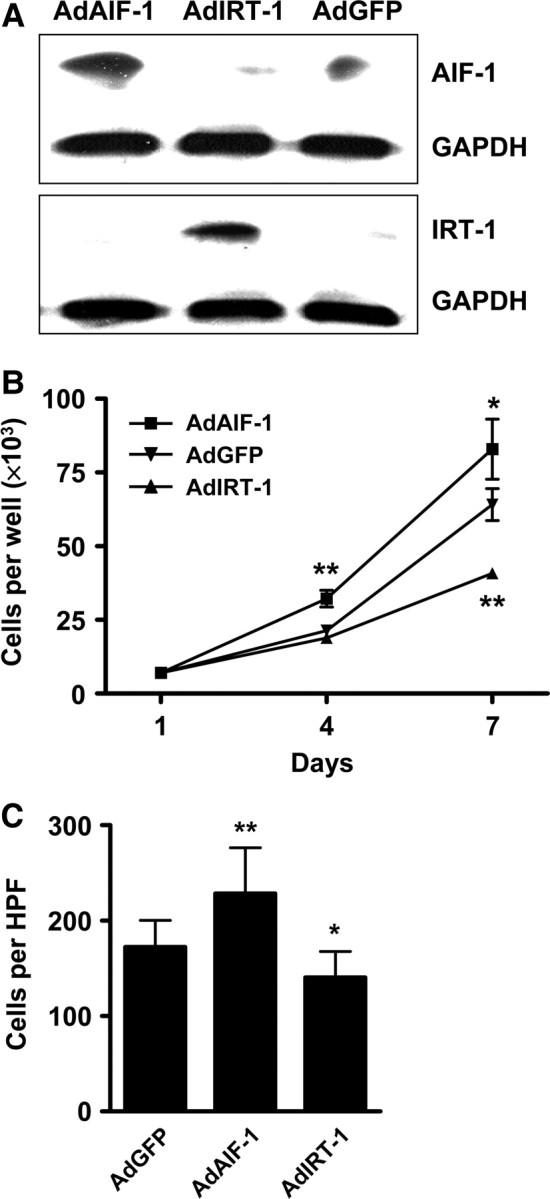
Opposite effects of AIF-1 and IRT-1 on VSMC proliferation and migration. (A) Western blots confirming overexpression of AIF-1 (upper panel) or IRT-1 (lower panel) in infected rat VSMCs. GAPDH was used to verify equal loading. (B) Effect of AdAIF-1 and AdIRT-1 overexpression on VSMC proliferation. Means are from three independent experiments performed in triplicate. (C) Migration of VSMCs infected with AdAIF-1, AdIRT-1, or AdGFP in response to PDGF-AB (40 ng) for 3 h. The mean number of migrated cells per high-power field (HPF) from three independently infected groups performed in triplicate are shown. *P< 0.05 and **P< 0.01 vs. AdGFP.
3.6. NFAT regulates AIF-1 and IRT-1 mRNA expression
Analysis of the murine and human AIF-1 and IRT-1 promoters reveals theoretical binding sites for a wide range of transcription factors;18 however, limited functional evidence is available. Sibinga et al.22 showed in murine macrophages that AIF-1 promoter activity was regulated by transcription factor binding to consensus Ets and interferon regulatory factor elements. In VSMCs, one transcription factor that has emerged as a regulator of cell proliferation and migration is NFAT.12,14 A search for NFAT consensus-binding elements (5′-T/AGGAAA-3′11) in the 5′-flanking region of AIF-1 and IRT-1 reveals the presence of four sites (see Supplementary material online, Figure S4).
