Abstract
The medicinal mushroom Ganoderma lucidum (G. lucidum) has been used for the treatment of various diseases, and is known for the immune-enhancing activity of its polysaccharide. However, little is known about another of its major constituents, triterpene. This study investigated the anticancer mechanism of a triterpene-enriched extract from G. lucidum. The triterpene-enriched extract, GLAI, was prepared from fruiting bodies of G. lucidum by sequential hot water extraction, removal of ethanol-insoluble polysaccharides and gel-filtration chromatography. The mechanisms of GLAI-induced apoptosis on SW620 human colorectal adenocarcinoma cells were investigated. Tumor cell lines in vitro were treated with different concentrations of GLAI. Cell proliferation was measured by the Alamar blue assay, morphology of cell apoptosis was observed, cell apoptosis was detected by flow cytometry (FCM) and caspase-3 activity was detected by Caspase-3 cellular activity assay. The results showed that GLAI inhibited the growth of different tumor cells and caused significant apoptosis in a dose-dependent manner. Marked morphological changes of cell apoptosis were observed after the cells had been exposed to GLAI for 24 h. The Caspase-3 assay results showed that the activity of the caspase-3 enzyme increased in both a time- and dose-dependent manner, whereas GLAI resulted in the down-regulation of Bcl-2 gene expression at the mRNA level and XIAP protein production at the protein level. Conversely, GLAI up-regulates the expression of the apoptosis enhancer Bax gene and p53 protein. These findings suggest that the triterpenes contained in G. lucidum are potential anticancer agents.
Keywords: apoptosis, Ganoderma lucidum, triterpene
Introduction
Ganoderma lucidum (G. lucidum), known as ‘Lingzhi’ in China, is a lamella-less basidiomycetous fungus that belongs to the Polyporaceae family. The medicinal properties of this mushroom are well known in China and other parts of Asia. Known as ‘miraculous Zhi’ or ‘auspicious herb’, Lingzhi is considered to ‘symbolize happy augury, and to bespeak good fortune, good health and longevity, even immortality’ (1). The fungus has been used as a traditional Chinese medicine to treat a variety of diseases for more than 4,000 years, and regular consumption of the mushroom extracts is believed to preserve human vitality and promote longevity (2,3).
A number of bioactive components have been identified from its fruit bodies, mycelia, spores and culture media. Polysaccharides and triterpenes are two major categories of the bioactive ingredients. It was previously identified that polysaccharides from G. lucidum exert their in vitro and in vivo anticancer effect via an immune-modulatory mechanism (4–6). Studies showed that triterpenes possess the bioactivities of antioxidation (7), hepatoprotection (8), cholesterol stasis (9), anti-hypertension (10,11) and inhibiting platelet aggregation (12) due to the inhibition of enzymes such as h-galactosidase, cholesterol synthase and angiotension-converting enzyme. Triterpenes isolated from G. lucidum were reported to exhibit cytotoxic activity against tumor cells (13–16). A triterpene from Ganoderma tsugae was found to induce cell apoptosis and cell cycle arrest in human hepatoma Hep3B cells, but its molecular mechanism was not investigated (13).
Apoptosis is a form of cell death defined by a characteristic set of morphological and biochemical changes. Previous studies identified a significant role for caspases, a family of cysteine-dependent aspartate-directed proteases, in apoptotic death, especially in the context of cancer cells (17). Individual members of the caspase family mediate apoptosis in different cell types, and different caspases have been found to mediate apoptosis even within a given cell type depending on the apoptotic stimulus received by the cells (18). Caspase-3 and -9 are reported to play key roles in caspase-mediated apoptosis, and variations in their activity were correlated with apoptosis in a variety of cancer cells (19,20).
In this study, we report that a triterpene-enriched fraction from mycelia of G. lucidum inhibits the growth of tumor cells and induces apoptosis in SW620 colorectal adenocarcinoma cells. Consequently, the anticancer mechanism of GLAI-induced apoptosis on SW620 human colorectal adenocarcinoma cells was examined. Findings present evidence of the signaling molecule involved in the anticancer activity of triterpene from G. lucidum.
