Abstract
CD44v6 has been causally associated with the development of metastases and with poor prognosis in various human malignancies. To elucidate the clinicopathological significance of CD44v6 expression in esophageal squamous cell carcinoma (ESCC), the present study aimed to investigate the expression of CD44v6 using immunohistological techniques. Using specific antibodies against CD44v6 and CD44s, expression of the proteins was analyzed immunohistochemically in 63 primary esophageal ESCCs, which were previously resected at the Nagoya City University Hospital without pre-operative induction therapy. Using light microscopy, the positive expression of CD44v6 was divided into a low- or high-expression group. The expression of CD44v6 in ESCC was analyzed with respect to various clinicopathological characteristics. The frequency of CD44v6 expression was 90.5% (57/63). The CD44v6 high-expression group comprised 55.6% of the patients (n=35) and the low expression group included 44.4% of the patients (n=28). In this study, no significant difference was observed between any clinicopathological factor and the immunohistochemical expression of CD44v6. In patients with high levels of CD44v6 expression, survival was markedly worse (p=0.0327). Favorable outcomes were observed for the clinicopathological characteristics of 6 patients whose tissue immunohistochemical expression of CD44v6 was not detected. Moreover, multivariate analysis confirmed that expression of CD44v6 was an independent prognostic indicator (risk ratio =2.793; p=0.0301). Overexpression of CD44v6 is a useful prognostic indicator of ESCC. Therefore, CD44v6 should be investigated as a potential target for therapy.
Keywords: CD44v6, esophageal squamous cell carcinoma, immunohistochemistry
Introduction
Esophageal squamous cell carcinoma (ESCC) is a malignant tumor belonging to the class of gastrointestinal carcinomas. Patients with ESCC have a poor prognosis despite intensive multimodality therapy such as surgery, radiation and chemotherapy. Almost 400,000 new cases of esophageal cancer are diagnosed annually worldwide, making it the eighth most common cancer and the sixth most common cause of cancer-related mortality (1). To improve patient survival, it is important to identify those relevant biomarkers in ESCC that are associated with adverse prognosis and to modify the therapeutic strategy for those patients accordingly. In the present study, expression of CD44v6 was assessed in the primary lesions of ESCC to elucidate its significance in clinical prognosis.
CD44 is a transmembrane glycoprotein involved in cell-cell and cell-extracellular matrix interactions, and whose role is to maintain cellular adhesion (2,3). CD44 is encoded by a single gene on chromosome 11p13, but actually represents a polymorphic group of transmembrane glycoproteins due to extensive alternative splicing and post-translational modifications (4). The human gene is composed of 20 exons, 10 of which (exons 1–5 and 16–20) are included in CD44 standard form (CD44s). CD44s is the smallest and most abundant member of this polymorphic and monogenic family of proteins. The remaining exons (exons 6–15) can be differentially inserted into the mature mRNA via alternative splicing and may give rise to hundreds of protein variants (5).
Overexpression of a number of CD44 variant isoforms was associated with tumor progression, suggesting that these CD44 isoforms have unique signaling properties (6–8). In colon cancer, CD44v3 was shown to promote invasion and resistance to apoptosis, while CD44v6 was associated with metastasis and decreased disease-free survival (6,7,9). In lung cancer, preferential CD44 variant expression occurs in squamous cell and bronchoalveolar carcinoma, where v5 and v6 variants appear to promote metastasis (8,10). Numerous reports showed that CD44 variants promote breast cancer progression, including the association of CD44v3-containing isoforms and breast cancer metastasis (6,11). The significance of CD44 variants in ESCC was discussed in previous investigations (2,12–14).
This study aimed to elucidate the clinicopathological significance of CD44v6 overexpression in ESCC.
Materials and methods
Patients and tissue samples
Samples were obtained from 63 patients with primary ESCCs. The patients had undergone radical esophagectomy without any pre-operative induction therapy at the Department of Surgery II, Nagoya City University Medical School, between 1997 and 2004. The study design was approved by the institutional review board of our university and written consent was obtained from all patients. Tumors were classified according to the guidelines for clinical and pathological studies on carcinoma of the esophagus established by the Japanese Society for Esophageal Diseases. Tissue specimens were collected from 51 males and 12 females, with a mean age of 63.5±8.3 years (range 46–78) (Table I). Tissues for immunohistochemistry were fixed in formalin and embedded in paraffin.