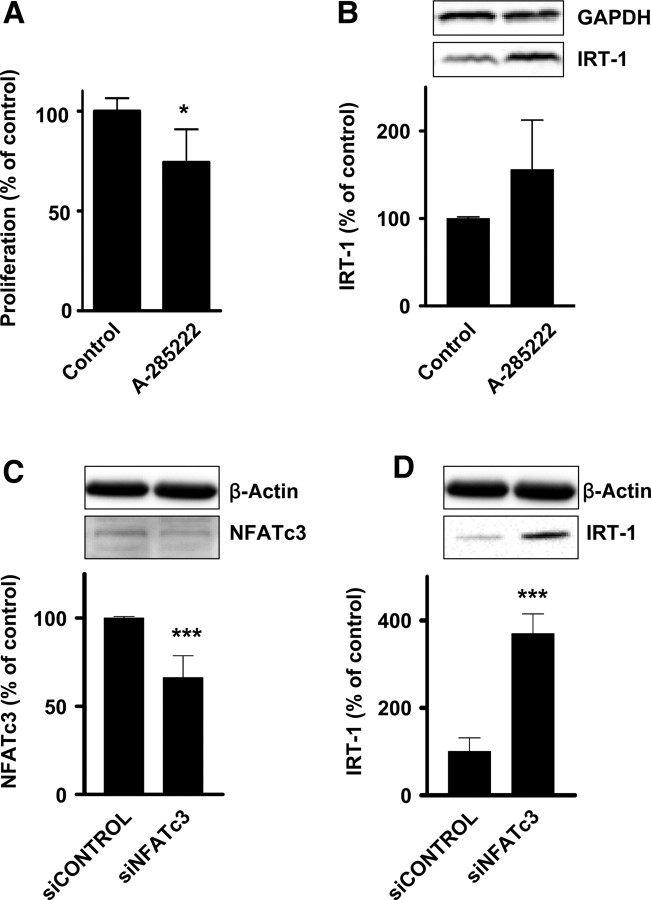
We next investigated whether changes in NFAT activity had any impact on the expression of AIF-1 and IRT-1, using primers that simultaneously detect both splice variants. Under basal conditions, AIF-1 mRNA is expressed in human VSMCs, whereas no or little IRT-1 mRNA is detected (Figure 4A). This is curious, since IRT-1 protein was detected in VSMCs under the same conditions (Figure 1C). Incubation with Et-1, which is a potent stimulus for NFAT activation in these cells,14 reduced the expression of AIF-1. This was prevented by the NFAT blocker A-285222. Et-1 stimulation had no apparent effect on IRT-1 levels; however, concomitant inhibition of NFAT increased expression of this transcript (Figure 4A). The same was true when VSMCs were cultured with A-285222 alone (see Supplementary material online, Figure S4). In contrast to cultured cells, both AIF-1 and IRT-1 transcripts were detected in freshly isolated human arteries, at apparently equal levels (Figure 4B). Upon organ culture of the arteries, which is associated with a well-characterized transition of the VSMCs towards a dedifferentiated phenotype,23 expression of these splice variants shifted, with a relative increase in pro-proliferative AIF-1 compared with IRT-1. Coincidentally, NFAT activity is increased during the culture of intact arteries, as well as the levels of IL-6.14 Inhibition of NFAT signalling with A-285222 prevented this shift, increasing instead the relative abundance of the anti-proliferative variant IRT-1 (Figure 4B). This effect of NFAT inhibition was further confirmed by real-time RT–PCR (Figure 4D) and was accompanied by increased IRT-1 protein as assessed by confocal immunofluorescence (Figure 4C). Changes in IRT-1 protein were already evident after 3 days of culture with A-285222, as assessed by confocal immunofluorescence and western blot (see Supplementary material online, Figure S5).
Figure 4.
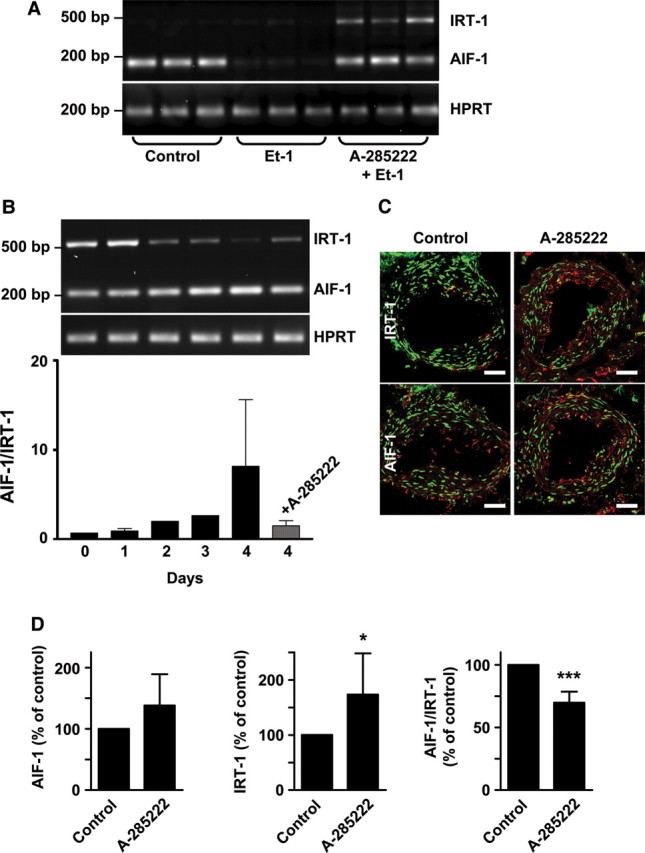
NFAT inhibition increases AIF-1 and IRT-1 expression. (A) Agarose gels showing expression of AIF-1, IRT-1, and HPRT in human VSMCs, incubated with or without Et-1 (10 nM) for 30 min in the presence or absence of A-285222 (1.0 μM). After stimulation, cells were further incubated in fresh medium for 18 h before RNA extraction. Samples were run in triplicates and experiments repeated twice using cells from different patients. (B) AIF-1 and IRT-1 mRNA expression in intact human myometrial arteries cultured for up to 4 days with or without A-285222 (1.0 μM). Primers detecting both AIF-1 and IRT-1 were used and the graph shows the ratio between AIF-1 and IRT-1. (C) Representative confocal images showing IRT-1 or AIF-1 protein expression (red) in sections from myometrial arteries that had been cultured for 4 days with or without A-285222 (0.1 µM). For each condition, sections from the same artery are shown in the upper and lower panels. Nuclei were stained with SYTOX Green. Scale bars = 50 µm (n= 4). (D) Summarized data from quantitative real-time PCR showing AIF-1 and IRT-1 mRNA expression in human myometrial arteries cultured for 4 days as in (B), normalized to HPRT and cyclophilin A and expressed as percentage of untreated control arteries. *P< 0.05 and ***P< 0.001 vs. control, n= 4–7/group.
