Abstract
OBJECTIVES
To examine associations between functional capacity estimated from cardiorespiratory fitness (CRF) and mortality risks in adults aged 60 and older.
DESIGN
Prospective study, averaging 13.6 years follow-up.
SETTING
Preventive medical clinic.
PARTICIPANTS
Four thousand sixty adults who completed preventive medical examinations between 1971 and 2001; 24.7% women, mean age ± standard deviation 64.6 ± 4.9, body mass index (BMI) 25.9 ± 3.8 kg/m2.
MEASUREMENTS
CRF was quantified as metabolic equivalents (METs) achieved during maximal treadmill exercise. The lowest 20% of the age- and sex-specific MET distribution was defined as having low CRF, the middle 40% moderate CRF, and the upper 40% high CRF. Cox regression was used to estimate death rates (per 1,000 person-years), hazard ratios (HRs), and their 95% confidence intervals (CIs).
RESULTS
Nine hundred eighty-nine deaths occurred during follow-up. Death rates adjusted for age, sex, and examination year were 30.9, 18.3, and 13.4 for all causes (P<.001); 15.9, 8.6, and 5.4 for cardiovascular disease (CVD) (P<.001); and 6.1, 4.9, and 4.2 for cancer (P=.04) for subjects with low, moderate, and high CRF, respectively. After adjusting for smoking, abnormal electrocardiograms at rest or while exercising, percentage of age-predicted maximal heart rate achieved during exercise testing, baseline medical conditions, BMI, hypercholesterolemia, and family CVD and cancer history, subjects with high CRF had notably lower mortality risk than those with low CRF from all causes (HR = 0.59, 95% CI = 0.47–0.74) and from CVD (HR = 0.57, 95% CI = 0.41–0.80).
CONCLUSION
CRF is an important independent predictor of death in older adults. The results add to the existing evidence that promoting physical activity in older adults provides substantial health benefits, even in the oldest old.
Keywords: functional capacity, exercise testing, mortality, cardiovascular diease, metabolic equivalents (METs)
By 2030, 70 million people in the United States will be aged 65 and older, constituting 22% of the population.1 To function independently and continue to perform activities of daily living, older people need to maintain muscle strength and sufficient cardiovascular functional capacity (FC).2 FC declines rapidly by age 75,2,3 with more than half of FC typically lost by that age.4
Cardiorespiratory fitness (CRF), an objective measure used to estimate FC, has been found to predict mortality from all causes, cardiovascular disease (CVD), and cancer across a broad spectrum of ages.5-10 Whether the relationship between CRF and mortality differs by age is unclear. A study of the protective effect of greater CRF for coronary events in initially healthy men found differences in this effect between younger (<65) and older (≥65) men. Their findings suggest the usefulness of a separate analysis for older adults, although in this age group, data on the prognostic value of CRF, which can be measured using exercise tests in routine clinical practice, are limited.11-14 Previous studies that included older participants were limited by small sample sizes,11,14 combined fatal and nonfatal cardiac outcomes,11,14 or did not evaluate cancer mortality risk.5,11,13,14 An earlier report demonstrated that there was an inverse association between functional testing and all-cause mortality in older individuals.15 This earlier report was regarded as a preliminary analysis, because it included a small number of deaths, which prevented a thorough investigation and a firm conclusion. The present study extends our previous observations and focuses on the importance of fitness, as estimated by maximal treadmill testing, as a predictor of mortality from all causes, CVD, and cancer in a cohort of men and women aged 60 and older.
METHODS
Study Sample
Participants were 3,057 men and 1,003 women aged 60 and older who completed a baseline clinical examination between 1971 and 2001 at the Cooper Clinic (Dallas, TX) and who were enrolled in the Aerobics Center Longitudinal Study (ACLS). Inclusion criteria for the current analyses required participants to have a maximal treadmill test at baseline, during which they must have achieved at least 85% of their age-predicted maximal heart rate (220 minus age in years). One thousand seven hundred forty men and 460 women reported a history of one or more of the following conditions at baseline: myocardial infarction, stroke, hypertension, diabetes mellitus, or cancer. The majority of participants were Caucasian from middle or upper socioeconomic strata. All participants provided written consent to participate in the follow-up study. The Cooper Institute institutional review board annually reviewed and approved the study protocol.
Clinical Data
The baseline examination was completed after an overnight fast of at least 12 hours and included a physician examination and an extensive array of clinical measurements.6,8 Body mass index (BMI, kg/m2) was computed from height and weight measured using a clinical scale and stadiometer. Systolic and diastolic blood pressures were recorded at rest as the first and fifth Korotkoff sounds using standard auscultation methods.16 Hypertension was defined as systolic blood pressure of 140 mmHg or greater, diastolic blood pressure of 90 mmHg or greater, or a history of physician diagnosis. Concentrations of total and high-density lipoprotein cholesterol, triglycerides, and glucose were measured using automated bioassays in accordance with the Centers for Disease Control and Prevention Lipid Standardization Program. Diabetes mellitus was defined as fasting plasma glucose concentration of 7.0 mmol/L or greater, a history of physician diagnosis, or insulin use. Hypercholesterolemia was defined as total cholesterol of 6.20 mmol/L or greater. Personal history of myocardial infarction, stroke, or cancer; family history of CVD or cancer; smoking; and physical activity habits were obtained from a standardized questionnaire.