Table I.
Relationship between the clinicopathological characteristics and immunostaining for CD44v6.
| CD44v6 expression | ||||
|---|---|---|---|---|
|
|
||||
| Total | High (n=35) | Low (n=28) | p-value | |
| Gender | ||||
| Male | 51 | 27 | 24 | |
| Female | 12 | 8 | 4 | 0.3893 |
| Age | ||||
| <65 | 35 | 22 | 13 | |
| ≥65 | 28 | 13 | 15 | 0.1922 |
| T factor | ||||
| T1 | 21 | 11 | 10 | |
| T2 | 10 | 5 | 5 | |
| T3 | 21 | 12 | 9 | |
| T4 | 11 | 7 | 4 | 0.9138 |
| T1 | 21 | 11 | 10 | |
| T2–4 | 42 | 24 | 18 | 0.7199 |
| N factor | ||||
| Negative | 20 | 10 | 10 | |
| Positive | 43 | 25 | 18 | 0.5450 |
| Stage | ||||
| 0 | 6 | 2 | 4 | |
| I | 11 | 6 | 5 | |
| II | 13 | 7 | 6 | |
| III | 16 | 9 | 7 | |
| IV | 17 | 11 | 6 | 0.7726 |
| 0–I | 17 | 8 | 9 | |
| II–IV | 46 | 27 | 19 | 0.4093 |
| Lymphatic invasion | ||||
| Negative | 19 | 9 | 10 | |
| Positive | 44 | 26 | 18 | 0.3901 |
| Vein invasion | ||||
| Negative | 28 | 16 | 12 | |
| Positive | 35 | 19 | 16 | 0.8206 |
| Differentiation | ||||
| Well | 18 | 10 | 8 | |
| Moderate | 40 | 24 | 16 | |
| Poor | 5 | 1 | 4 | 0.2369 |
Immunohistochemistry
Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded ESCC tissues. Paraffin-embedded tumor sections were deparaffinized, rehydrated, heat-treated by microwaving in 10 mM citrate buffer for 15 min for antigen retrieval and cooled to room temperature. Sections were then treated with 0.3% H2O2 in methanol for 30 min to neutralize endogenous peroxidases, blocked with non-specific goat serum for 10 min and incubated with the primary monoclonal antibodies for CD44v6 (1:200; Serotec, UK) and CD44s (1:500; Enzo, Miami, FL, USA) overnight at 4°C. Immunoreactive protein was detected with a Dako Envision™ + System, HRP (DAB), and the sections were then counterstained with hematoxylin.
The immunostaining of CD44v6 and CD44s was subjectively assessed by two independent investigators (M.S. and H.I.) using light microscopy. The CD44v6-positive cells were counted and the positive expression was classified as: low-expression group with positive cells <50% and high-expression group with positive cells >50%.
Statistical analysis
Statistical analysis was performed using the Stat-View software package (Abacus Concepts, Berkeley, CA, USA). The Chi-square test was used to analyze the association between the immunohistochemical analysis and the clinical histopathological parameters of the patients. The survival of ESCC patients following surgery was assessed using the Kaplan-Meier method and survival times were compared using the log-rank test. The data are expressed as the mean ± SD. Multivariate analysis was performed using the Cox regression model and logistic multivariate regression model. In all analyses, p<0.05 was considered to be statistically significant.
Results
The frequency of CD44v6 expression was 90.5% (57/63). Representative cases of immunostaining are shown in Fig. 1. The CD44v6 high-expression group comprised 55.6% (n=35) of the patients and the low expression group included 44.4% of the patients (n=28). CD44v6 was observed only in epithelial and tumor cells. By contrast, CD44s was not solely expressed in epithelial and tumor cells in ESCCs. CD44s was also strongly present in lymphocytic cells and was loosely stained in the interstitium. Therefore, we could not evaluate the immunohistochemical expression for CD44s in the same manner. CD44v6 and CD44s staining was shown directly in consecutive serial sections of the same samples (Fig. 2).