Materials and methods
Preparation of ganoderma extracts
G. lucidum fruiting bodies were extracted with 95% (v/v) aqueous ethanol at room temperature. Combined ethanolic extracts were evaporated to dryness, redissolved in water and extracted with chloroform. After addition of a saturated NaHCO3 solution, the chloroform layer containing non-acidic triterpenoids was collected. The crude extracts were purified using silica gel (200–300 mesh) column chromatography. The column was eluted with petrol ether/acetone (v/v=1:0, 50:1, 30:1, 10:1, 3:1 sequentially) and eight fractions were collected. The second fraction was separated further by MCI chromatography and the elution gradient was 40–100% methanol in water. The fifth fraction, obtained with 80% methanol elution, was termed GLAI. Triterpenes were visualized as fluorescent spots under long wavelength UV light.
Cell cultures
Human tumor cell lines, SW620 cells (colon), MCF-7 (breast), K562 (bone marrow), and mouse lymphocytic leukemia cell line L1210 were obtained from the American Type Culture Collection (ATCC) and maintained at 37°C in RPMI-1640 containing 10% fetal calf serum (FCS) (Kraeber, Wedel, Germany), 100 U/ml penicillin and 100 μg/ml streptomycin.
Cell proliferation assay
Cells were adjusted to a concentration of 1×104 cells/ml. Cell suspension (180 μl) and different test agents (20 μl) were added to each well of a 96-well microplate reader. After incubation at 37°C in a 5% CO2 atmosphere for a defined time, 20 μl Alamar Blue reagent (Biosource, Nivelles, Belgium) were added to each well and incubation continued for another 6 h. The extinction was measured using a micro ELISA autoreader at 570 and 600 nm. The proliferation rate was calculated according to the Biosource protocol.
Microscopic observation and nuclear staining with Hoechst 33258
SW620 cells (1×104 cells/ml) were treated for 24 h with a control and 10 μM GLAI. Morphological observations of cultured cells were made by inverted, phase-contrast microscopy. Samples treated with DMSO only served as controls. For nuclear staining, SW620 cells (1×105 cells/ml) were treated for 24 h with DMSO and 10, 50 and 100 μM GLAI. After treatment, cells were harvested and washed with ice-cold phosphate-buffered saline (PBS). The cells were then incubated in nuclear fluorochrome Hoechst 33258 at a final concentration of 10 μg/ml at room temperature for 10 min in the dark. Nuclear morphology was then examined with an Olympus fluorescent microscope.
Flow cytometric analysis of apoptosis
To confirm the nature of the effects of GLAI on SW620 cells, dual-staining [propidium iodide (PI) and annexin V (AV)] flow cytometry was used to measure the externalization of phosphatidylserine (PS). Aliquots (5×106) of SW620 cells cultured as described above were treated with 20, 50 or 100 μmol/l GLAI for 24 h. Controls were treated with DMSO only. After washing and trypsinization, cell samples were collected by centrifugation (400 g, 3 min, 4°C) and double-stained using the apoptosis detection kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. Cells were incubated for 30 min at 25°C in 100 μl 1X buffer solution, 5 μl AV-FITC and 5 μl PI, and then a further 400 μl of 1X solution was added. The green fluorescence of AV-FITC-bound PS and the red fluorescence of DNA-bound PI in individual cells were measured at 525 and 575 nm, respectively, using a BD FACSCalibur. Cell populations were classified as: AV−/PI−, viable cells; AV+/PI−, early apoptotic cells; AV+/PI+, apoptotic cells; and AV−/PI+, residual damaged cells.
Caspase-3 activity assay
Caspase-3 activity in the lysates of SW620 cells was measured using the Caspase-3 cellular activity assay (Calbiochem, Darmstadt, Germany). SW620 cells were cultured as described above and suspensions (2×107 cells) were treated with different concentrations of GLAI (0, 10, 25 and 50 μmol/l) for 24 h. Controls were treated with DMSO only. After washing and trypsinization, cell suspensions were centrifuged (400 g, 3 min, 4°C) and cell pellets were re-suspended in 1 ml ice-cold cell lysis buffer for 5 min. Following centrifugation (400 g, 3 min, 4°C), cytosol supernatants were collected and enzyme activity was measured according to the manufacturer’s instructions. Reaction mixtures (total volume 100 μl) were incubated at 37°C for 10 min and the optical density value was measured for 15 h at 405 nm using an ELISA reader (Bio-Tek, Atlanta, GA, USA).