CRF was quantified as the duration of a symptom-limited maximal treadmill exercise test using a modified Balke protocol.8,17 The treadmill test began with the patient walking 88 m per minute at 0% grade. At the end of the first minute, elevation was increased to 2% and thereafter increased 1% per minute until the 25th minute. Only 38 patients were able to continue past 25 minutes. For these patients, the treadmill speed was increased by 5.4 m per minute for each minute after the 25th. The test endpoint was volitional exhaustion or termination by the physician for medical reasons. Patients were encouraged to give a maximal effort. The high maximal heart rates achieved suggest that they did so. The mean percentage ± standard deviation of age-predicted maximal heart rate achieved during exercise was 101.4 ± 10.7 in women and 100.7 ± 12.1 in men. Exercise duration on this protocol is highly correlated with measured maximal oxygen uptake in men18 (correlation coefficient (r) = 0.92) and women19 (r = 0.94). Thus, CRF in this study is analogous to maximal aerobic power and was used as an objective laboratory marker of exercise participation during the months before the examination. Maximal metabolic equivalents (METs, 1 MET = 3.5 mL oxygen uptake/kg per minute) were estimated from the final treadmill speed and grade.20 One MET is an individual’s resting metabolic rate when sitting or lying quietly; walking at a moderate pace on a level surface uses approximately 3.3 METs, race walking approximately 6.5 METs, and running at 12 minutes per mile approximately 8.0 METs. MET values for a wide variety of physical activities have been published.21
There is no widely accepted clinical categorization of the CRF phenotype. Low, moderate, and high CRF exposures have previously been defined as the lower 20%, middle 40%, and upper 40%, respectively, of the age- and sex-specific distribution of treadmill duration in the overall ACLS population.22,23 The same approach was used in the current analysis to maintain consistency in the study methods and because it has been found that low CRF, defined in this way, is an independent predictor of mortality.6-8 CRF exercise durations for the three fitness categories for men were low (<7.8 minutes), moderate (7.8–13.1 minutes), and high (>13.1 minutes) and for women were low (<5.5 minutes), moderate (5.5–9.0 minutes), and high (>9.0 minutes). In equivalent MET values, the thresholds that defined these categories were 7.2 and 9.5 METs for men and 5.8 and 7.6 METs for women.
Abnormal exercise electrocardiogram (ECG) responses included rhythm and conduction disturbances and ischemic ST-Twave abnormalities, as described in detail elsewhere.24 Previously, 90% agreement has been found between the ECG interpretation recorded in the database and a group of three physicians who read a random sample of 357 patient records.24
Mortality Surveillance
All participants were followed from the date of their baseline examination until their death or December 31, 2003. The National Death Index was the primary data source for mortality surveillance, augmented with death certificates. The underlying cause of death was determined from the National Death Index report or by a nosologist’s review of official death certificates obtained from the department of vital records in the decedent’s state of residence. CVD mortality was defined as International Classification of Diseases, Ninth Revision (ICD-9) codes 390 to 449.9 before 1999 and ICD-Tenth Revision (ICD-10) codes I00 to I78 during 1999 to 2003. Cancer mortality was defined as ICD-9 codes 140 to 208 and ICD-10 codes C00 to C97. The mean duration of follow-up was 13.6 ± 7.4 (range 1–33.0). Person-years of exposure was computed as the sum of follow-up time of decedents and survivors. In 55,337 person-years of exposure, 989 deaths were identified (453 CVD, 263 cancer).
Statistical Analysis
Baseline characteristics of the population were estimated according to CRF category and vital status. Differences in covariates were tested using Student t tests, chi-square tests, and F-tests. Cox proportional hazards regression was used to estimate hazard ratios (HRs), 95% confidence intervals (CIs), and mortality rates (deaths per 1,000 person-years of follow-up), according to exposure categories. Multivariable analyses included controls for baseline measures: age, sex, examination year, smoking status (current smoker or not), BMI (kg/m2), hypercholesterolemia (yes or no), abnormal exercise ECG responses (present or not), percentage maximal heart rate achieved during exercise testing, medical conditions (the presence or absence, separately measured, of myocardial infarction, stroke, hypertension, diabetes mellitus, or cancer), and family history of CVD or cancer (present or not).
Cumulative hazard plots grouped according to exposure suggested no appreciable violations of the proportional hazards assumption. Events that occurred during the first year of follow-up were excluded to reduce potential confounding caused by procedure-related deaths and potential influence of undetected subclinical disease at baseline. Kaplan–Meier survival curves were generated according to CRF for all-cause, CVD, and cancer mortality. The log-rank test was used to determine whether any differences between the curves representing low, moderate, and high CRF within each mortality outcome were statistically significant. Supplementary analyses were performed to test for age- or sex-related interactions on the association between CRF and outcomes using interaction terms. All P-values were calculated assuming two-sided alternative hypotheses; P-values <.05 were taken to indicate statistically significant comparisons. All analyses were performed using SAS statistical software, version 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
At baseline, average age was 64.6 ± 4.9 in men and 64.8 ± 5.2 in women. Average BMI was 26.5 ± 3.5 in men and 24.2 ± 4.0 in women. There were 844 deaths (389 CVD, 225 cancer) during 42,278 person-years of exposure in men and 145 deaths (64 CVD, 38 cancer) during 13,059 person-years of exposure in women. Table 1 shows baseline descriptive characteristics based on vital status and CRF categories. As expected, exercise duration and maximal METs were higher in survivors and the higher CRF groups, whereas age, systolic blood pressure, serum cholesterol, sedentary behavior, current smoking, and chronic diseases were greater in decedents and the low CRF group.
Table 1.