Figure 1.
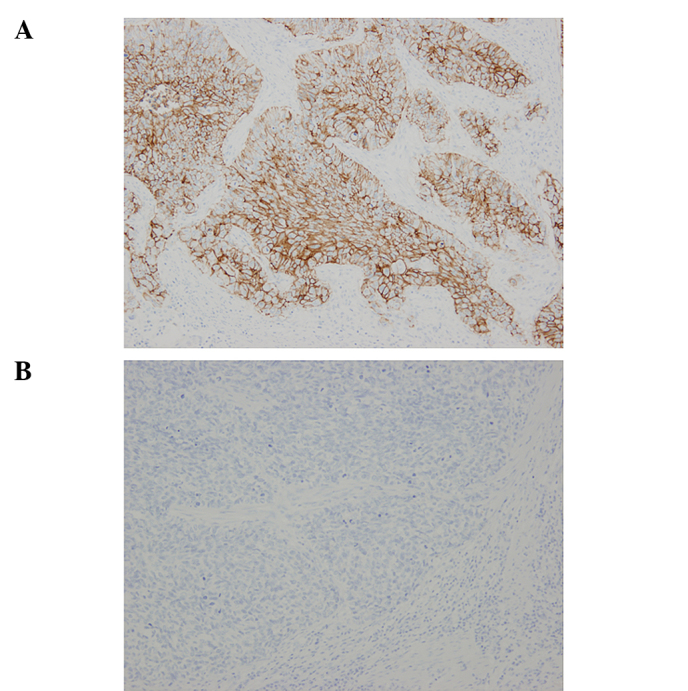
Representative immunostaining of CD44v6 in ESCC. (A) Positive for CD44v6 expression. (B) Negative for CD44v6 expression (original magnification, ×100).
Figure 2.
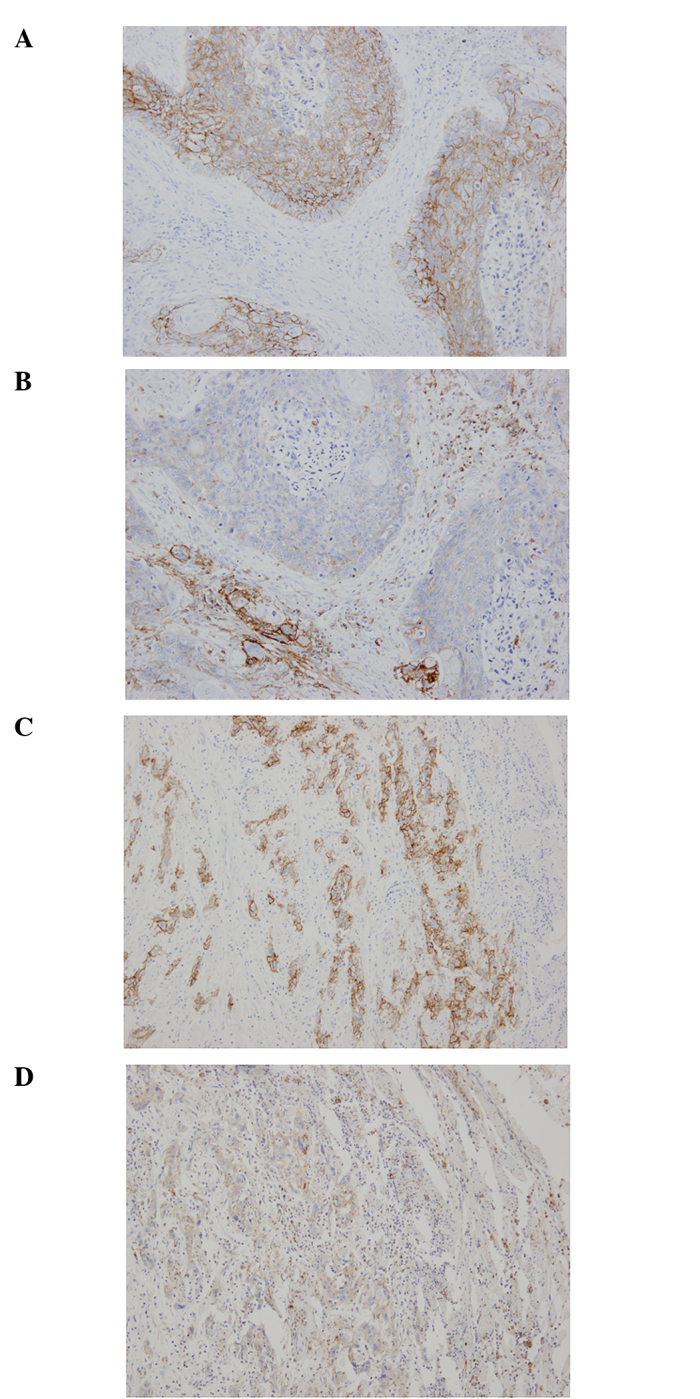
Comparative expression of CD44v6 and CD44s in ESCC. Carcinoma cells were positive for CD44v6 (A and C) and CD44s (B and D). CD44s was observed, not only in CD44s tumor cells, but also in lymphocytic cells and the interstitium (B and D). Cell nuclei were stained with hematoxylin (blue). Original magnification, ×100.
The correlation between immunostaining for CD44v6 and the clinicopathological characteristics of the patients are shown in Table I. No significant correlation was observed between clinicopathological characteristics such as age, gender, T factor, N factor, stage, lymphatic invasion, venous invasion and differentiation, and the immunohistochemical expression of CD44v6 (Table I).
