Abstract
BACKGROUND
Few prospective studies have evaluated the risk for incident hypertension (HTN) across the normal range of body mass index (BMI). Even fewer studies included body composition and fat distribution measurements in their analyses. In the Aerobics Center Longitudinal Study, we examined HTN risk in women across a wide spectrum of baseline BMI (kg/m2) values and also studied waist circumference (WC, cm), percent body fat, fat mass (FM, kg), and fat-free mass (FFM, kg) on incident HTN in subgroup analyses.
METHODS
A total of 5,296 healthy normotensive women between 20 and 77 years of age completed a baseline examination during 1971–2004, and were followed for HTN incidence. Incident HTN was identified using mail-back surveys.
RESULTS
A total of 592 women reported HTN during a mean 16.7 years of follow-up. Higher BMI, even within the “normal” range, was associated with greater risk of HTN. Compared with women in the lowest fifth of BMI (18.5–20.0 kg/m2), the hazard ratios (HRs) (95% confidence interval (CI)) of developing HTN for women with a BMI of 20.1–21.2, 21.3–22.5, 22.6–24.7, and >24.7 were 1.19 (0.89–1.60), 1.33 (0.99–1.78), 1.36 (1.03–1.81), and 2.01 (1.52–2.66), respectively (Ptrend < 0.001). In a subgroup (n = 3,189) with complete data on all the five adiposity measures, significant positive associations with HTN were seen across incremental fifths of BMI, percent body fat, and FM (Ptrend < 0.05 each), but not WC and FFM.
CONCLUSIONS
Clinicians should emphasize the importance of weight management for the primary prevention of HTN in women.
Hypertension (HTN) affects ~58 million individuals, or one in three US adults.1 The estimated direct and indirect cost of HTN for 2007 is $66.4 billion dollars.1 From age 45 to 54, the percentages of men and women with HTN are similar,1 but at older ages a higher percentage of women have HTN than men.
A number of important contributory factors for HTN have been identified, including overweight/obesity, excessive dietary sodium intake, low physical activity, smoking, and high alcohol intake.2 Previous studies have shown that being overweight or obese is associated with a higher risk for HTN.3-15 However, few prospective epidemiological studies have investigated the association between body mass index (BMI) and incidence of HTN in women at the lower end of the BMI distribution.3-6 Further, we know of no published study that concurrently examined overall obesity (reflected by higher BMI), abdominal obesity (reflected by higher waist circumference (WC)), and total body fat (reflected by percent body fat and fat-free mass (FFM)) in predicting incident HTN.
Our primary aim was to investigate the association between BMI and incident HTN in a cohort of normotensive, healthy women, most of whom were normal weight at baseline. In addition, we studied BMI, WC, percent body fat, fat mass (FM), and FFM to explore their associations with HTN development in a subgroup of women with complete data on all the five adiposity measurements.
METhOdS
Study population
The study included 5,296 women participants between 20 and 77 years of age, who completed a preventive medical examination and who were enrolled in the Aerobics Center Longitudinal Study at the Cooper Clinic (Dallas, TX) during 1971–2004. Study participants came to the clinic for periodic preventive health examinations and for counseling regarding diet, exercise, and other lifestyle factors associated with increased risk of chronic disease. Many participants were sent by their employers for the examination. Some were referred by their personal physicians. Others were self-referred. At baseline, all participants included in this analysis were free of known cardiovascular disease (CVD), cancer, or diabetes, had normal resting and exercise electrocardiograms, were able to complete an exercise stress test to at least 85% of their age-predicted maximal heart rate, and had a BMI ≥18.5 kg/m2. They also reported no physician diagnosis of HTN and had resting blood pressure <140/90 mm Hg at baseline and responded to at least one mail-back health survey during follow-up. Most participants were white, well educated, and from middle and upper socioeconomic strata. This study protocol was approved annually by the Institutional Review Board of the Cooper Institute.
Baseline examination
The baseline clinical examination was performed after receiving written informed consent from each participant and included fasting blood chemistry analyses, personal and family health history, anthropometry, resting blood pressure and electrocardiogram, and a maximal-graded exercise test on a treadmill. Previous studies have described the baseline examination in detail.16 All procedures were administered by trained technicians who followed standardized protocols. Height and weight were measured using a stadiometer and standard physician’s scale. BMI (in kg/m2) was computed as weight in kilograms divided by height squared in meters. Participants were grouped into fifths of the BMI distribution as follows: BMI 18.5–20.0, 20.1–21.2, 21.3–22.5, 22.6–24.7, and >24.7 kg/m2 respectively. Percent body fat was assessed with hydrostatic weighing, with the sum of seven skinfold measures, or with both assessments, following standardized protocols. Detailed description of our hydrodensitometry procedures has been published elsewhere.17 FM (kg) was computed as weight (in kg) × (percent body fat)/100. FFM (kg) was computed as weight (kg) – FM (kg).17 WC was measured at the level of the umbilicus with a plastic tape measure. Adiposity was grouped based on standard clinical definitions for: BMI (normal weight 18.5–24.9, overweight 25.0–29.9, and obese ≥30.0); WC (normal <88.0 cm; abdominal obesity ≥88.0 cm); and percent body fat (normal <30%; obese ≥30%).18 In addition, we examined tenths of BMI to better illustrate the association between BMI and the risk of developing HTN. In a subgroup with complete data on BMI, WC, percent body fat, FM, and FFM (n = 3,189), women were also grouped into fifths of each these adiposity measures.