Baseline Characteristics According to Vital Status and Cardiorespiratory Fitness (CRF) Groups, Aerobics Center Longitudinal Study, 1971-2003
| Characteristic | Survivors (n = 3,071) |
Decedents (n = 989) |
P-Value | Low CRF (n = 572) |
Moderate CRF (n = 1,559) |
High CRF (n = 1,929) |
P-Value |
|---|---|---|---|---|---|---|---|
| Age, mean ± SD | 64.4 ± 4.9 | 65.4 ± 5.1 | <.001 | 66.2 ± 5.3 | 64.8 ± 4.9 | 64.0 ± 4.8 | <.001 |
| 60-69 | 88.3 | 81.4 | 77.0 | 85.6 | 90.3 | ||
| 70-79 | 10.5 | 16.8 | <.001 | 20.5 | 13.3 | 8.5 | < .001 |
| ≥80 | 1.2 | 1.8 | 2.5 | 1.1 | 1.2 | ||
| Female, % | 27.9 | 14.7 | <.001 | 23.1 | 22.7 | 26.8 | .01 |
| Follow-up, years, mean ± SD | 13.4 ± 7.4 | 14.3 ± 7.2 | .001 | 13.9 ± 7.8 | 14.3 ± 7.5 | 13.0 ± 7.1 | <.001 |
| Body mass index, kg/m2, mean ± SD | 26.0 ± 3.8 | 25.8 ± 3.8 | .27 | 27.9 ± 4.9 | 26.7 ± 3.7 | 24.8 ± 3.1 | <.001 |
| Maximal metabolic equivalents achieved during treadmill test, mean ± SD |
9.1 ± 2.2 | 8.3 ± 2.3 | <.001 | 5.8 ± 0.7 | 7.9 ± 0.9 | 10.6 ± 1.7 | <.001 |
| Treadmill time, minutes, mean ± SD | 12.5 ± 4.7 | 10.8 ± 4.9 | <.001 | 5.2 ± 1.6 | 10.0 ± 2.0 | 15.8 ± 3.8 | <.001 |
| Lipids, mmol/L, mean ± SD | |||||||
| Total cholesterol* | 5.7 ± 1.1 | 5.8 ± 1.1 | .02 | 5.8 ± 1.1 | 5.7 ± 1.1 | 5.6 ± 1.1 | <.001 |
| High-density lipoprotein cholesterol† | 1.3 ± 0.4 | 1.2 ± 0.4 | <.001 | 1.2 ± 0.4 | 1.3 ± 0.4 | 1.4 ± 0.4 | <.001 |
| Triglycerides‡ | 1.5 ± 1.0 | 1.5 ± 0.9 | .49 | 1.8 ± 1.1 | 1.7 ± 1.0 | 1.3 ± 0.8 | <.001 |
| Fasting blood glucose, mmol/L, mean ± SD§ | 5.8 ± 1.3 | 5.9 ± 1.4 | .19 | 6.3 ± 2.1 | 5.9 ± 1.4 | 5.7 ± 1.0 | <.001 |
| Blood pressure, mmHg, mean ± SD | |||||||
| Systolic∥ | 129 ± 17 | 133 ± 18 | <.001 | 133 ± 18 | 131 ± 17 | 128 ± 17 | <.001 |
| Diastolic# | 82 ± 10 | 83 ± 10 | .03 | 83 ± 11 | 83 ± 10 | 81 ± 10 | <.001 |
| Physically inactive, %** | 29.1 | 40.8 | <.001 | 56.3 | 42.3 | 16.4 | < .001 |
| Current smoker, % | 8.3 | 13.4 | <.001 | 16.4 | 11.0 | 6.4 | < .001 |
| Abnormal electrocardiogram during exercise, % | 26.3 | 39.9 | <.001 | 48.6 | 31.9 | 22.1 | < .001 |
| Baseline medical conditions, % | |||||||
| Myocardial infarction | 3.5 | 8.3 | <.001 | 9.8 | 5.7 | 2.3 | < .001 |
| Stroke | 0.6 | 0.5 | .69 | 1.2 | 0.6 | 0.4 | .09 |
| Diabetes mellitus | 4.6 | 6.3 | .03 | 10.0 | 4.9 | 3.6 | < .001 |
| Hypertension | 45.3 | 55.1 | <.001 | 58.0 | 52.2 | 41.0 | < .001 |
| Cancer | 6.1 | 1.6 | <.001 | 3.9 | 3.9 | 6.3 | .002 |
| Family history of premature cardiovascular disease, % | 15.1 | 7.2 | <.001 | 13.5 | 11.7 | 14.3 | .07 |
| Family history of cancer (%) | 31.8 | 5.8 | <.001 | 20.3 | 22.1 | 29.7 | < .001 |
Normal range 2.59–5.15 mmol/L (100–199 mg/dL).
Normal range 0–2.56 mmol/L (0–99mg/dL).
Normal range 0–1.68 mmol/L (0–149 mg/dL).
Normal range 3.89–6.11mmoll/L (70–110mg/dL).
Normal range<120mmHg.
Normal range < 80 mmHg.
Individuals reporting no leisure-time physical activity in the 3 months before the examination. SD = standard deviation.