3.7. NFAT inhibition leads to decreased proliferation and increased IRT-1 protein expression
Pharmacological inhibition of NFAT with A-285222 for 48 h reduced VSMC proliferation (Figure 5A). Under the same conditions, IRT-1 protein was increased compared with control cells (Figure 5B). To investigate the specific role of the NFATc3 isoform, siRNA against NFATc3 was used to down-regulate its expression (Figure 5C). IRT-1 protein expression was increased in cells expressing lower levels of NFATc3 (Figure 5D).
Figure 5.
NFATc3 inhibits IRT-1 expression. (A) Human VSMCs were incubated for 2 days in 15% FBS, with or without A-285222 (1.0 μM). Proliferation was measured by MTS assay and normalized to control (n= 4). (B) Representative western blot and summarized data from experiments showing IRT-1 protein levels in human VSMCs treated as in (A). Data were normalized to GAPDH and expressed as percentage of control (n= 4). (C and D) Summarized data from western blot experiments showing NFATc3 (C) and IRT-1 (D) expression in human VSMCs 5 days after transfection with siNFATc3 (100 nM). Representative blots from the same gel show simultaneous down-regulation of NFATc3 and up-regulation of IRT-1 expression. Data were normalized to β-actin and expressed as percentage of control (siCONTROL, n= 5). *P < 0.05 and ***P < 0.001 vs. controls.
3.8. Mutation of NFAT-binding site at the IRT-1 transcriptional start compromises IFNγ- and A-285222-induced transcription
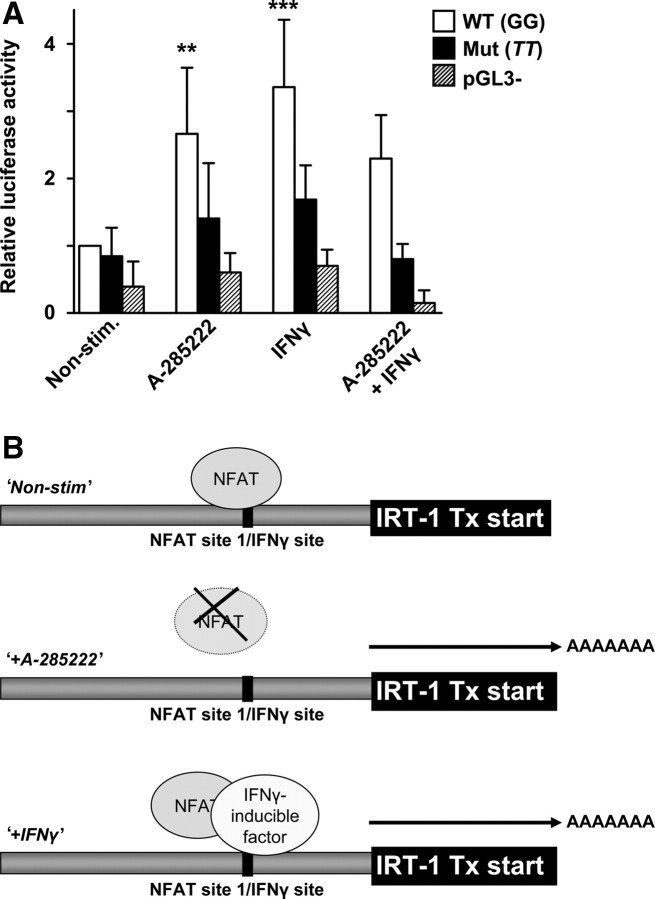
One of the four NFAT consensus-binding elements found in the AIF-1/IRT-1 promoter is located at the IRT-1 transcriptional start. To test its potential functional relevance, we used luciferase constructs driven by an ∼2 kb region of the AIF-1/IRT-1 promoter containing a mutation in this site (see Supplementary material online, Figure S3). Incubation of VSMCs carrying the wild-type construct with A-285222 resulted in enhanced luciferase activity, whereas the response was abrogated in cells transfected with the mutated construct (Figure 6A). This NFAT-binding site overlaps with a GAAANN motif, which is prevalent in IFN-induced genes.24 Since IRT-1 is strongly up-regulated by IFNγ,10 the functional relevance of this site for IFNγ-induced transcription was tested. Stimulation of VSMCs carrying the wild-type construct with IFNγ resulted in enhanced luciferase activity, whereas no response was observed in cells carrying the mutated one. Hence, under non-stimulated conditions, NFAT appears to act on this site as a transcriptional repressor of IRT-1. Incubation with A-285222 would release the block, allowing IRT-1 transcription. This site may also be important for IFNγ-induced IRT-1 transcription, probably via displacement of NFAT from this site, as suggested by the lack of synergy between A-285222 and IFNγ. A cartoon summarizing the proposed mechanism is depicted in Figure 6B.
Figure 6.