Resting blood pressure was measured in the seated position and was recorded as the first and fifth Korotkoff sounds by auscultatory methods after at least 5 min of sitting quietly. Two or more readings separated by 2 min were averaged. If the first two readings differed by >5 mm Hg, additional readings were obtained and averaged. Serum samples were analyzed for lipids and glucose using standardized automated bioassays. Hypercholesterolemia was defined as total cholesterol of ≥6.20 mmol/l (≥240 mg/dl)19 or previous diagnosis by a physician. Information on smoking habits (current smoker or not), alcohol intake (drinks per week), physical activity habits (physically inactive or not), and family history of CVD or HTN was obtained from a standardized questionnaire. Participants were defined as physically inactive if they reported no leisure-time physical activity in the 3 months before baseline examination.
Cardiorespiratory fitness (CRF), which can be measured objectively in a laboratory and thereby provides quantifiable data that are correlated with habitual physical activity, was measured as the duration of a symptom-limited maximal treadmill exercise test using a modified Balke protocol.16,20 Exercise duration on this protocol is highly correlated with directly measured maximal oxygen uptake in women (r = 0.94).21 Patients were encouraged to give a maximal effort during the test. The test end point was volitional exhaustion or test termination by the supervising physician for medical reasons. Maximal metabolic equivalents (METs, 1 MET = 3.5 ml O2 uptake/kg/min) were estimated from the final treadmill speed and grade.22
Ascertainment of incident HTN
The incidence of HTN was ascertained from responses to mail-back health surveys in 1982, 1986, 1990, 1995, 1999, and 2004. The aggregate survey response rate across all survey periods in the Aerobics Center Longitudinal Study is ≈65%. Nonresponse bias is a concern in epidemiological surveillance. This issue has been investigated in the Aerobics Center Longitudinal Study,23 and was found not to present a major source of bias. Baseline health histories and clinical measures were similar between responders and nonresponders and between early and late responders.23 The end point was defined as a participant report of a physician diagnosis of HTN and has been described in detail elsewhere.24-26 Our methods of case ascertainment are similar to those used in other established epidemiological studies on HTN.5,7,27 Sensitivity and specificity of self-reported diagnosis of HTN in this cohort were 98 and 99%, respectively.24
Statistical analysis
Follow-up time among noncases was computed as the difference between the date of the baseline examination and the date of the last returned survey where the participant reported being free of HTN. Follow-up time among cases was computed as the difference between the baseline examination date and the reported date of the HTN event. If a diagnosis date was not provided, we used the midpoint between the date of the case-finding survey and either the baseline examination date or the date of the last returned survey where the participant reported being free of HTN. Cox proportional hazards regression analysis was used to estimate hazard ratios (HRs) (in hours) and 95% confidence intervals (CIs) of HTN events according to exposure categories. Multivariate-adjusted models controlled for the potential confounding effects of baseline age (in years), current smoker (yes/no), alcohol intake (≥5 drinks/week or not), resting systolic and diastolic blood pressure (mm Hg), hypercholesterolemia (yes/no), family history of CVD or HTN (present or not for each), and physically inactive (yes/no). We also constructed indicator variables (yes/no) for each survey period to account for differences in survey response patterns to reduce the influence of ascertainment bias.25,26 To standardize for surveillance period and length of follow-up, we entered these variables, as well as the year of the baseline examination, into our analyses as covariables. Additional stratum-specific analyses were performed according to baseline age (<55 and ≥55 years) and CRF (low, moderate, and high fit). Tests of linear trends across exposure categories were computed using ordinal scoring. The potential influence of smoking and undetected subclinical disease at baseline was evaluated by restricting analyses to women who were not current smokers (n = 4,834) and by excluding incident HTN that occurred during the first 2 years of follow-up (n = 4,785). Statistical analyses were performed using SAS (version 9.1; SAS Institute, Cary, NC) software. All reported P values are two-tailed, and CIs were calculated at the 95% level.
RESULTS
The mean BMI of the participants was 22.7 ± 3.6 (range 18.5–43.6). The prevalence of overweight (BMI: 25.0–29.9) and obese (BMI ≥30.0) was 14.2 and 4.0%, respectively. During a mean (s.d.) 16.7 (7.8) years of follow-up and 87,992 woman-years of observation, there were 592 incident cases of HTN. On average, women with higher BMI were older, had lower CRF, greater levels of adiposity, and higher systolic and diastolic blood pressure at baseline; and were less likely to be a current smoker and were less physically active (Table 1).
Table 1. Baseline characteristics of study participants by body mass index, Aerobics Center Longitudinal Study, 1971–2004.