Table 2 shows unadjusted death rates for all causes, CVD, and cancer; HRs adjusted for age, sex, and examination year (Model 1); and HRs with additional adjustments for baseline measures of smoking, BMI, hypercholesterolemia, medical conditions, abnormal exercise ECG, percentage maximal heart rate during exercise testing, and family history of CVD or cancer (Model 2). The unadjusted all-cause mortality rates were 1.69 (30.9/13.4) and 2.31 (30.9/18.3) times as great for those with low CRF as for those with moderate and high CRF, respectively. The analogous differences for CVD mortality were 1.85 and 2.94 times as great. The adjusted results of Model 1 similarly showed a notably declining risk of all-cause and CVD mortality with increasing CRF (P<.001). After the additional adjustments of Model 2, the all-cause and CVD mortality risks remained progressively lower with increasing levels of CRF (both P≤.001). For example, the all-cause mortality risk for subjects with moderate CRF in the fully adjusted model was 71% of the corresponding mortality risk for those with low CRF (95% CI = 59–85%), whereas the risk for those with high CRF was 59% of the corresponding mortality risk for those with low CRF (95% CI = 47–74%). The results also provide some evidence that cancer mortality rates may have been greater in individuals with low CRF in Model 1 (P =.04), although in risk factor adjustment Model 2 (P =.45) attenuated the borderline significance of this association. Sex-related interactions, which would modify the association between low CRF and mortality, were not found.
Table 2.
All-Cause, Cardiovascular Disease (CVD), and Cancer Mortality According to Cardiorespiratory Fitness (CRF) Group in Older Adults
| Cause of Death | Deaths, n (rate)* |
Model † | Model 2‡ |
|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | |||
| All causes | |||
| Low CRF | 256 (30.9) | 1.00 (Referent) | 1.00 (Referent) |
| Moderate CRF | 415 (18.3) | 0.59 (0.51-0.69) | 0.71 (0.59-0.85) |
| High CRF | 318 (13.4) | 0.43 (0.37-0.51) | 0.59 (0.47-0.74) |
| Pfor linear trend | <.001 | < .001 | |
| CVD | |||
| Low CRF | 134 (15.9) | 1.00 (Referent) | 1.00 (Referent) |
| Moderate CRF | 194 (8.6) | 0.54 (0.44-0.68) | 0.77 (0.59-1.00) |
| High CRF | 125 (5.4) | 0.34 (0.27-0.44) | 0.57 (0.41-0.80) |
| P for linear trend | < .001 | .001 | |
| Cancer | |||
| Low CRF | 49 (6.1) | 1.00 (Referent) | 1.00 (Referent) |
| Moderate CRF | 111 (4.9) | 0.80 (0.57-1.13) | 0.92 (0.61-1.37) |
| High CRF | 103 (4.2) | 0.70 (0.49-0.99) | 0.84 (0.52-1.34) |
| P for linear trend | .04 | .45 | |
Rate per 1,000 person-years adjusted for age, sex, and examination year.
Adjusted for age, sex, and examination year.
Adjusted for the above plus current smoking (yes or no), body mass index, hypercholesterolemia (yes or no), baseline medical conditions (myocardial infarction, stroke, hypertension, diabetes mellitus, or cancer, yes or no for each), family history of CVD or cancer (yes or no), abnormal exercise electrocardiogram response (yes or no), and percentage maximal heart rate achieved during exercise testing.
Whether other risk predictors modified the association between CRF and all-cause, CVD, and cancer mortality was examined next (Table 3). After adjusting for differences in baseline age, sex, examination year, and the other variables in Table 3, each 1-MET increase in maximal exercise was, on average, associated with 9% to 43% lower all-cause mortality risk, depending on the stratum (P<.05 in most strata), and 6% to 67% lower CVD mortality risk (P<.05 in most strata). The consistency of the direction and magnitude of association between fitness and all-cause and CVD mortality suggested that there was generally little effect modification across risk factor categories.
Table 3.
Risk of All-Cause, Cardiovascular Disease (CVD), and Cancer Mortality per Metabolic Equivalent Increment in Maximal Exercise Test Within Strata of Other Personal Characteristics
| Characteristic | All Causes | CVD | Cancer | |||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) P-Value* | ||||||
| Age | ||||||
| 60-69 | 0.91 (0.88-0.95) | <.001 | 0.90 (0.85-0.96) | < .001 | 0.98 (0.90-1.06) | .54 |
| 70-79 | 0.80(0.74-0.88) | < .001 | 0.78 (0.69-0.89) | < .001 | 0.88 (0.73-1.06) | .19 |
| ≥80 | 0.57 (0.37-0.90) | .02 | 0.33 (0.12-0.92) | .03 | NA† | NA† |
| Current smoker | ||||||
| No | 0.88(0.85-0.91) | <.001 | 0.85 (0.80-0.90) | < .001 | 0.97 (0.90-1.04) | .35 |
| Yes | 0.81 (0.72-0.91) | <.001 | 0.83 (0.70-0.97) | .02 | 0.69 (0.51-0.92) | .01 |
| Body mass index, kg/m2 | ||||||
| 18.5-24.9 | 0.89(0.85-0.93) | < .001 | 0.89 (0.82-0.95) | .002 | 0.96 (0.87-1.06) | .42 |
| ≥25.0 | 0.83 (0.79-0.88) | < .001 | 0.81 (0.75-0.88) | < .001 | 0.90 (0.81-1.00) | .05 |
| Exercise electrocardiogram responses | ||||||
| Normal | 0.87 (0.83-0.91) | < .001 | 0.82 (0.76-0.89) | < .001 | 0.95 (0.87-1.03) | .20 |
| Abnormal | 0.87(0.82-0.93) | < .001 | 0.88 (0.81-0.95) | .002 | 0.93 (0.81-1.06) | .27 |
| Total cholesterol, mmol/L | ||||||
| <6.20 | 0.84 (0.73-0.98) | .03 | 0.71 (0.53-0.96) | .03 | 0.87 (0.64-1.20) | .40 |
| ≥6.20 | 0.88(0.85-0.91) | <.001 | 0.86(0.81-0.91) | <.001 | 0.94 (0.87-1.01) | .10 |
| Medical conditions, n‡ | ||||||
| 0 | 0.89 (0.84-0.94) | < .001 | 0.84 (0.76-0.94) | .002 | 0.97 (0.87-1.08) | .57 |
| 1 | 0.85(0.81-0.90) | <.001 | 0.83 (0.77-0.89) | < .001 | 0.90 (0.81-1.00) | .05 |
| ≥2 | 0.89 (0.78-1.00) | .06 | 0.94 (0.80-1.11) | .45 | 0.97 (0.74-1.27) | .83 |
| Family history of CVD | ||||||
| No | 0.87 (0.84-0.91) | < .001 | 0.85 (0.80-0.90) | < .001 | 0.94 (0.87-1.02) | .12 |
| Yes | 0.86 (0.76-0.97) | .02 | 0.85 (0.72-1.01) | .06 | 0.91 (0.71-1.17) | .48 |
Adjusted for age, sex, examination year, and each of the other variables in the table.