Inhibition of NFAT increases promoter activity. (A) HCASMC were transfected with plasmid DNA containing either the wild-type (WT) or mutated (mut) AIF-1/IRT-1 promoter, or empty pGL3 basic vector. After 24 h, transfected cells were stimulated with 0.5–1 µg/mL (10–20 000 U/mL) IFNγ, 1 µM A-285222 or IFNγ and A-285222 together. Non-stimulated transfected cells served as a control. After 6 h, cells were harvested and luciferase activity normalized to total protein content measured. Results are expressed relative to the untreated WT plasmid. The experiment was performed four times, each in triplicates. (B) Proposed mechanism underlying NFAT- and IFNγ-dependent regulation of the AIF-1/IRT-1 promoter. **P < 0.01 and ***P < 0.001 vs. non-stimulated WT.
3.9. Differential associations of AIF-1 and IRT-1 expression with human plaque phenotype
Human carotid plaques and plasma were collected from 158 patients undergoing endarterectomy to assess potential associations between plaque AIF-1 or IRT-1 mRNA expression with plaque and clinical characteristics. All patients had advanced atherosclerotic disease given that the indications for surgery were either ipsilateral symptoms (TIA, stroke, or amaurosis fugax) and stenosis >70%, or stenosis >80%. A brief clinical description of the patients is shown in Supplementary material online, Table S1.
Inflammation is pivotal in the development of arterial lesions and a high content of inflammatory cells and/or mediators is an indicator of plaque vulnerability.25 Here, we found a positive association between AIF-1 mRNA and levels of plaque macrophage inflammatory protein-1β and plasma RANTES. Instead, IRT-1 mRNA associated positively with plaque levels of the anti-inflammatory cytokine IL-10 and negatively with plasma monocyte chemoattractant protein-1 (MCP-1). Both transcripts exhibited positive associations with plaque IL-1β.
Analysis of extracellular matrix components in plaque homogenates revealed that AIF-1 levels were associated to lower collagen and elastin contents, whereas both transcripts showed negative associations with levels of glycosaminoglycans. Examination of histological sections from the most stenotic region of the plaque showed that both transcripts were positively associated with the size of the lipid pool (Oil Red O) and degree of macrophage infiltration (CD68) and negatively associated with collagen (Masson). Interestingly, we found a negative association between IRT-1 mRNA and VSMC content (α-actin), maybe reflecting a decreased VSMC proliferation in patients with high IRT-1 levels. Sections also showed a positive association between AIF-1 mRNA and elastin, in contrast to that found in plaque homogenates from below and above the most stenotic region. Further, AIF-1 mRNA levels were positively associated with blood haemoglobin and age. A complete list of the parameters analysed is shown in Supplementary material online, Table S2.
4. Discussion
This study describes the alternative transcription and splicing of the AIF-1 gene, which results in the expression of two different proteins: AIF-1 and IRT-1. These proteins have opposite impact on angioplasty-induced neointima formation and on VSMC activation. We demonstrate that expression of these AIF-1 gene products is modulated by changes in NFAT activity. Moreover, we found differential associations of AIF-1 and IRT-1 mRNA levels with parameters defining human atherosclerotic plaque phenotype.