| Baseline BMI Fifth (kg/m2) |
|||||||
|---|---|---|---|---|---|---|---|
| Total (n = 5,296) |
18.5–20.0 (n = 1,061) |
20.1–21.2 (n = 1,060) |
21.3–22.5 (n = 1,057) |
22.6–24.7 (n = 1,060) |
>24.7 (n = 1,058) |
P for trend | |
| Age (mean ± s.d., y) | 43.3 ± 9.7 | 40.5 ± 9.0 | 42.1 ± 9.3 | 43.3 ± 9.6 | 45.1 ± 9.8 | 45.8 ± 9.7 | <0.001 |
| Waist circumferences (mean ± s.d., cm) | 67.2 ± 20.0 | 60.4 ± 16.2 | 61.8 ± 18.7 | 65.0 ± 18.6 | 68.9 ± 17.5 | 78.7 ± 22.1 | <0.001 |
| Percent body fats (mean ± s.d.) | 26.0 ± 6.8 | 20.4 ± 5.3 | 22.6 ± 5.1 | 25.3 ± 5.0 | 28.3 ± 4.9 | 33.5 ± 5.1 | <0.001 |
| Fat masss(mean ± s.d., kg) | 16.4 ± 6.6 | 10.8 ± 2.9 | 12.7 ± 3.0 | 15.1 ± 3.2 | 18.0 ± 3.4 | 25.5 ± 6.6 | <0.001 |
| Fat free masss (mean ± s.d., kg) | 45.1 ± 5.6 | 42.0 ± 4.2 | 43.4 ± 4.2 | 44.4 ± 4.4 | 45.4 ± 4.7 | 50.0 ± 6.6 | <0.001 |
| Exercise tolerance (mean ± s.d., METs) | 9.8 ± 2.1 | 10.7 ± 2.2 | 10.3 ± 2.1 | 9.9 ± 2.0 | 9.5 ± 1.8 | 8.5 ± 1.8 | <0.001 |
| Treadmill test duration (mean ± s.d., minutes) | 13.9 ± 4.6 | 15.9 ± 4.7 | 15.1 ± 4.5 | 14.3 ± 4.3 | 13.4 ± 4.0 | 11.2 ± 3.8 | <0.001 |
| Lipids (mean ± s.d., mg/dl) | |||||||
| Total cholesterol | 198.3 ± 42.3 | 192.0 ± 56.5 | 191.8 ± 34.4 | 195.9 ± 38.1 | 202.2 ± 36.7 | 209.1 ± 39.3 | <0.001 |
| HDL-C | 62.2 ± 17.5 | 66.3 ± 26.2 | 64.2 ± 14.5 | 62.6 ± 14.2 | 61.9 ± 14.2 | 56.9 ± 14.3 | <0.001 |
| Triglycerides | 86.2 ± 82.6 | 75.2 ± 45.7 | 73.0 ± 34.6 | 78.2 ± 40.3 | 89.7 ± 48.7 | 114.0 ± 80.0 | <0.001 |
| Fasting blood glucose (mean ± s.d., mg/dl) | 95.0 ± 13.1 | 100.7 ± 29.8 | 92.0 ± 11.3 | 92.6 ± 13.2 | 93.8 ± 9.1 | 95.8 ± 10.5 | <0.001 |
| Blood pressure (mean ± s.d., mm Hg) | |||||||
| Systolic | 109 ± 11 | 107 ± 11 | 108± 10 | 109 ± 11 | 111 ± 11 | 113 ± 10 | <0.001 |
| Diastolic | 73 ± 7 | 72 ± 7 | 72 ± 7 | 73 ± 7 | 74 ± 7 | 76 ± 7 | <0.001 |
| Physically inactiveb (%) | 27.5 | 24.2 | 24.8 | 25.6 | 29.3 | 33.3 | <0.001 |
| Current smoker (%) | 8.7 | 8.0 | 11.3 | 8.3 | 8.7 | 7.3 | 0.01 |
| Alcohol consumption (>5 drinks/week) (%) | 19.0 | 23.7 | 21.8 | 20.9 | 16.1 | 12.6 | <0.001 |
| Hypercholesterolemiac (%) | 11.6 | 6.6 | 7.6 | 11.5 | 12.8 | 19.5 | <0.001 |
| Family history of hypertension (%) | 17.0 | 13.3 | 15.9 | 16.2 | 18.0 | 21.8 | <0.001 |
| Family history of CVD (%) | 26.1 | 19.0 | 25.0 | 26.7 | 30.7 | 28.9 | <0.001 |
SI conversion factors: To convert total cholesterol and HDL-C values to mmol/l, multiply by 0.0259; triglycerides values to mmol/l, by 0.0113; glucose values to mmol/l, by 0.0555. BMI, body mass index; CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; METs, maximal metabolic equivalents achieved during the treadmill test.
Waist circumference: n = 3,193 (294 hypertension), percent body fat (fat mass, fat free mass): n = 4,732 (502 hypertension).
Women reporting no leisure-time physical activity in the 3 months before the examination. hypercholesterolemia was defined as total cholesterol >6.20 mmol/l (240 mg/dl).
We observed a positive gradient (Ptrend < 0.001) of HTN incidence rates across incremental BMI groups (Table 2). After adjusting for baseline covariates (age, examination year, survey response pattern, current smoking, alcohol intake, resting systolic and diastolic blood pressure, hypercholesterolemia, family history of HTN and CVD, and physically inactive), higher baseline BMI was associated with greater risk of developing HTN (Ptrend < 0.001) even within the range of “normal” BMI. Women in the highest fifth of BMI quintile (>24.7) were more than two times as likely to report HTN during follow-up as women in the lowest fifth. Similar patterns of association were observed between BMI and HTN when we restricted analysis to those who were not current smokers at baseline or to those with >2 years of follow-up. In addition, we examined the multivariable-adjusted HRs for incident HTN, according to tenths of baseline BMI. Across incremental tenths of baseline BMI, the multivariable-adjusted HRs of HTN were 1.00 (referent), 1.16 (95% CI: 0.75, 1.80), 1.40 (95% CI: 0.91, 2.14), 1.19 (95% CI: 0.77, 1.84), 1.94 (95% CI: 1.30, 2.91), 0.99 (95% CI: 0.63, 1.56), 1.39 (95% CI: 0.92, 2.09), 1.58 (95% CI: 1.04, 2.38), 1.83 (95% CI: 1.22, 2.76), 2.58 (95% CI: 1.73, 3.85), Ptrend < 0.001.