NA5not applicable; there were no recorded deaths from cancer among those ≥80.
Number of baseline medical conditions, including diabetes mellitus, cancer, hypertension, stroke, and myocardial infarction.
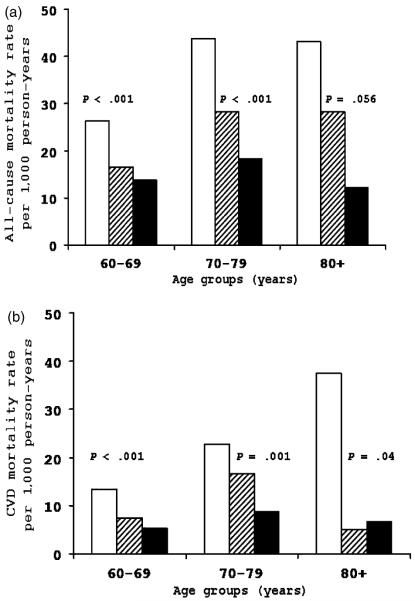
One notable exception to this generalization was the much greater effect size observed in those aged 80 and older at baseline, in whom, for example, the risk of CVD mortality was 67% greater in those with low fitness (HR for subjects aged ≤80 = 0.33, 95% CI = 0.12–0.92, P 5.03). Figure 1 was created to examine whether baseline age modified the association between CRF and mortality. As in Table 3, age was grouped into three categories. There was weak statistical evidence of interaction between CRF and age in predicting all-cause death (χ2df=1 = 3.8, P = 5.05) and CVD death (χ2df=1 = 1.14, P = .29), although it is likely that statistical power to examine interaction effects was inadequate. Adjusted for sex and examination year, there was a significant inverse gradient of all-cause death rates across incremental CRF levels within each age group. Across age strata, individuals in the lowest CRF group had a 1.9 to 3.5 times higher rate of total mortality than those in the highest CRF group (Figure 1A). CRF was also inversely associated with CVD mortality rates within age groups (Figure 1B).
Figure 1.
Sex- and examination year–adjusted death rates per 1,000 person-years according to cardiorespiratory fitness (CRF) and age groups. (A) All-cause mortality, (B) cardiovascular disease (CVD) mortality. White bars represent low CRF, striped bars moderate CRF, and black bars high CRF.
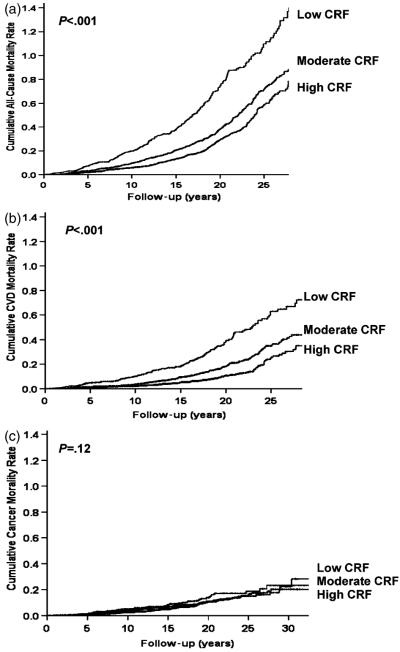
Figure 2 shows the Kaplan-Meier curves for all-cause, CVD, and cancer mortality for individuals with low, moderate, and high CRF. The corresponding log-rank test statistics were 103.4 (P<.001), 87.5 (P<.001), and 4.2 (P =.12), respectively, again suggesting that the differences in the survival curves were statistically significant for all-cause and CVD mortality.
Figure 2.
Kaplan–Meier plots for mortality due to (A) all causes, (B) cardiovascular diseases (CVD), and (C) cancer.