The major findings are: (i) IRT-1 mRNA contains an atypical 5′-UTR which affects translational efficiency; (ii) IRT-1 is primarily nuclear as opposed to a predominantly cytosolic AIF-1; (iii) overexpression of the anti-proliferative splice variant IRT-1 reduces neointima formation after angioplasty, whereas overexpression of the pro-proliferative variant AIF-1 exerts the opposite effect; (iv) consensus NFAT-binding elements are present in the AIF-1/IRT-1 promoters; (v) changes in NFAT activity modulate AIF-1 and IRT-1 expression, with inhibition of NFAT resulting in a lowered AIF-1/IRT-1 ratio and favouring an anti-proliferative outcome; (vi) NFAT acts as a repressor on IRT-1 transcriptional start, a site also sensitive to IFNγ stimulation; and (vii) expression of AIF-1 mRNA in human carotid plaques associates with less extracellular matrix contents and a more pro-inflammatory plaque and plasma profile, features that may predispose to plaque rupture. Instead, expression of IRT-1 mRNA associates with a less aggressive phenotype and less VSMCs at the most stenotic region of the plaque. Thus, AIF-1 and IRT-1 seem to behave as yin and yang variants with opposite impact on neointima formation and atherosclerosis.
Clear differences were observed in the length and predicted secondary structure of the 5′-UTRs of AIF-1 and IRT-1. The long and highly structured IRT-1 5′-UTR and the presence of two upstream ORFs are sequence characteristics which can decrease translational efficiency and are shared by growth regulatory genes.19 A third attribute of IRT-1 is the long 3′-UTR containing an ATTTA sequence, typically found in the mRNA of cytokines and proto-oncogenes and thought to confer mRNA instability.10 The presence of AU-rich elements that can increase mRNA turnover via deadenylation and degradation may explain the discrepancy between IRT-1 mRNA and protein levels observed (Figure 4A vs. Figure 1C). IRT-1 mRNA is indeed unstable, with 50% degradation after 8.5 h and 75% degradation after 16 h.26 The described regulatory elements are consistent with a role for IRT-1 in the regulation of cell proliferation.
Fundamental differences are also found between AIF-1 and IRT-1 at the protein level. The structure of AIF-1 is of a cytoplasmic, calcium-binding signal transduction/scaffold protein.20 IRT-1 instead has sequence signatures of a nuclear, DNA-binding protein. At the present time, the structure/function relationships for AIF-1 and IRT-1 are uncharacterized and beyond the scope of the present study. However, these protein characteristics may explain the differences in their intracellular distribution.
Overexpression of AIF-1 and IRT-1 regulates VSMC proliferation and migration and this is likely one mechanism underlying the in vivo changes in neointima formation after balloon injury. We cannot rule out that other cell types in the vessel wall apart from VSMCs are infected during the incubation with the different adenoviruses, but the endothelium was almost completely removed after angioplasty and no inflammatory cells were observed at the time of infection, making this alternative explanation less likely. Furthermore, when ligation injury of the carotid artery was performed in transgenic mice in which AIF-1 expression is restricted to VSMCs (driven by a modified SM22α promoter), an enhanced response to injury was still obtained.7
NFAT activity has a pronounced inhibitory effect on IRT-1, which would suggest that NFAT acts as a repressor. Three of the NFAT-binding elements (NFAT sites 2–4) are located in a region that was described to have predominantly repressive effects on promoter activity.22 Abundant evidence supports the role of NFAT as a transcriptional activator, but a regulatory role for NFAT as suppressor is also emerging. An interesting example is NFATc2, which was shown in immune cells to suppress cyclin-dependent kinase 4 expression through binding to a site immediately downstream of the transcriptional start site.27 The promoter studies described here support the idea of NFAT acting as a repressor of IRT-1 at NFAT site 1, located immediately downstream of the IRT-1 transcriptional start site. This site may also be relevant for IFNγ-induced transcription of IRT-1. IFNγ has anti-proliferative actions on VSMC28,29 and inhibits angioplasty-induced arterial restenosis.28,30 It is possible that part of the anti-proliferative programme of IFNγ may involve regulation of AIF-1 alternative splicing to favour IRT-1 expression and that this utilizes the NFAT site 1 described here.