Table 2. Rates and hazard ratios for developing hypertension, according to baseline body mass index.
| Fifths of baseline BMI (kg/m2) |
||||||
|---|---|---|---|---|---|---|
| 18.5–20.0 | 20.1–21.2 | 21.3–22.5 | 22.6–24.7 | >24.7 | P for trend | |
| Mean weight—lb (kg) | 116 (53) | 124 (56) | 131 (60) | 140 (63) | 167 (76) | |
| All women (n = 5,296) | ||||||
| No. of hypertension cases | 84 | 101 | 111 | 133 | 163 | |
| Ratea | 48.4 | 56.0 | 63.8 | 73.1 | 103.2 | <0.001 |
| Model 1b HR (95% CI) | 1.00 | 1.19 (0.89–1.60) | 1.45 (1.09–1.93) | 1.66 (1.25–2.20) | 2.58 (1.96–3.39) | <0.001 |
| Model 2cHR (95% CI) | 1.00 | 1.19 (0.89–1.60) | 1.33 (0.99–1.78) | 1.36 (1.03–1.81) | 2.01 (1.52–2.66) | <0.001 |
| Women who were not current smoker (n = 4,834) | ||||||
| No. of hypertension cases | 80 | 92 | 100 | 127 | 148 | |
| Ratea | 50.5 | 59.5 | 62.7 | 77.7 | 102.1 | <0.001 |
| Model 1b HR (95% CI) | 1.00 | 1.19 (0.88–1.61) | 1.36 (1.01–1.84) | 1.66 (1.25–2.21) | 2.42 (1.83–3.22) | <0.001 |
| Model 2d HR (95% CI) | 1.00 | 1.18 (0.87–1.60) | 1.27 (0.94–1.71) | 1.35 (1.01–1.80) | 1.89 (1.42–2.53) | <0.001 |
| Women who were not current smoker with >2 y of follow-up (n = 4,785) | ||||||
| No. of hypertension cases | 73 | 87 | 94 | 117 | 128 | |
| Ratea | 45.8 | 56.0 | 57.6 | 72.1 | 90.2 | <0.001 |
| Model 1b HR (95% CI) | 1.00 | 1.23 (0.90–1.69) | 1.39 (1.02–1.89) | 1.71 (1.27–2.30) | 2.38 (1.77–3.20) | <0.001 |
| Model 2d HR (95% CI) | 1.00 | 1.23 (0.90–1.68) | 1.30 (0.95–1.78) | 1.40 (1.03–1.89) | 1.89 (1.40–2.55) | <0.001 |
BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
Rate per 10,000 woman-years adjusted for age and examination year.
Model 1: adjusted for baseline age, examination year, and survey response pattern.
Model 2: adjusted for all variables in model 1 plus physically inactive (yes or no), current smoking (yes or no), alcohol intake (=5 drinks/week, yes or no), resting systolic and diastolic blood pressure (mm Hg), hypercholesterolemia (yes or no), family history of hypertension (present or not), and family history of CVD (present or not).
Model 2: adjusted for all variables in model 1 plus physically inactive (yes or no), alcohol intake (=5 drinks/week, yes or no), resting systolic and diastolic blood pressure (mm Hg), hypercholesterolemia (yes or no), family history of hypertension (present or not), and family history of CVD (present or not).
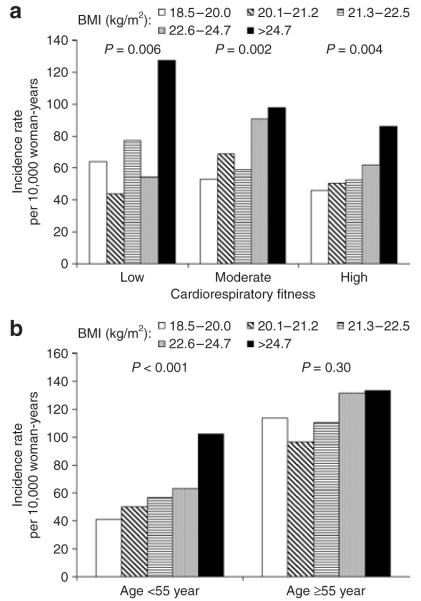
We also conducted stratified analyses by baseline CRF (Figure 1a) and age groups (Figure 1b) to examine whether other risk predictors modified the association between BMI and HTN. Higher levels of BMI were associated with higher incidence rates of HTN across low, moderate, and high CRF (Ptrend < 0.01 each). Similar patterns of association were also observed among women who were <55 years of age (Ptrend < 0.001). However, the risk of developing HTN was not significant across BMI quintiles among women who were ≥55 years (Ptrend = 0.30). Although the trend was not significant among older women, we did not detect effect modification by baseline age (P interaction = 0.93).
Figure 1.
Age and examination year–adjusted hypertension incidence rates (per 10,000 woman-years) by fifths of body mass index (BMI) across cardiorespiratory (a) fitness levels and (b) age groups in 5,296 women. The P values are for a test of linear trend across BMI groups.
To better understand the roles of other adiposity measures on incident HTN, we analyzed a subgroup which had complete measurement data on all the five adiposity variables (BMI, WC, percent body fat, FM, and FFM) (n = 3,189) (Table 3). Significant positive associations with incident HTN were seen across incremental fifths of BMI, percent body fat, and FM (Ptrend < 0.05 each).