Reverse transcription-polymerase chain reaction (RT-PCR)
For RNA extraction, two experiments were performed. Firstly, SW620 cells (3.6×106 cells/ml) were incubated in RPMI-1640 containing 10% FCS and different concentrations of GLAI (10, 50 and 100 μg/ml). The entry with 0 μg/ml GLAI, treated with DMSO, was used as a negative control. The cells were cultured for 24 h at 37°C, 5% CO2, in a humidified incubator. Secondly, SW620 cells (2×106/ml) were incubated in RPMI-1640 containing 10% FCS and GLAI (50 μg/ml). The cells were cultured for 0, 6, 12, 18 and 24 h at 37°C, 5% CO2.
The cells were lysed in TRIzol reagent (Invitrogen Life Technologies). Total RNA was extracted according to the manufacturer’s instructions. The RNA pellet was dissolved in diethyl pyrocarbonate (DEPC)-treated water prior to use for reverse transcription, electrophoresed on a 1.5% agarose gel and visualized by ethidium bromide (EB) staining under an ultraviolet light, and two bands (28S and 18S) are evident.
RNA was primed with oligo(dT)15 and converted into complementary DNA (cDNA) by Moloney murine leukemia virus (MMLV) reverse transcriptase. The reaction mixture for reverse transcription contained 5.0X buffer, 2.5 mM of each deoxynucleotriphosphate (dNTP, i.e., dATP, dCTP, dGTP and dTTP), 40 U/μl RNase inhibitor, 200 U/μl reverse transcriptase, 10 μM oligo(dT)15 and 2 μg total RNA (equal amounts of starting RNA were used for each condition in the different experiments). The final volume of the reaction was 30 μl. The program parameters were 70°C for 5 min to heat, 37°C for 1.5 h for reverse transcription reaction and 95°C for 5 min to inactivate the reverse transcriptase. cDNA generated by reverse transcription was either used immediately for PCR experiments for the cytokines of interest or stored at −20°C.
Oligonucleotide primers for GAPDH, Bcl-2 and Bax were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). Table I shows the sequence primers and the sizes of the fragments generated by the PCR reactions.
Table I.
List of polymerase chain reaction primers.
| Genes | Primer sequences | Size (bp) |
|---|---|---|
| GAPDH | ||
| Sense | 5′ TGA AGG TCG GAG TCA ACG GAT TTG GT 3′ | 566 |
| Antisense | 5′ CAT GTG GGC CAT GAG GTC CAC CAC 3′ | |
| Bcl-2 | ||
| Sense | 5′ TGC ACC TGA CGC CCT TCA C 3′ | 293 |
| Antisense | 5′ AGA CAG CCA GGA GAA ATC AAA CAG 3′ | |
| Bax | ||
| Sense | 5′ ACC AAG AAG CTG AGC GAG TGT C 3′ | 332 |
| Antisense | 5′ ACA AAG ATG GTC ACG GTC TGC C 3′ | |
Glyceraldehyde 3-phosphate dehydrogenase.
The components added to the sample to make up 20 μl reaction mixture were: 1 μl cDNA, 2 μl 10X Taq DNA polymerase buffer, 2 μl 25 mM Mg2+ (Promega, Madison, WI, USA), 0.5 μl 10 mM mixture of all four deoxynucleotide triphosphates (Promega), 0.5 μl each of 5′ and 3′ primer (10 μM) and 1 unit Taq DNA polymerase (Promega). PCR was performed for 35 cycles. Temperature cycling was initiated with each cycle as follows: for GAPDH, 95°C for 45 sec (denaturation), 58°C for 45 sec (annealing), 72°C for 30 sec (extension); for Bcl-2, 95°C for 45 sec, 56°C for 45 sec, 72°C for 30 sec; for Bax, 95°C for 45 sec, 58°C for 45 sec and 72°C for 30 sec. The amplified products were detected in 1.5% agarose gels stained with EB and visualized under UV light.