The opposite roles of AIF-1 and IRT-1 may not be just limited to the context of neointima formation. Their differential associations with atherosclerotic plaque phenotype and plasma parameters in patients undergoing endarterectomies suggest that they may also exert different roles in human atherosclerosis. Higher AIF-1 expression associated with a more pro-inflammatory profile than IRT-1, which instead associated positively with anti-inflammatory IL-10 in the plaque. Endogenous production of IL-10 in the vessel wall limits angiotensin II-mediated oxidative stress and vascular dysfunction and may account for the protective effects exerted by this cytokine.31
Collagen contents increase in parallel with the evolution of the lesions, but in advanced plaques as the ones examined here, extensive degradation of collagen occurs in the lipid and necrotic core, contributing to weakening and fragility.32 AIF-1 but not IRT-1 levels associated negatively with plaque collagen. A similar pattern was observed for elastin measured from plaque homogenates (i.e. patients with high expression of AIF-1 had lower elastin contents). Higher elastin breakdown results in loss of medial elasticity, increased luminal wall stress, and, subsequently, increased endothelial damage and predisposition to atherosclerosis.33 This is in line with recent work showing that AIF-1 transgenic mice had increased atherosclerosis and higher expression of the matrix metalloproteinases MMP2 and MMP9 in VSMCs,34 both strongly correlated with plaque instability.35 On the other hand, AIF-1 associated positively with histologically determined elastin contents from sections at the most stenotic region of the plaque. This is the area where VSMCs are most abundant and these cells are the main producers of elastin in the plaque.32 Results are intriguing, but at the moment, we cannot provide an explanation for the opposite relationships between AIF-1 and elastin at different sites of the plaque.
Additionally, a positive association between AIF-1 and blood haemoglobin was found. Just recently, the Apolipoprotein MOrtality RISk (AMORIS) Study including over 114 000 patients established high blood haemoglobin as a risk factor for major atherosclerotic cardiovascular events, including acute myocardial infarction, congestive heart failure, and stroke.36 Haemoglobin seems to contribute to plaque instability by triggering chemotaxis of monocytes and dendritic cells into the vascular wall.37 In summary, plaque expression of AIF-1 associates with a more inflammatory and rupture prone plaque phenotype, whereas IRT-1 associates with more beneficial parameters. Inhibition of the NFAT signalling pathway, by shifting the AIF-1/IRT-1 ratio, may be an attractive target to regulate the VSMC response to injury and manipulate plaque stability in atherosclerosis.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the Swedish Heart and Lung Foundation (HLF20070459 and HLF20080843 to M.F.G., HLF20090419 to I.G.); the Swedish Research Council (2006-5287 and 2009-4120 to M.F.G.; 2010-2932 to I.G.); the National Heart Lung, and Blood Institute (HL-63810 to M.V.A.); the American Heart Association (0455562U to M.V.A.), and the Roche Organ Transplant Research Foundation (146643428 to M.V.A.). Also by the Swedish Medical Society; Crafoord; Påhlsson; Hierta Memorial; Wiberg; Zoéga; Lundström; Lundgren; Nilsson; Segerfalk; M&M Wallenberg; K&A Wallenberg foundations; Royal Physiographic Society in Lund; Skåne Hospital Research Funds; Regional Research Funds; Swedish Society for Medical Research, Vascular Wall Programme at the Medical Faculty in Lund; and Lund University Diabetes Centre.
Supplementary Material
Acknowledgements
We thank Catarina Larsson, Mihaela Nitulescu, Ana Persson, and Marie Nilsson for technical assistance. A-285222 was kindly provided by Abbott Laboratories (Abbott Park, IL, USA).
Conflict of interest: none declared.