Table 3. Risk of hypertension incidence according to fifths of adiposity measures in a subgroupa.
| Adiposity measures |
N | No. of Cases |
HR (95% CI)b | HR (95% CI)c |
|---|---|---|---|---|
| BMI fifths (kg/m2) | ||||
| 18.5–20.0 | 635 | 42 | 1.00 | 1.00 |
| 20.1–21.2 | 632 | 49 | 1.17 (0.77–1.79) | 1.13 (0.74–1.73) |
| 21.3–22.6 | 647 | 58 | 1.48 (0.99–2.22) | 1.36 (0.91–2.04) |
| 22.7–24.9 | 639 | 67 | 1.71 (1.15–2.55) | 1.50 (1.00–2.24) |
| ≥25.0 | 636 | 78 | 2.46 (1.66–3.65) | 1.83 (1.22–2.73) |
| P for linear trend | <0.001 | 0.001 | ||
| WC fifths (cm) | ||||
| <64.0 | 618 | 49 | 1.00 | 1.00 |
| 64.0–67.0 | 658 | 56 | 1.00 (0.67–1.47) | 0.89 (0.60–1.32) |
| 67.1–71.0 | 685 | 66 | 1.23 (0.85–1.79) | 1.10 (0.75–1.60) |
| 71.1–77.0 | 609 | 47 | 0.98 (0.65–1.47) | 0.81 (0.53-1.22) |
| >77.0 | 619 | 76 | 1.25 (0.86–1.84) | 1.91 (1.31–2.78) |
| P for linear trend | 0.002 | 0.32 | ||
| Percent body fat fif | ths | |||
| <20.7 | 644 | 29 | 1.00 | 1.00 |
| 20.7–24.5 | 641 | 52 | 1.59 (1.00–2.53) | 1.57 (0.99–2.50) |
| 24.6–27.9 | 634 | 58 | 1.75 (1.11–2.77) | 1.74 (1.10–2.76) |
| 28.0–32.0 | 634 | 63 | 1.85 (1.17–2.91) | 1.70 (1.08–2.70) |
| >32.0 | 636 | 92 | 2.83 (1.82–4.39) | 2.25 (1.44–3.50) |
| P for linear trend | <0.001 | <0.001 | ||
| Fat mass fifths (kg) | ||||
| <11.5 | 652 | 36 | 1.00 | 1.00 |
| 11.5–14.1 | 624 | 52 | 1.41 (0.91–2.18) | 1.39 (0.90–2.16) |
| 14.2–16.8 | 635 | 59 | 1.60 (1.05–2.45) | 1.52 (0.99–2.33) |
| 16.9–21.1 | 641 | 63 | 1.54 (1.00–2.36) | 1.37 (0.89–2.11) |
| >21.1 | 637 | 84 | 2.59 (1.71–3.90) | 2.00 (1.32–3.04) |
| P for linear trend | <0.001 | 0.003 | ||
| Fat free mass fifths (kg) | ||||
| <40.6 | 640 | 70 | 1.00 | 1.00 |
| 40.6–43.1 | 632 | 61 | 0.97 (0.69–1.37) | 0.93 (0.66–1.32) |
| 43.2–45.5 | 643 | 59 | 1.00 (0.70–1.42) | 0.99 (0.69–1.41) |
| 45.6–48.8 | 636 | 45 | 0.90 (0.61–1.32) | 0.82 (0.56–1.21) |
| >48.8 | 638 | 59 | 1.50 (1.04–2.17) | 1.17 (0.81–1.71) |
| Pfor linear trend | 0.13 | 0.72 | ||
BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease, HR, hazard ratio; WC, waist circumference.
Adiposity measures with complete data on BMI, WC, percent body fat, fat mass, and fat free mass (n = 3,189).
Adjusted for baseline age, examination year, and survey response pattern.
Adjusted for above plus physically inactive (yes or no), current smoking (yes or no), alcohol intake (≥5 drinks/week, yes or no), resting systolic and diastolic blood pressure (mm Hg), hypercholesterolemia (yes or no), family history of hypertension (present or not), and family history of CVD (present or not).
To place our findings into a more clinically relevant perspective, we examined risk of HTN incidence on adiposity exposures grouped according to standardized definitions (Table 4). HTN incidence risk was significantly higher in BMI-defined overweight (HR: 1.65; 95% CI: 1.33–2.04) and obese (HR: 1.85; 95% CI: 1.30–2.63) compared with normal weight women, in WC-defined abdominal obese (HR: 1.51; 95% CI: 1.00–2.27) compared with normal WC women, and in percent body fat defined obese (HR: 1.32; 95% CI: 1.09–1.60) compared with normal percent body fat women, respectively.
Table 4. Risk of hypertension incidence according to clinical cutpoints of adiposity measures.
| Adiposity measures |
N | No. of cases |
HR (95% CI)a | HR (95% CI)b |
|---|---|---|---|---|
| BMI (kg/m2) | ||||
| 18.5–24.9 | 4,330 | 437 | 1.00 | 1.00 |
| 25.0–29.9 | 754 | 117 | 1.87 (1.52–2.31) | 1.65 (1.33–2.04) |
| ≥30.0 | 212 | 38 | 2.80 (1.98–3.96) | 1.85 (1.30–2.63) |
| P for linear trend |
<0.001 | <0.001 | ||
| WCc (cm) | ||||
| Normal (<88) | 2,998 | 265 | 1.00 | 1.00 |
| Abdominal obesity (≥88) |
195 | 29 | 2.16 (1.45–3.22) | 1.51 (1.00–2.27) |
| P for difference | <0.001 | 0.048 | ||
| Percent body fatd | ||||
| Normal (<30) | 3,390 | 309 | 1.00 | 1.00 |
| Obese (≥30) | 1,342 | 193 | 1.48 (1.23–1.79) | 1.32 (1.09–1.60) |
| P for difference | <0.001 | 0.004 | ||
BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; WC, waist circumference.
Adjusted for baseline age, examination year, and survey response pattern.