We subsequently investigated the correlation between immunostaining for CD44v6 and survival in ESCC patients after surgery (median follow-up, 28 months). The patients in the CD44v6 high-expression group had a significantly shorter survival following surgery than patients whose expression was low (p=0.0301, log-rank test) (Fig. 3). Moreover, favorable outcomes were noted for the clinicopathological characteristics of 6 patients whose expression of CD44v6 was not detected, (Table II).
Figure 3.
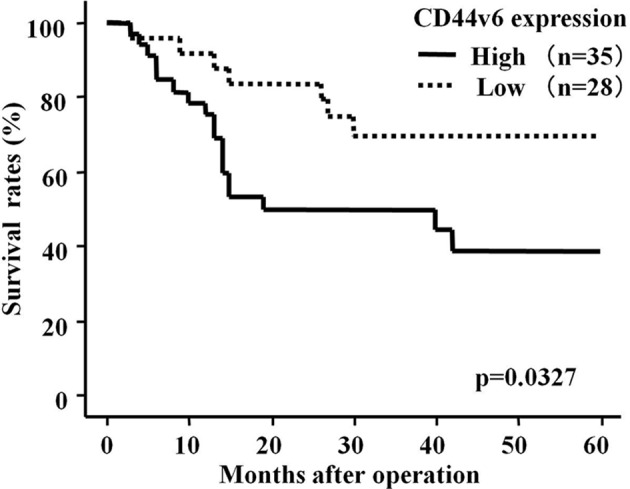
Overall survival rate of patients with ESCC according to CD44v6 immunostaining. Patients with overexpression of CD44v6 had a significantly shorter survival after surgery than patients with a low expression of CD44v6 (p=0.0327).
Table II.