References
- 1.Crook MF, Akyurek LM. Gene transfer strategies to inhibit neointima formation. Trends Cardiovasc Med. 2003;13:102–106. doi: 10.1016/s1050-1738(02)00255-4. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu K, Mitchell RN. The role of chemokines in transplant graft arterial disease. Arterioscler Thromb Vasc Biol. 2008;28:1937–1949. doi: 10.1161/ATVBAHA.107.161232. [DOI] [PubMed] [Google Scholar]
- 3.Zargham R. Preventing restenosis after angioplasty: a multistage approach. Clin Sci (Lond) 2008;114:257–264. doi: 10.1042/CS20070228. [DOI] [PubMed] [Google Scholar]
- 4.Utans U, Arceci RJ, Yamashita Y, Russell ME. Cloning and characterization of allograft inflammatory factor-1: a novel macrophage factor identified in rat cardiac allografts with chronic rejection. J Clin Invest. 1995;95:2954–2962. doi: 10.1172/JCI118003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autieri MV. cDNA cloning of human allograft inflammatory factor-1: tissue distribution, cytokine induction, and mRNA expression in injured rat carotid arteries. Biochem Biophys Res Commun. 1996;228:29–37. doi: 10.1006/bbrc.1996.1612. [DOI] [PubMed] [Google Scholar]
- 6.Autieri MV, Kelemen S, Thomas BA, Feller ED, Goldman BI, Eisen HJ. Allograft inflammatory factor-1 expression correlates with cardiac rejection and development of cardiac allograft vasculopathy. Circulation. 2002;106:2218–2223. doi: 10.1161/01.cir.0000035652.71915.00. [DOI] [PubMed] [Google Scholar]
- 7.Sommerville LJ, Kelemen SE, Autieri MV. Increased smooth muscle cell activation and neointima formation in response to injury in AIF-1 transgenic mice. Arterioscler Thromb Vasc Biol. 2008;28:47–53. doi: 10.1161/ATVBAHA.107.156794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sommerville LJ, Xing C, Kelemen SE, Eguchi S, Autieri MV. Inhibition of allograft inflammatory factor-1 expression reduces development of neointimal hyperplasia and p38 kinase activity. Cardiovasc Res. 2009;81:206–215. doi: 10.1093/cvr/cvn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishima T, Iwabuchi K, Fujii S, Tanaka SY, Ogura H, Watano-Miyata K, et al. Allograft inflammatory factor-1 augments macrophage phagocytotic activity and accelerates the progression of atherosclerosis in ApoE-/- mice. Int J Mol Med. 2008;21:181–187. [PubMed] [Google Scholar]
- 10.Autieri MV, Agrawal N. IRT-1, a novel interferon-gamma-responsive transcript encoding a growth-suppressing basic leucine zipper protein. J Biol Chem. 1998;273:14731–14737. doi: 10.1074/jbc.273.24.14731. [DOI] [PubMed] [Google Scholar]
- 11.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Dronadula N, Rao GN. A novel role for nuclear factor of activated T cells in receptor tyrosine kinase and G protein-coupled receptor agonist-induced vascular smooth muscle cell motility. J Biol Chem. 2004;279:41218–41226. doi: 10.1074/jbc.M406917200. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Zhang C, Dronadula N, Li Q, Rao GN. Blockade of nuclear factor of activated T cells activation signaling suppresses balloon injury-induced neointima formation in a rat carotid artery model. J Biol Chem. 2005;280:14700–14708. doi: 10.1074/jbc.M500322200. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson LM, Sun Z-W, Nilsson J, Nordstrom I, Chen Y-W, Molkentin JD, et al. Novel blocker of NFAT activation inhibits IL-6 production in human myometrial arteries and reduces vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2007;292:C1167–C1178. doi: 10.1152/ajpcell.00590.2005. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson-Berglund LM, Zetterqvist AV, Nilsson-Ohman J, Sigvardsson M, Gonzalez Bosc LV, Smith ML, et al. Nuclear factor of activated T cells regulates osteopontin expression in arterial smooth muscle in response to diabetes-induced hyperglycemia. Arterioscler Thromb Vasc Biol. 2010;30:218–224. doi: 10.1161/ATVBAHA.109.199299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Autieri MV, Carbone CM. Overexpression of allograft inflammatory factor-1 promotes proliferation of vascular smooth muscle cells by cell cycle deregulation. Arterioscler Thromb Vasc Biol. 2001;21:1421–1426. doi: 10.1161/hq0901.095566. [DOI] [PubMed] [Google Scholar]
- 17.Goncalves I, Moses J, Dias N, Pedro LM, Fernandes e Fernandes J, Nilsson J, et al. Changes related to age and cerebrovascular symptoms in the extracellular matrix of human carotid plaques. Stroke. 2003;34:616–622. doi: 10.1161/01.STR.0000058157.69113.F6. [DOI] [PubMed] [Google Scholar]
- 18.Deininger MH, Meyermann R, Schluesener HJ. The allograft inflammatory factor-1 family of proteins. FEBS Lett. 2002;514:115–121. doi: 10.1016/s0014-5793(02)02430-4. [DOI] [PubMed] [Google Scholar]