Adjusted for above plus physically inactive (yes or no), current smoking (yes or no), alcohol intake (≥5 drinks/wk, yes or no), resting systolic and diastolic blood pressure (mm Hg), hypercholesterolemia (yes or no), family history of hypertension (present or not), and family history of CVD (present or not).
Waist circumference: n = 3,193 (294 hypertension).
Percent body fat : n = 4,732 (502 hypertension).
DISCUSSION
Principle findings
In this large prospective study of women, we found that a higher BMI was positively associated with a greater risk of incident HTN. After adjusting for multiple confounders, including CRF, this association remained across all BMI categories, even within the “normal” BMI range. Women in the highest fifth of BMI (>24.7 kg/m2) had a more than twofold higher risk of developing HTN when compared with women in the lowest fifth (18.5–20.0 kg/m2). Similar patterns of association were observed between BMI and HTN when we restricted our analyses to those who were not current smokers and to those with >2 years of follow-up. The positive pattern of association was present in younger women (<55 years) and also in low, moderate, and high CRF categories, but not in older women (≥55 years). The latter finding is likely because of the small number of HTN outcomes in some BMI groups (for example, there were only 15 and 16 HTN outcomes in the first and second fifths of BMI, respectively). Our exploratory findings in the subgroup (Table 3) of women indicated that BMI, percent body fat, and FM, but not WC and FFM, were significantly associated with incident HTN. However, when BMI, WC, and percent body fat are grouped according to the National Institutes of Health obesity guidelines, high-risk value of all the three adiposity measurements shows a significant predictor of HTN risk.
Comparison with previous reports
Previous studies have found that weight gain or being overweight or obese are independent risk factors for the development of HTN.6 Weight reduction is beneficial for both primary prevention and treatment of HTN.7 Data collected from previous longitudinal studies3-7 confirmed the adverse effects of a higher BMI on greater risk of developing HTN. However, data on women are limited.3-6 Jousilahti et al.4 found that among normotensive female subjects at baseline, BMI-associated risk for new anti-hypertensive drug treatment was 1.11/kg/m2 (P < 0.0001).4 Another prospective cohort study by Huang et al.5 reported that higher BMI was strongly associated with greater risk for HTN and each unit of BMI (per kg/m2) was associated with a 12% higher risk of incident HTN. In a study of 9,139 Finnish women who were 25–64 years old, 813 women who developed HTN had a higher BMI and were more often obese or overweight compared with those who remained normotensive.6 This study had some characteristics that differentiate it from these previous studies: all the women free of HTN and other forms of CVD at baseline, the effect of fitness on the association between BMI and HTN, and various objective measures of BMI and other adiposity markers. We believe this is the first study of BMI and HTN in women that meets all of these criteria.
In this study, the positive association between BMI and HTN was consistent across strata of CRF. However, the correlation between BMI and HTN was stronger in younger age groups than among older women (Figure 1b). This finding was consistent not only with previous cross-sectional study where HTN was an outcome,11 but also with several studies where mortality was the primary outcome.28-30 A few hypotheses have been suggested to explain the lack of association between BMI and mortality in older people.31,32 In this study, the nonsignificant finding in women older than 55 years is likely because of the small number of events in some BMI groups. However, even with this small number of events, there was a positive trend across incremental fifths of BMI. Therefore, future studies with a larger sample size will be needed to address this important issue on incident HTN or other CVD risk factors.
In a subgroup of women with complete data on all adiposity measurements, we found that BMI remains a strong predictor for developing HTN. In addition, incremental fifths of percent body fat and FM show a positive association with incident HTN. Whether percent body fat and FM maintain associations with HTN compared with simple measurements, such as BMI or WC, is largely unknown.15,33-35 Menke et al.15 did not observe an association between fourths of percent body fat with prevalence HTN in women. Although many studies have reported that WC is a more powerful marker than BMI for CVD risk factors,10,11,13,15 we did not find this similar association with incident HTN across fifths of WC (Table 3). However, when the clinical cutoff point was used to group WC into abdominal obesity and normal categories, women with abdominal obesity had a 1.51 times higher risk of future HTN than women with normal WC (P = 0.048) (Table 4). Our inconsistent findings may be partly due to differences in study design. The principal limitation of previous studies was the use of cross-sectional data to identify factors associated with CVD risks. This study is the only study to our knowledge using longitudinal data to investigate these issues. Another difference is the study population. The women in the current study are not a representative sample of US female population. Finally, we extensively controlled a variety of baseline potential confounders including clinical measurements, physical activity, and CRF. From a practical perspective, findings from the current study indicate that simple, readily available and inexpensive obesity measures such as BMI may be useful in assessing HTN risk. Nonetheless, future prospective studies with a representative sample need to confirm findings reported here with HTN as an outcome.
Mechanisms underlying the observed associations
The biological mechanism by which higher BMI increases the risk of developing HTN is not completely known. Complex interactions between metabolic and neurohormonal pathways may be the underlying mechanism by which HTN develops.36-40 Insulin resistance, the renin–angiotensin–aldosterone system, and sympathetic tone may be altered by increases in BMI, and these alterations may play a causative role in increasing blood pressure among persons who are overweight and obese.40 Investigators have reported decreases in plasma renin activity and plasma aldosterone levels after weight loss, which suggests that the renin–angiotensin–aldosterone system may mediate the relationship between BMI and HTN.38,39 Future research is needed to further explore possible biological mechanisms by which higher BMI, especially within the normal range, increases the risk of developing HTN.