Clinicopathological characteristics of 6 patients.
| Patient | Gender | Age | T factor | N factor | Stage | Lympatic invasion | Vein invasion | Differrentiation (months) | Follow-up | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 50 | T3 | n2 | III | + | + | Poor | 60 | Alive |
| 2 | Male | 74 | T1 | n1 | II | + | + | Well | 30 | Dead |
| 3 | Male | 67 | T1 | n0 | 0 | − | − | Moderate | 44 | Alive |
| 4 | Female | 57 | T3 | n1 | III | + | + | Well | 59 | Alive |
| 5 | Male | 62 | T1 | n0 | 0 | − | − | Moderate | 58 | Alive |
| 6 | Male | 72 | T1 | n0 | I | + | − | Moderate | 55 | Alive |
Univariate analysis revealed that among the clinicopathological factors, tumor status [risk ratio (RR)=9.346; p=0.0025], lymph node status (RR=6.211; p=0.0001), lymphatic invasion (RR=6.623; p=0.0105), venous invasion (RR=2.809; p=0.0209) and CD44v6 expression (RR=2.491; p=0.0410) were all statistically significant prognostic factors (Table III).
Table III.
Univariate analysis.
| Characteristics | Risk ratio | 95% CI | p-value |
|---|---|---|---|
| Age at surgery | |||
| <65 | 1 | ||
| ≥65 | 0.915 | 0.415–2.018 | 0.8261 |
| Gender | |||
| Female | 1 | ||
| Male | 0.872 | 0.326–2.331 | 0.7843 |
| Histological grade | |||
| Well | 1 | ||
| Moderately/poorly | 0.642 | 0.288–1.431 | 0.2784 |
| Tumor status | |||
| T1 | 1 | ||
| T2–4 | 9.346 | 2.193–40.000 | 0.0025 |
| Lymph node status | |||
| n0–1 | 1 | ||
| n2–4 | 6.211 | 2.222–15.625 | 0.0001 |
| Lymphatic invasion | |||
| Negative | 1 | ||
| Positive | 6.623 | 1.558–28.571 | 0.0105 |
| Vein invasion | |||
| Negative | 1 | ||
| Positive | 2.809 | 1.170–6.757 | 0.0209 |
| CD44v6 expression | |||
| Low | 1 | ||
| High | 2.491 | 1.038–5.975 | 0.0410 |
CI, confidence interval.
Multivariate analysis revealed that CD44v6 overexpression (RR=2.793; p=0.0301) as well as tumor status (RR=9.259; p=0.0275) and lymph node status (RR=4.785; p=0.0023) were factors independently associated with an unfavorable prognosis of patients with ESCC (Table IV).
Table IV.
Multivariate analysis.
| Parameter | Risk ratio | 95% CI | p-value |
|---|---|---|---|
| Tumor status | |||
| T1 | 1 | ||
| T2–4 | 9.259 | 1.279–66.667 | 0.0275 |
| Lymph node status | |||
| n0–1 | 1 | ||
| n2–4 | 4.785 | 1.748–13.158 | 0.0023 |
| Lymphatic invasion | |||
| Negative | 1 | ||
| Positive | 0.369 | 0.038–3.559 | 0.3880 |
| Vein invasion | |||
| Negative | 1 | ||
| Positive | 2.037 | 0.718–5.780 | 0.1809 |
| CD44v6 expression | |||
| Low | 1 | ||
| High | 2.793 | 1.104–7.066 | 0.0301 |
CI, confidence interval.
Discussion
The present study aimed to determine the clinicopathological significance of CD44v6 expression in ESCC. In this study, three main findings were noted. First, CD44v6 was expressed at a high frequency in patients with ESCC. Of the 63 ESCC cases, positivity for CD44v6 was observed in 57 cases (90.5%) (Table I). Second, the survival of patients whose expression of CD44v6 was high was significantly less favorable than that of patients whose expression was low (p=0.0301; Fig. 3). Third, immunohistochemical overexpression of CD44v6 was an independent prognostic indicator of patients with ESCC (Table III).
The first major finding involving CD44 in the metastatic process was the identification of a CD44 variant isoform containing exons v4-v7 in a highly metastasizing rat pancreatic carcinoma cell line (BSp73ASML). Transfection of this specific variant into related BSp73AS cells that did not metastasize conferred metastatic potential to those cells upon injection into syngeneic rats (15). Moreover, a CD44 exon v6-specific antibody blocked the metastatic propensity of these cells. When animals injected with metastatic BSpASv4-v7 cells were treated with anti-CD44v6 antibody, lymph node and lung metastases were blocked (16). Since these findings, CD44v6 has attracted increasing interest and investigations are currently underway regarding the physiological significance of CD44v6 as a prognostic factor for tumor progression. The exact role that CD44v6 plays in ESCC has yet to be elucidated, but has become an area of active study.