- 19.Willis AE. Translational control of growth factor and proto-oncogene expression. Int J Biochem Cell Biol. 1999;31:73–86. doi: 10.1016/s1357-2725(98)00133-2. [DOI] [PubMed] [Google Scholar]
- 20.Autieri MV, Chen X. The ability of AIF-1 to activate human vascular smooth muscle cells is lost by mutations in the EF-hand calcium-binding region. Exp Cell Res. 2005;307:204–211. doi: 10.1016/j.yexcr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Amoutzias GD, Robertson DL, Van de Peer Y, Oliver SG. Choose your partners: dimerization in eukaryotic transcription factors. Trends Biochem Sci. 2008;33:220–229. doi: 10.1016/j.tibs.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Sibinga NES, Feinberg MW, Yang H, Werner F, Jain MK. Macrophage-restricted and interferon gamma-inducible expression of the allograft inflammatory factor-1 gene requires Pu.1. J Biol Chem. 2002;277:16202–16210. doi: 10.1074/jbc.M200935200. [DOI] [PubMed] [Google Scholar]
- 23.Hellstrand P. Long-term effects of intracellular calcium and growth factors on excitation and contraction in smooth muscle. Acta Physiol Scand. 1998;164:637–644. doi: 10.1111/j.1365-201x.1998.tb10707.x. [DOI] [PubMed] [Google Scholar]
- 24.Sims SH, Cha Y, Romine MF, Gao PQ, Gottlieb K, Deisseroth AB. A novel interferon-inducible domain: structural and functional analysis of the human interferon regulatory factor 1 gene promoter. Mol Cell Biol. 1993;13:690–702. doi: 10.1128/mcb.13.1.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Libby P, Aikawa M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med. 2002;8:1257–1262. doi: 10.1038/nm1102-1257. [DOI] [PubMed] [Google Scholar]
- 26.Del Galdo F, Maul GG, Jimenez SA, Artlett CM. Expression of allograft inflammatory factor 1 in tissues from patients with systemic sclerosis and in vitro differential expression of its isoforms in response to transforming growth factor beta. Arthritis Rheum. 2006;54:2616–2625. doi: 10.1002/art.22010. [DOI] [PubMed] [Google Scholar]
- 27.Baksh S, Widlund HR, Frazer-Abel AA, Du J, Fosmire S, Fisher DE, et al. Nfatc2-mediated repression of cyclin-dependent kinase 4 expression. Mol Cell. 2002;10:1071–1081. doi: 10.1016/s1097-2765(02)00701-3. [DOI] [PubMed] [Google Scholar]
- 28.Hansson GK, Jonasson L, Holm J, Clowes MM, Clowes AW. Gamma-interferon regulates vascular smooth muscle proliferation and ia antigen expression in vivo and in vitro. Circ Res. 1988;63:712–719. doi: 10.1161/01.res.63.4.712. [DOI] [PubMed] [Google Scholar]
- 29.Rolfe BE, Campbell JH, Smith NJ, Cheong MW, Campbell GR. T lymphocytes affect smooth muscle cell phenotype and proliferation. Arterioscler Thromb Vasc Biol. 1995;15:1204–1210. doi: 10.1161/01.atv.15.8.1204. [DOI] [PubMed] [Google Scholar]
- 30.Hansson GK, Holm J. Interferon-gamma inhibits arterial stenosis after injury. Circulation. 1991;84:1266–1272. doi: 10.1161/01.cir.84.3.1266. [DOI] [PubMed] [Google Scholar]
- 31.Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension. 2009;54:619–624. doi: 10.1161/HYPERTENSIONAHA.109.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsuda S, Kaji T. Atherosclerosis and extracellular matrix. J Atheroscler Thromb. 2003;10:267–274. doi: 10.5551/jat.10.267. [DOI] [PubMed] [Google Scholar]
- 33.Krettek A, Sukhova GK, Libby P. Elastogenesis in human arterial disease: a role for macrophages in disordered elastin synthesis. Arterioscler Thromb Vasc Biol. 2003;23:582–587. doi: 10.1161/01.ATV.0000064372.78561.A5. [DOI] [PubMed] [Google Scholar]
- 34.Sommerville LJ, Kelemen SE, Ellison SP, England RN, Autieri MV. Increased atherosclerosis and vascular smooth muscle cell activation in AIF-1 transgenic mice fed a high-fat diet. Atherosclerosis. doi: 10.1016/j.atherosclerosis.2011.07.095. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heo SH, Cho C-H, Kim HO, Jo YH, Yoon K-S, Lee JH, et al. Plaque rupture is a determinant of vascular events in carotid artery atherosclerotic disease: involvement of matrix metalloproteinases 2 and 9. J Clin Neurol. 2011;7:69–76. doi: 10.3988/jcn.2011.7.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. High blood hemoglobin concentration as risk factor of major atherosclerotic cardiovascular events in 114,159 healthy men and women in the apolipoprotein mortality risk study (AMORIS) Ann Med. 2011 doi: 10.3109/07853890.2011.573804. May 17. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Buttari B, Profumo E, Di Cristofano C, Pietraforte D, Lionetti V, Capoano R, et al. Haemoglobin triggers chemotaxis of human monocyte-derived dendritic cells: possible role in atherosclerotic lesion instability. Atherosclerosis. 2011;215:316–322. doi: 10.1016/j.atherosclerosis.2010.12.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.