Strengths and limitations
Strengths of the current study include the relatively large sample size, a follow-up period of 17 years, objectively measured baseline BMI and other measures of adiposity and fat distribution, and the extensive adjustment for potential confounders, including both self-reported physical activity and objectively measured fitness. In addition, this study had an extensive baseline examination, which is important since undetected subclinical disease at baseline is a concern in prospective studies.
There are some limitations that should be considered when interpreting our data. First, the homogeneity of the Aerobics Center Longitudinal Study population on sociodemographic factors deserves comment. This sample of women was mainly white ethnicity and of middle to upper socioeconomic status. They were relatively fit and mostly normal weight. While this enhances the internal validity of our findings by reducing the degree of confounding by these factors, our findings may not be generalized to the general population. Second, we did not have sufficient information on medication usage, menopausal status, or dietary sodium intake to control for these potential covariates. Lastly, because of the widespread geographical distribution of participants, we were unable to verify the onset of HTN in all cases. However, it appears that an acceptable level of agreement exists between the participants’ self-reported histories and their medical records based on a validation substudy.24-26
Conclusions and perspectives
Higher levels of BMI, even within the normal range, are a strong predictor for future development of HTN in a large cohort of women who were normotensive and healthy at baseline. Baseline BMI is positively and linearly associated with risk for developing HTN, which may have a substantial public health impact on HTN prevalence and related health sequellae. We believe that clinicians should counsel their patients to maintain a healthy BMI or lose weight if they are overweight or obese in order to reduce the risk of HTN and subsequent CVD. Future research is needed to clarify the relationship between other adiposity measures (such as WC and percent body fat) and HTN.
Acknowledgments
This work was supported by the National Institutes of Health grants AG06945 and HL62508. We thank the Cooper Clinic physicians and technicians for collecting the baseline data, and staff at the Cooper Institute for data entry and data management.
Dr Blair receives book royalties (<$5,000/year) from Human Kinetics; honoraria for service on the Medical Advisory Boards for Matria Health Care, Magellan Health Services, and jenny Craig; and honoraria for lectures from scientific, educational, and lay groups. He gives these fees to the University of South Carolina Educational Foundation or to other nonprofit groups. During the past 2-year period he has received a research grant from BodyMedia Inc. Dr Church receives honoraria for lectures from scientific, educational, and lay groups. Dr Church is a consultant for Trestle Tree Inc and has a book in publication from which he will receive royalties. Dr Church has received research funding from the American Heart Association and the National Institutes of Health. Dr Church has overseen study sites for large pharmaceutical trials funded by Sanofi Aventis, Orexigen, and Amylin.
Footnotes
Disclosures: The other authors declared no conflict of interest.
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Dyer AR, Elliott P, INTERSALT Co-operative Research Group The INTERSALT study: relations of body mass index to blood pressure. J Hum Hypertens. 1989;3:299–308. [PubMed] [Google Scholar]
- 4.Jousilahti P, Tuomilehto J, Vartiainen E, Valle T, Nissinen A. Body mass index, blood pressure, diabetes and the risk of anti-hypertensive drug treatment: 12-year follow-up of middle-aged people in eastern Finland. J Hum Hypertens. 1995;9:847–854. [PubMed] [Google Scholar]
- 5.Huang Z, Willett WC, Manson JE, Rosner B, Stampfer MJ, Speizer FE, Colditz GA. Body weight, weight change, and risk for hypertension in women. Ann Intern Med. 1998;128:81–88. doi: 10.7326/0003-4819-128-2-199801150-00001. [DOI] [PubMed] [Google Scholar]
- 6.Hu G, Barengo NC, Tuomilehto J, Lakka TA, Nissinen A, Jousilahti P. Relationship of physical activity and body mass index to the risk of hypertension: a prospective study in Finland. Hypertension. 2004;43:25–30. doi: 10.1161/01.HYP.0000107400.72456.19. [DOI] [PubMed] [Google Scholar]
- 7.Gelber RP, Gaziano JM, Manson JE, Buring JE, Sesso HD. A prospective study of body mass index and the risk of developing hypertension in men. Am J Hypertens. 2007;20:370–377. doi: 10.1016/j.amjhyper.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu FB, Wang B, Chen C, Jin Y, Yang J, Stampfer MJ, Xu X. Body mass index and cardiovascular risk factors in a rural Chinese population. Am J Epidemiol. 2000;151:88–97. doi: 10.1093/oxfordjournals.aje.a010127. [DOI] [PubMed] [Google Scholar]
- 9.Wilsgaard T, Schirmer H, Arnesen E. Impact of body weight on blood pressure with a focus on sex differences: the Tromso Study, 1986–1995. Arch Intern Med. 2000;160:2847–2853. doi: 10.1001/archinte.160.18.2847. [DOI] [PubMed] [Google Scholar]
- 10.Zhu S, Wang Z, Heshka S, Heo M, Faith MS, Heymsfield SB. Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. Am J Clin Nutr. 2002;76:743–749. doi: 10.1093/ajcn/76.4.743. [DOI] [PubMed] [Google Scholar]
- 11.Foucan L, Hanley J, Deloumeaux J, Suissa S. Body mass index (BMI) and waist circumference (WC) as screening tools for cardiovascular risk factors in Guadeloupean women. J Clin Epidemiol. 2002;55:990–996. doi: 10.1016/s0895-4356(02)00430-4. [DOI] [PubMed] [Google Scholar]