In previous studies, high expression frequencies exceeding 90% have been found in squamous cell carcinomas (SCCs) derived from head and neck, esophagus, skin and lung (10, 17–20). Our studies are in agreement with those results. In addition to CD44v6, CD44s was expressed in epithelial and tumor cells in ESCCs at high frequencies. CD44s stained strongly in lymphocytic cells and was loosely stained in the stroma. On the other hand, CD44v6 was expressed only in carcinoma and non-cancerous epithelial cells. Therefore, CD44v6 appears to be an ideal target antigen for the majority of SCCs. This frequent and homogeneous expression of CD44v6 renders SCC a suitable target for therapeutic approaches using CD44v6-specific antibodies.
There has been some controversy regarding the relationship of CD44v6 to the prognoses of various malignancies. It was previously reported that overexpression of CD44v6 is associated with metastasis of prostate cancer (21) and the prognosis of thymic epithelial neoplasms (22). In breast cancer, Kaufmann et al proposed that CD44v6 is a good marker for prognosis independent of progesterone receptor, lymph node status, tumor size and grade (23). By contrast, it has been suggested that CD44v6 is negatively associated with the progression of various malignancies. Lipponen et al studied 173 patients with bladder tumors and found that those with negative CD44v6 immunoreactivity had poorer prognoses (24). We showed the survival rates of ESCC patients with overexpression of CD44v6 to be markedly worse and that CD44v6 expression was a significant independent prognostic factor for patients with ESCC. Notably, in 6 patients lacking CD44v6 expression, favorable outcomes were found for their clinicopathological characteristics (Table II). These results suggest that ESCC patients lacking CD44v6 expression have more favorable prognoses, even when disease is advanced. CD44v6 is potentially a co-receptor for c-Met and VEGFR-2 (28,29). Further studies are required in order to clarify the role of CD44v6 protein in ESCC.
CD44v6 is a good prognostic marker and a suitable target for anticancer therapy for ESCC patients. Consequently, patients suffering from head and neck squamous cell carcinoma have entered phase I clinical trials utilizing CD44v6 antibodies that were either radiolabeled or covalently linked to a toxin (25,26). Although the phase I clinical trials appeared to be promising, 1 patient developed toxic epidermal necrolysis and succumbed to the disease. For this reason, the development of this drug has been terminated (27). Despite the termination of the trials, CD44v6 remains a valid target for anticancer therapy. Alternative strategies targeting CD44v6 functions have been presented.
In conclusion, overexpression of CD44v6 is an independent prognostic indicator for patients with ESCC. CD44v6 may be used as a prognostic marker for various malignancies, including ESCC. Although the precise molecular mechanism of up-regulated CD44v6 expression has yet to be clarified, our data have clearly indicated that CD44v6 may be a favorable candidate as a prognostic marker as well as a molecular target for the development of an effective therapeutic reagent for patients with esophageal cancer.
Acknowledgements
The authors would like to thank Ms. Shinobu Makino for the excellent technical assistance.