Western-blot analysis
For the analysis of p53 and XIAP, SW620 cells (3.6×106/ml) were incubated in RPMI-1640 containing 10% FCS and different concentrations of GLAI (10, 20, 50 and 100 μg/ml). The entry with 0 μg/ml GLAI, treated with DMSO, was used as a negative control. The cells were cultured for 24 h at 37°C, 5% CO2, in a humidified incubator. Cells were washed twice with PBS and lysed in lysis buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% Triton X-100 and 100 μg/ml PMSF at 4°C overnight. The suspensions were then centrifuged at 12,000 rpm for 5 min; All lysates were subjected to BCA protein assay reagent (Pierce, Rockford, IL, USA) for the quantification of protein concentration, and then Western blot analysis was performed. Total proteins (20–50 μg) were separated by sodium dodecyl sulfate-polyacrylamide gelelectrophoresis using a 10% polyacrylamide gel. The proteins in the gel were transferred to a PVDF membrane. The membrane was blocked with 0.1% BSA in TBST for 1 h. Membranes were incubated with primary antibody (1:2,000) at 4°C overnight and then with secondary antibody (1:2,000) for 1 h. The membranes were washed three times in TBST for 10 min between each step. The signal was detected using the Amersham ECL system (Amersham-Pharmacia Biotech, Arlington Heights, IL, USA).
Statistical analysis
The data are shown as the means ± SD. The Student’s t-test was used to determine the significance of differences between population means with results considered as: significant, p<0.05; very significant, p<0.01; extremely significant, p<0.001.
Results
Cell proliferation assay
To determine the effect of GLAI on different tumor cells, the proliferation assay was performed using the Alamar blue. As shown in Fig. 1, GLAI at a concentration of 2 μmol/l inhibited the growth of SW620, K562 and L1210 cells to ~19, 12 and 60%, respectively. Exposure of the SW620, K562 and L1210 cells to 10 μmol/l GLAI inhibited cell growth by 35, 52 and 86%. respectively. However, no additional effect was observed with MCF7 cells at these concentrations (Fig. 1). However, increased growth inhibition of SW620, K562, MCF7 and L1210 cells (to 55, 90 and 98%, respectively) was observed following exposure to 50 μmol/l GLAI. Treatment with 100 μmol/l GLAI resulted in almost total inhibition of cell growth and few viable cells (see below) in all cases. Positive controls treated with 5-fluorouracil were inhibited 88–90% under these conditions.
Figure 1.
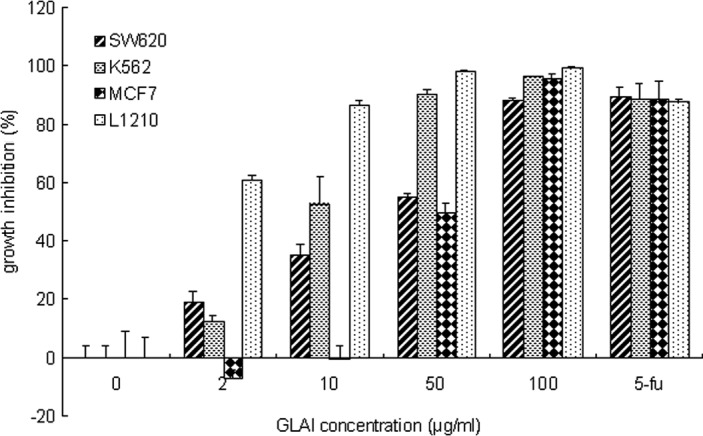
Effect of GLAI on the growth of different tissue cell lines. Cells were treated for 72 h with different concentrations of GLAI (2, 10, 50 and 100 μg/ml) dissolved in DMSO. Negative controls were treated with DMSO only and positive controls were treated with 5-fluorouracil (1 μg/ml). Values are the mean ± SD. Experiments were performed in triplicate, p<0.001.
Microscopic observation
Normally adhesive SW620 cells were readily suspended following treatment with 10 μmol/l GLAI for 24 h, and few viable cells were observed following exposure to 50 μmol/l GLAI (data not shown). Light microscopy showed that cells exposed to DMSO (Fig. 2A) or 10 μmol/l GLAI exhibited distinct morphological features, such as apoptotic bodies, associated with programmed cell death (Fig. 2B).