- 12.Sharabi Y, Grotto I, Huerta M, Grossman E. Susceptibility of the influence of weight on blood pressure in men versus women: lessons from a large-scale study of young adults. Am J Hypertens. 2004;17:404–408. doi: 10.1016/j.amjhyper.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79:379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 14.Field AE, Cook NR, Gillman MW. Weight status in childhood as a predictor of becoming overweight or hypertensive in early adulthood. Obes Res. 2005;13:163–169. doi: 10.1038/oby.2005.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menke A, Muntner P, Wildman RP, Reynolds K, He J. Measures of adiposity and cardiovascular disease risk factors. Obesity (Silver Spring) 2007;15:785–795. doi: 10.1038/oby.2007.593. [DOI] [PubMed] [Google Scholar]
- 16.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 17.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69:373–380. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 18.Bray GA. Fat distribution and body weight. Obes Res. 1993;1:203–205. doi: 10.1002/j.1550-8528.1993.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 19.Cleeman JI, Lenfant C. The National Cholesterol Education Program: progress and prospects. JAMA. 1998;280:2099–2104. doi: 10.1001/jama.280.24.2099. [DOI] [PubMed] [Google Scholar]
- 20.Balke B, Ware RW. An experimental study of physical fitness in Air Force personnel. US Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 21.Pollock ML, Foster C, Schmidt D, Hellman C, Linnerud AC, Ward A. Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. Am Heart J. 1982;103:363–373. doi: 10.1016/0002-8703(82)90275-7. [DOI] [PubMed] [Google Scholar]
- 22.American College of Sports Medicine . ACSM’s guidelines for exercise testing and prescription. 6th edn Lippincott Williams & Wilkins; Philadelphia, PA: 2000. [Google Scholar]
- 23.Macera CA, Jackson KL, Davis DR, Kronenfeld JJ, Blair SN. Patterns of non-response to a mail survey. J Clin Epidemiol. 1990;43:1427–1430. doi: 10.1016/0895-4356(90)90112-3. [DOI] [PubMed] [Google Scholar]
- 24.Blair SN, Goodyear NN, Gibbons LW, Cooper KH. Physical fitness and incidence of hypertension in healthy normotensive men and women. JAMA. 1984;252:487–490. [PubMed] [Google Scholar]
- 25.Barlow CE, LaMonte MJ, FitzGerald SJ, Kampert JB, Perrin JL, Blair SN. Cardiorespiratory fitness is an independent predictor of hypertension incidence among initially normotensive healthy women. Am J Epidemiol. 2006;163:142–150. doi: 10.1093/aje/kwj019. [DOI] [PubMed] [Google Scholar]
- 26.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness and risk of nonfatal cardiovascular disease in women and men with hypertension. Am J Hypertens. 2007;20:608–615. doi: 10.1016/j.amjhyper.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paffenbarger RS, Jr, Wing AL, Hyde RT, Jung DL. Physical activity and incidence of hypertension in college alumni. Am J Epidemiol. 1983;117:245–257. doi: 10.1093/oxfordjournals.aje.a113537. [DOI] [PubMed] [Google Scholar]
- 28.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338:1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 29.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 30.Dolan CM, Kraemer H, Browner W, Ensrud K, Kelsey JL. Associations between body composition, anthropometry, and mortality in women aged 65 years and older. Am J Public Health. 2007;97:913–918. doi: 10.2105/AJPH.2005.084178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med. 2001;161:1194–1203. doi: 10.1001/archinte.161.9.1194. [DOI] [PubMed] [Google Scholar]
- 32.Elia M. Obesity in the elderly. Obes Res. 2001;9(Suppl 4):244S–2448S. doi: 10.1038/oby.2001.126. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi N, Nakamura K, Suzuki K, Matsuo Y, Tatara K. Associations of body mass index and percentage body fat by bioelectrical impedance analysis with cardiovascular risk factors in Japanese male office workers. Ind Health. 2000;38:273–279. doi: 10.2486/indhealth.38.273. [DOI] [PubMed] [Google Scholar]
- 34.Bonora E, Zenere M, Branzi P, Bagnani M, Maggiulli L, Tosi F, Travia D, Cacciatori V, Querena M, Moghetti P. Influence of body fat and its regional localization on risk factors for atherosclerosis in young men. Am J Epidemiol. 1992;135:1271–1278. doi: 10.1093/oxfordjournals.aje.a116233. [DOI] [PubMed] [Google Scholar]
- 35.Richelsen B, Pedersen SB. Associations between different anthropometric measurements of fatness and metabolic risk parameters in non-obese, healthy, middle-aged men. Int J Obes Relat Metab Disord. 1995;19:169–174. [PubMed] [Google Scholar]
- 36.Kolanowski J. Obesity and hypertension: from pathophysiology to treatment. Int J Obes Relat Metab Disord. 1999;23(Suppl 1):42–46. doi: 10.1038/sj.ijo.0800794. [DOI] [PubMed] [Google Scholar]
- 37.DeFronzo RA. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidaemia and atherosclerosis. Neth J Med. 1997;50:191–197. doi: 10.1016/s0300-2977(97)00012-0. [DOI] [PubMed] [Google Scholar]
- 38.Ikeda T, Gomi T, Hirawa N, Sakurai J, Yoshikawa N. Improvement of insulin sensitivity contributes to blood pressure reduction after weight loss in hypertensive subjects with obesity. Hypertension. 1996;27:1180–1186. doi: 10.1161/01.hyp.27.5.1180. [DOI] [PubMed] [Google Scholar]
- 39.Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med. 1981;304:930–933. doi: 10.1056/NEJM198104163041602. [DOI] [PubMed] [Google Scholar]
- 40.Hsueh WA, Buchanan TA. Obesity and hypertension. Endocrinol Metab Clin North Am. 1994;23:405–427. [PubMed] [Google Scholar]