References
- 1.Parkin DM, Bray F. Evaluation of data quality in the cancer registry: principles and methods Part II. Completeness. Eur J Cancer. 2009;45:756–764. doi: 10.1016/j.ejca.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 2.Lyons AJ, Jones J. Cell adhesion molecules, the extracellular matrix and oral squamous carcinoma. Int J Oral Maxillofac Surg. 2007;36:671–679. doi: 10.1016/j.ijom.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Rautava J, Soukka T, Inki P, Leimola-Virtanen R, Saloniemi I, Happonen RP, Heikinheimo K. CD44v6 in developing, dysplastic and malignant oral epithelia. Oral Oncol. 2003;39:373–379. doi: 10.1016/s1368-8375(02)00140-9. [DOI] [PubMed] [Google Scholar]
- 4.Naor D, Wallach-Dayan SB, Zahalka MA, Sionov RV. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin Cancer Biol. 2008;18:260–267. doi: 10.1016/j.semcancer.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Van Weering DH, Baas PD, Bos JL. A PCR-based method for the analysis of human CD44 splice products. PCR Methods Appl. 1993;3:100–106. doi: 10.1101/gr.3.2.100. [DOI] [PubMed] [Google Scholar]
- 6.Iida N, Bourguignon LY. New CD44 splice variants associated with human breast cancers. J Cell Physiol. 1995;162:127–133. doi: 10.1002/jcp.1041620115. [DOI] [PubMed] [Google Scholar]
- 7.Kuniyasu H, Oue N, Tsutsumi M, Tahara E, Yasui W. Heparan sulfate enhances invasion by human colon carcinoma cell lines through expression of CD44 variant exon 3. Clin Cancer Res. 2001;7:4067–4072. [PubMed] [Google Scholar]
- 8.Pirinen R, Hirvikoski P, Bohm J, Kellokoski J, Moisio K, Viren M, Johansson R, Hollmen S, Kosma VM. Reduced expression of CD44v3 variant isoform is associated with unfavorable outcome in non-small cell lung carcinoma. Hum Pathol. 2000;31:1088–1095. doi: 10.1053/hupa.2000.16277. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn S, Koch M, Nubel T, Ladwein M, Antolovic D, Klingbeil P, Hildebrand D, Moldenhauer G, Langbein L, Franke WW, Weitz J, Zoller M. A complex of EpCAM, claudin-7, CD44 variant isoforms, and tetraspanins promotes colorectal cancer progression. Mol Cancer Res. 2007;5:553–567. doi: 10.1158/1541-7786.MCR-06-0384. [DOI] [PubMed] [Google Scholar]
- 10.Mizera-Nyczak E, Dyszkiewicz W, Heider KH, Zeromski J. Isoform expression of CD44 adhesion molecules, Bcl-2, p53 and Ki-67 proteins in lung cancer. Tumour Biol. 2001;22:45–53. doi: 10.1159/000030154. [DOI] [PubMed] [Google Scholar]
- 11.Iida N, Bourguignon LY. Coexpression of CD44 variant (v10/ex14) and CD44S in human mammary epithelial cells promotes tumorigenesis. J Cell Physiol. 1997;171:152–160. doi: 10.1002/(SICI)1097-4652(199705)171:2<152::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 12.Koyama S, Maruyama T, Adachi S. Expression of epidermal growth factor receptor and CD44 splicing variants sharing exons 6 and 9 on gastric and esophageal carcinomas: a two-color flow-cytometric analysis. J Cancer Res Clin Oncol. 1999;125:47–54. doi: 10.1007/s004320050241. [DOI] [PubMed] [Google Scholar]
- 13.Gotoda T, Matsumura Y, Kondo H, Ono H, Kanamoto A, Kato H, Watanabe H, Tachimori Y, Nakanishi Y, Kakizoe T. Expression of CD44 variants and prognosis in oesophageal squamous cell carcinoma. Gut. 2000;46:14–19. doi: 10.1136/gut.46.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li DM, Li SS, Zhang YH, Zhang HJ, Gao DL, Wang YX. Expression of human chorionic gonadotropin, CD44v6 and CD44v4/5 in esophageal squamous cell carcinoma. World J Gastroenterol. 2005;11:7401–7404. doi: 10.3748/wjg.v11.i47.7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- 16.Seiter S, Arch R, Reber S, Komitowski D, Hofmann M, Ponta H, Herrlich P, Matzku S, Zoller M. Prevention of tumor metastasis formation by anti-variant CD44. J Exp Med. 1993;177:443–455. doi: 10.1084/jem.177.