Figure 2.
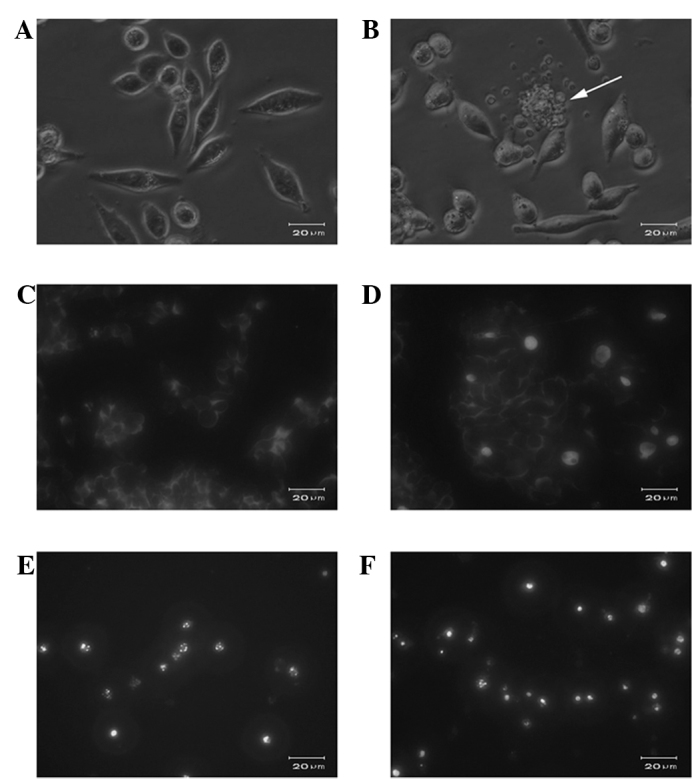
Photomicrographs of SW620 exposed to (A) DMSO and (B) 10 μg/ml GLAI for 24 h; the arrow indicates apoptotic bodies. Fluorescence microscopy of SW620 cells using DNA fluorochrome Hoechst 33258. SW620 cells were treated with different concentrations of GLAI for 24 h. (C) DMSO, (D) 10 μg/ml GLAI, (E) 50 μg/ml GLAI and (F) 100 μg/ml GLAI.
To further determine the nuclear morphology of SW620 cells treated with GLAI, Hoechst 33258 staining was performed. Following treatment of SW620 cells with different concentrations of GLAI for 24 h, the chromatin stained with Hoechst 33258 had a characteristic condensed and fragmented appearance (Fig. 2C-F).
Flow cytometry
The staining patterns of SW620 cells exposed to GLAI for 24 h and treated with AV-FITC and PI are shown in Fig. 3. More than 94% of cells remained viable following treatment for 24 h with DMSO alone (negative control) and almost no apoptotic events were detected (Fig. 3, lower left quadrant). However, the proportion of cells with externalized PS increased in cells following treatment for 24 h with different concentrations of GLAI (Fig. 3).
Figure 3.
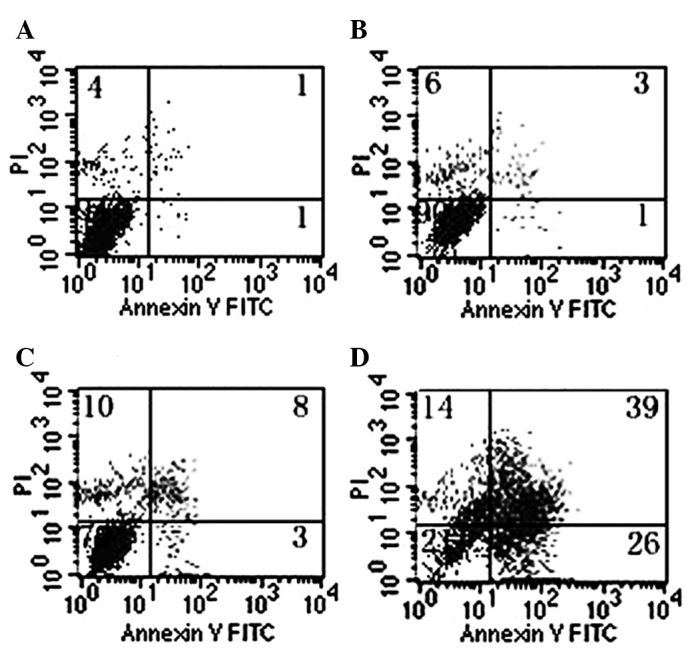
FACS analysis of annexin V (AV) and propidium iodide (PI) binding. SW620 cells were treated with DMSO or GLAI (20, 50 or 100 μg/ml) for 24 h as described in Materials and methods. PI and AV-FITC fluorescence was measured by flow cytometry and analyzed (dot-plots). Viable (AV−/PI−), early apoptotic (AV+/PI−), apoptotic (AV+/PI+) and residual damaged (AV−/PI+) cells are shown in the respective quadrants.