DISCUSSION
In the cohort of older adults followed for an average of 13.6 years, low CRF was strongly associated with adjusted risk of death from all causes and CVD. These results are consistent with previous findings that fitness level is an independent predictor of mortality from all causes and CVD in middle-aged adults.8,25,26
It is well established that higher levels of CRF are related to lower risk of coronary heart disease, some cancers, and physical disability. The various health benefits associated with physical exercise include lowering BMI, blood pressure, and blood glucose levels and improving the lipid profile; physical exercise also reduces effects of osteoporosis and helps maintain mobility and prolong independence.27 The precise mechanisms conferring protection from physical exercise are unknown and may differ between younger and older adults.11 Studies reveal that biological aging may increase oxidative stress, which contributes to higher levels of inflammation,28 both of which may be reduced with exercise.29,30
Studies have examined CRF and mortality in many diverse populations,5,6,8-10,31,32 although there are insufficient data in older people.11,13,14 The current study extends previous investigations of healthy middle-aged men and women by showing that CRF has a strong inverse association with all-cause and CVD mortality, not only for older adults in general, but also for those aged 70 and older with multiple morbidities.6-8 In a study from the Baltimore Longitudinal Study of Aging,11 FC was as strong a predictor of coronary events in older men as in younger ones. Another study from Olmstead County had similar findings.14 Recently, it was reported that impaired FC was strongly related to death from all causes.13 An earlier study reached similar conclusions,5 although these previous studies of older adults enrolled few participants,11,14 had short follow-up time (<4 years),5,13 combined fatal and nonfatal cardiac outcomes,11,14 and did not evaluate specific mortality risks such as mortality from CVD or cancer, both of which are common chronic diseases in later life.5,11,13,14 The present study also extends an earlier preliminary report15 on fitness and mortality in older individuals, showing that the inverse association was similar to that observed in the overall ACLS population and that it remained after adjustments for multiple potential confounding factors.
In contrast with other well-established traditional risk factor–mortality relationships, the lack of age attenuation of the relationship between fitness and mortality is remarkable and deserves comment. A significant trend was observed between CRF and all-cause and CVD mortality across age strata, and the relationship was stronger with older age (Figure 1). Studies have found a protective33-35 or no relationship36,37 between higher blood pressure and mortality and an inverse relationship38 between BMI and mortality in older people. The direction of these relationships is the reverse of what is typically found in younger and middle-aged adults. The age attenuation in older adults may be attributable to competing causes of mortality that become larger factors with older age. It may also reflect selection factors that have allowed survival to older age. The lack of a similar age-attenuated association between CRF and mortality risk in the current study provides further evidence of the importance of enhancing functional capacity to achieve longevity.
CRF is a surrogate measure of physical activity. There is increasing evidence that physical activity and higher CRF protect against cancers of the colon, lung, prostate, and breast.39-42 The small number of cause-specific cancer deaths (such as 7 breast cancer and 28 colon cancer) in the current study prevented any meaningful analyses of the relationship between CRF and cause-specific cancer mortality. Nevertheless unlike in previous reports from the ACLS,7,8 the current data did not suggest that higher CRF levels protect older adults against total cancer mortality, although the trend in the hazard analysis suggested that the risk of death from cancer may be higher in those with low CRF. There are some possible explanations for the lack of statistically significant findings with regard to CRF and cancer. First, there was a small number of deaths in the low CRF group (n = 49), which limits statistical power. Second, associations between physical activity and cancer tend to be moderate, not always statistically significant, and some times only evident in subgroups or for substantially high levels of activity.40,43 Finally, cancers are not homogenous. Differences exist in etiology between different populations (e.g., younger or older subgroups) or by type of end point (e.g., high vs low grade, incident vs fatal). It would be useful to confirm these findings related to cancer and CRF in older adults with a prospective study with a larger sample.
Several factors should be considered when interpreting these results. A major strength of this analysis, in contrast to most other reports on this topic, was the extensive baseline clinical examination. Although undetected subclinical disease is always a concern in observational studies, it is less likely to have occurred in this cohort because of the comprehensive physical examination by a physician and the thorough clinical assessment completed by each participant. Other strengths include use of maximal exercise testing to quantify CRF, long follow-up, and multiple mortality outcomes. The study population was limited to predominantly white, well-educated, middle- to upper-class older adults. This limits the generalizability of the study’s findings, although this limitation should not affect the study’s internal validity. Moreover, there is no compelling reason to assume that the benefits of moderate and high fitness levels would be less in other socioeconomic groups. There was insufficient information about medication use or dietary habits to include these factors in the analysis. The inability to control for these factors may have biased the results through residual confounding, although it seems unlikely that these factors would account for all of the observed association between CRF and mortality. Future studies should include such information whenever possible.
Although CRF has a genetic component (25–40%),44,45 usual physical activity habits are the major determinant of fitness. The correlation between CRF and detailed records of regular physical activity habits is high (r = 0.70–0.90).46,47 Numerous randomized and controlled studies have shown that exercise training can improve fitness up to 30%20 and that bed rest can reduce fitness.48 More recently, a striking finding was reported from the Dose-Response to Exercise in postmenopausal Women Trial that even activity at the 4-kcal/kg per week level (approximately 72 min/wk of moderate-intensity walking) was associated with a significantly greater improvement in fitness than in women in the nonexercise control group.49 That report found no significant difference in the effect of fitness improvement between women younger than 55 and those aged 55 and older. These results also suggest that older persons can increase fitness through regular physical activity.
The current report suggests a strong inverse association for older adults between estimated FC and mortality for all causes and for CVD. This result was based on objective measures and persisted after adjustment for clinical variables. More than 30% of the older survivors in this study were not physically active; nor were more than 40% of the decedents. Clinicians should pay particular attention to CRF when assessing risks of all-cause and CVD death. They should strongly encourage their older patients to maintain an active lifestyle. These findings also support current national and international physical activity promotion campaigns by public health organizations. Older adults benefit from active lifestyles.