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Hal NL, van Dongen GA, Stigter-van Walsum M, Snow GB, Brakenhoff RH. Characterization of CD44v6 isoforms in head-and-neck squamous-cell carcinoma. Int J Cancer. 1999;82:837–845. doi: 10.1002/(sici)1097-0215(19990909)82:6<837::aid-ijc12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 18.Kanke M, Fujii M, Kameyama K, Kanzaki J, Tokumaru Y, Imanishi Y, Tomita T, Matsumura Y. Clinicopathological significance of expression of CD44 variants in head and neck squamous cell carcinoma. Jpn J Cancer Res. 2000;91:410–415. doi: 10.1111/j.1349-7006.2000.tb00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heider KH, Sproll M, Susani S, Patzelt E, Beaumier P, Ostermann E, Ahorn H, Adolf GR. Characterization of a high-affinity monoclonal antibody specific for CD44v6 as candidate for immunotherapy of squamous cell carcinomas. Cancer Immunol Immunother. 1996;43:245–253. doi: 10.1007/s002620050329. [DOI] [PubMed] [Google Scholar]
- 20.Simon JC, Heider KH, Dietrich A, Wuttig C, Schopf E, Adolf GR, Ponta H, Herrlich P. Expression of CD44 isoforms in human skin cancer. Eur J Cancer. 1996;32A:1394–1400. doi: 10.1016/0959-8049(96)00196-7. [DOI] [PubMed] [Google Scholar]
- 21.Ekici S, Cerwinka WH, Duncan R, Gomez P, Civantos F, Soloway MS, Lokeshwar VB. Comparison of the prognostic potential of hyaluronic acid, hyaluronidase (HYAL-1), CD44v6 and microvessel density for prostate cancer. Int J Cancer. 2004;112:121–129. doi: 10.1002/ijc.20368. [DOI] [PubMed] [Google Scholar]
- 22.Sonobe S, Miyamoto H, Nobukawa B, Izumi H, Futagawa T, Ishikawa N, Yamazaki A, Uekusa T, Abe H, Suda K. Prognostic value of CD44 isoform expression in thymic epithelial neoplasms. Cancer. 2005;103:2015–2022. doi: 10.1002/cncr.21046. [DOI] [PubMed] [Google Scholar]
- 23.Kaufmann M, Heider KH, Sinn HP, von Minckwitz G, Ponta H, Herrlich P. CD44 variant exon epitopes in primary breast cancer and length of survival. Lancet. 1995;345:615–619. doi: 10.1016/s0140-6736(95)90521-9. [DOI] [PubMed] [Google Scholar]
- 24.Lipponen P, Aaltoma S, Kosma VM, Ala-Opas M, Eskelinen M. Expression of CD44 standard and variant-v6 proteins in transitional cell bladder tumours and their relation to prognosis during a long-term follow-up. J Pathol. 1998;186:157–164. doi: 10.1002/(SICI)1096-9896(1998100)186:2<157::AID-PATH169>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Heider KH, Kuthan H, Stehle G, Munzert G. CD44v6: a target for antibody-based cancer therapy. Cancer Immunol Immunother. 2004;53:567–579. doi: 10.1007/s00262-003-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stroomer JW, Roos JC, Sproll M, Quak JJ, Heider KH, Wilhelm BJ, Castelijns JA, Meyer R, Kwakkelstein MO, Snow GB, Adolf GR, van Dongen GA. Safety and biodistribution of 99mTechnetium-labeled anti-CD44v6 monoclonal antibody BIWA 1 in head and neck cancer patients. Clin Cancer Res. 2000;6:3046–3055. [PubMed] [Google Scholar]
- 27.Riechelmann H, Sauter A, Golze W, Hanft G, Schroen C, Hoermann K, Erhardt T, Gronau S. Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral Oncol. 2008;44:823–829. doi: 10.1016/j.oraloncology.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 2002;16:3074–3086. doi: 10.1101/gad.242602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tremmel M, Matzke A, Albrecht I, Laib AM, Olaku V, Ballmer-Hofer K, Christofori G, Heroult M, Augustin HG, Ponta H, Orian-Rousseau V. A CD44v6 peptide reveals a role of CD44 in VEGFR-2 signaling and angiogenesis. Blood. 2009;114:5236–5244. doi: 10.1182/blood-2009-04-219204. [DOI] [PubMed] [Google Scholar]