ACKNOWLEDGMENTS
We thank the Cooper Clinic physicians and technicians for collecting the baseline data, and staff at the Cooper Institute for data entry and data management.
Sponsor’s Role: The funding body played no role in the formulation of the design, methods, subject recruitment, data collection, analysis, or preparation of this paper.
Footnotes
Conflict of Interest: Supported by National Institutes of Health Grants AG06945 and HL62508 and by the Communities Foundation of Texas on recommendation of Nancy Ann and Ray L. Hunt.
Author Contributions: Xuemei Sui: study conceptualization and design, data analysis, interpretation of data, and writing of the manuscript. James N. Laditka: study conceptualization, interpretation of results, and writing of manuscript. James W. Hardin: interpretation of results and editing the manuscript. Steven N. Blair: study conceptualization, interpretation of data, and editing and writing the manuscript.
REFERENCES
- 1.Lurie N. Healthy people 2010: Setting the nation’s public health agenda. Acad Med. 2000;75:12–13. doi: 10.1097/00001888-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Fleg JL, Morrell CH, Bos AG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 3.Hollenberg M, Yang J, Haight TJ, et al. Longitudinal changes in aerobic capacity: Implications for concepts of aging. J Gerontol A Biol Sci Med Sci. 2006;61A:M851–M858. doi: 10.1093/gerona/61.8.851. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins S, Wiswell R. Rate and mechanism of maximal oxygen consumption decline with aging: Implications for exercise training. Sports Med. 2003;33:877–888. doi: 10.2165/00007256-200333120-00002. [DOI] [PubMed] [Google Scholar]
- 5.Snader CE, Marwick TH, Pashkow FJ, et al. Importance of estimated functional capacity as a predictor of all-cause mortality among patients referred for exercise thallium single-photon emission computed tomography: Report of 3,400 patients from a single center. J Am Coll Cardiol. 1997;30:641–648. doi: 10.1016/s0735-1097(97)00217-9. [DOI] [PubMed] [Google Scholar]
- 6.Blair SN, Kampert JB, Kohl HW, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210. [PubMed] [Google Scholar]
- 7.Kampert JB, Blair SN, Barlow CE, et al. Physical activity, physical fitness, and all-cause and cancer mortality: A prospective study of men and women. Ann Epidemiol. 1996;6:452–457. doi: 10.1016/s1047-2797(96)00059-2. [DOI] [PubMed] [Google Scholar]
- 8.Blair SN, Kohl HW, III, Paffenbarger RS, Jr, et al. Physical fitness and all-cause mortality: A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 9.Ekelund LG, Haskell WL, Johnson JL, et al. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men: The Lipid Research Clinic’s mortality follow-up study. N Engl J Med. 1988;319:1379–1384. doi: 10.1056/NEJM198811243192104. [DOI] [PubMed] [Google Scholar]
- 10.Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 11.Talbot LA, Morrell CH, Metter EJ, et al. Comparison of cardiorespiratory fitness versus leisure time physical activity as predictors of coronary events in men aged < or = 65 years and >65 years. Am J Cardiol. 2002;89:1187–1192. doi: 10.1016/s0002-9149(02)02302-0. [DOI] [PubMed] [Google Scholar]
- 12.Nishime EO, Cole CR, Blackstone EH, et al. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284:1392–1398. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- 13.Messinger-Rapport B, Pothier Snader CE, Blackstone EH, et al. Value of exercise capacity and heart rate recovery in older people. J Am Geriatr Soc. 2003;51:63–68. doi: 10.1034/j.1601-5215.2002.51011.x. [DOI] [PubMed] [Google Scholar]
- 14.Goraya TY, Jacobsen SJ, Pellikka PA, et al. Prognostic value of treadmill exercise testing in elderly persons. Ann Intern Med. 2000;132:862–870. doi: 10.7326/0003-4819-132-11-200006060-00003. [DOI] [PubMed] [Google Scholar]
- 15.Blair SN, Wei M. Sedentary habits, health, and function in older women and men. Am J Health Prom. 2000;15:1–8. doi: 10.4278/0890-1171-15.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 17.Balke B, Ware RW. An experimental study of physical fitness in air force personnel. US Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 18.Pollock ML, Bohannon RL, Cooper KH, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 19.Pollock ML, Foster C, Schmidt D, et al. Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. Am Heart J. 1982;103:363–373. doi: 10.1016/0002-8703(82)90275-7. [DOI] [PubMed] [Google Scholar]
- 20.American College of Sports Medicine . ACSM’s Guidelines for Exercise Testing and Prescription. 7th Ed Lippincott Williams and Wilkins; Philadelphia: 2005. [Google Scholar]
- 21.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(Suppl 9):S498–S516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 22.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. Am J Epidemiol. 2007;165:1413–1423. doi: 10.1093/aje/kwm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness and risk of nonfatal cardiovascular disease in women and men with hypertension. Am J Hypertens. 2007;20:608–615. doi: 10.1016/j.amjhyper.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibbons LW, Mitchell TL, Wei M, et al. Maximal exercise test as a predictor of risk for mortality from coronary heart disease in asymptomatic men. Am J Cardiol. 2000;86:53–58. doi: 10.1016/s0002-9149(00)00827-4. [DOI] [PubMed] [Google Scholar]
- 25.Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: A 20-year follow-up of the lipid research clinics prevalence study. JAMA. 2003;290:1600–1607. doi: 10.1001/jama.290.12.1600. [DOI] [PubMed] [Google Scholar]
- 26.Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005;353:468–475. doi: 10.1056/NEJMoa044154. [DOI] [PubMed] [Google Scholar]
- 27.Landi F, Cesari M, Onder G, et al. Physical activity and mortality in frail, community-living elderly patients. J Gerontol A Biol Sci Med Sci. 2004;59A:M833–M837. doi: 10.1093/gerona/59.8.m833. [DOI] [PubMed] [Google Scholar]
- 28.Chung HY, Kim HJ, Kim JW, et al. The inflammation hypothesis of aging: Molecular modulation by calorie restriction. Ann NY Acad Sci. 2001;928:327–335. [PubMed] [Google Scholar]
- 29.Goto S, Radak Z, Nyakas C, et al. Regular exercise: An effective means to reduce oxidative stress in old rats. Ann NY Acad Sci. 2004;1019:471–474. doi: 10.1196/annals.1297.085. [DOI] [PubMed] [Google Scholar]
- 30.Pedersen BK, Bruunsgaard H, Ostrowski K, et al. Cytokines in aging and exercise. Int J Sports Med. 2000;21(Suppl 1):S4–S9. doi: 10.1055/s-2000-1444. [DOI] [PubMed] [Google Scholar]
- 31.Slattery ML, Jacobs DR., Jr. Physical fitness and cardiovascular disease mortality: The U.S. Railroad Study. Am J Epidemiol. 1988;127:571–580. doi: 10.1093/oxfordjournals.aje.a114832. [DOI] [PubMed] [Google Scholar]
- 32.Ellestad MH, Wan MK. Predictive implications of stress testing: Follow-up of 2700 subjects after maximum treadmill stress testing. Circulation. 1975;51:363–369. doi: 10.1161/01.cir.51.2.363. [DOI] [PubMed] [Google Scholar]
- 33.Langer RD, Ganiats TG, Barrett-Connor E. Paradoxical survival of elderly men with high blood pressure. BMJ. 1989;298:1356–1357. doi: 10.1136/bmj.298.6684.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodwin JS. Embracing complexity: A consideration of hypertension in the very old. J Gerontol A Biol Sci Med Sci. 2003;58A:M653–M658. doi: 10.1093/gerona/58.7.m653. [DOI] [PubMed] [Google Scholar]
- 35.Oates DJ, Berlowitz DR, Glickman ME, et al. Blood pressure and survival in the oldest old. J Am Geriatr Soc. 2007;55:383–388. doi: 10.1111/j.1532-5415.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 36.Elia M. Obesity in the elderly. Obes Res. 2001;9(Suppl 4):S244–S248. doi: 10.1038/oby.2001.126. [DOI] [PubMed] [Google Scholar]
- 37.Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med. 2001;161:1194–1203. doi: 10.1001/archinte.161.9.1194. [DOI] [PubMed] [Google Scholar]
- 38.Janssen I, Katzmarzyk PT, Ross R. Body mass index is inversely related to mortality in older people after adjustment for waist circumference. J Am Geriatr Soc. 2005;53:2112–2118. doi: 10.1111/j.1532-5415.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 39.Lee I-M, Bouchard C. Physical activity, fitness, and cancer. In: Shephard RJ, Stephens T, editors. Physical Activity, Fitness, and Health. Human Kinetics; Champaign, IL: 1994. pp. 814–831. [Google Scholar]
- 40.Batty D, Thune I. Does physical activity prevent cancer? Evidence suggests protection against colon cancer and probably breast cancer. BMJ. 2000;321:1424–1425. doi: 10.1136/bmj.321.7274.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wannamethee SG, Shaper AG, Walker M. Physical activity and risk of cancer in middle-aged men. Br J Cancer. 2001;85:1311–1316. doi: 10.1054/bjoc.2001.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colbert LH, Hartman TJ, Malila N, et al. Physical activity in relation to cancer of the colon and rectum in a cohort of male smokers. Cancer Epidemiol Biomarkers Prev. 2001;10:265–268. [PubMed] [Google Scholar]
- 43.Lee I-M, Paffenbarger RS, Jr, Hsieh C-C. Physical activity and risk of prostatic cancer among college alumni. Am J Epidemiol. 1992;135:169–179. doi: 10.1093/oxfordjournals.aje.a116269. [DOI] [PubMed] [Google Scholar]
- 44.Bouchard C, Daw EW, Rice T, et al. Familial resemblance for VO2max in the sedentary state: The HERITAGE Family Study. Med Sci Sports Exerc. 1998;30:252–258. doi: 10.1097/00005768-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Bouchard C, An P, Rice T, et al. Familial aggregation of VO2max response to exercise training: Results from the HERITAGE Family Study. J Appl Physiol. 1999;87:1003–1008. doi: 10.1152/jappl.1999.87.3.1003. [DOI] [PubMed] [Google Scholar]
- 46.Blair SN, Mulder RT, Kohl HW. Reaction to “secular trends in adult physical activity: Exercise boom or bust? Res Q Exerc Sport. 1987;58:106–110. [Google Scholar]
- 47.Paffenbarger RS, Jr, Blair SN, Lee I-M, et al. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Smorawinski J, Nazar K, Kaciuba-Uscilko H, et al. Effects of 3-day bed rest on physiological responses to graded exercise in athletes and sedentary men. J Appl Physiol. 2001;91:249–257. doi: 10.1152/jappl.2001.91.1.249. [DOI] [PubMed] [Google Scholar]
- 49.Church TS, Earnest CP, Skinner JS, et al. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: A randomized controlled trial. JAMA. 2007;297:2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]