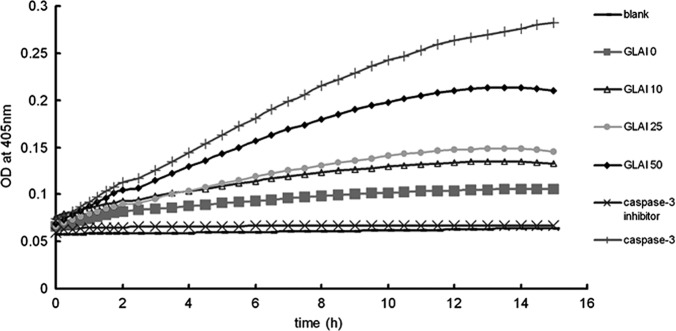
Activation of caspase-3 by treatment with GLAI
Caspases are significant mediators of apoptosis in mammalian cells (21), therefore, we measured caspase-3 activities. When SW620 cells were treated with different concentrations of GLAI, intracellular caspase-3 activity was analyzed (Fig. 4). Caspase-3 activity was found to be up-regulated in SW620 cells in a dose-dependent manner.
Figure 4.
Analysis of caspase-3 activity. SW620 cells were treated with different concentrations of GLAI (10, 25 and 50 μg/ml). Caspase-3 activity was monitored and three negative controls were observed: the blank well had only assay buffer added, the GLAI 0 well had DMSO-treated cell extract added and the inhibitor well had inhibitor-treated cell extract added. The positive control was purified caspase-3.
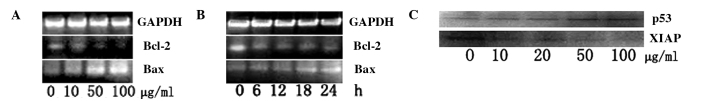
Molecular events responding to GLAI-treatment
In order to understand the induction mechanism of apoptosis by GLAI, we examined the expression levels of Bcl-2 and Bax using RT-PCR, as well as p53 and XIAP using Western blotting. The treatment of the SW620 cells with GLAI resulted in a marked decrease of Bcl-2 at mRNA levels in a dose-dependent manner (Fig. 5A). By contrast, the Bax mRNA expression level was increased in a dose-dependent manner (Fig. 5B). The results of Western blotting showed that the p53 protein expression level was up-regulated after SW620 cells were treated with different concentrations of GLAI. By contrast, the XIAP protein expression level was down-regulated (Fig. 5C).
Figure 5.
Molecular responses in SW620 cells treated with GLAI. Expression of apoptosis mRNA proteins in SW620 cells exposed to GLAI. SW620 cells were treated with different concentrations of GLAI for 24 h (A) or 50 μg/ml GLAI for different times (B), internal standard gene was GAPDH. (C) Expression of apoptosis proteins in SW620 cells exposed to different concentrations of GLAI. SW620 cells were treated with different concentrations of GLAI (10, 20, 50 and 100 μg/ml). DMSO served as the negative control.
Discussion
Colon cancer is a common cause of death among cancer patients worldwide. Dysregulation of the normal colonic epithelium is the causative factor of neoplastic transformation caused by alterations in various parameters, including epithelial cell proliferation and apoptosis. The latter two processes are highly regulated in the constantly regenerating non-transformed colonic epithelium and involve adhesion molecules, cytoskeletal proteins, cell cycle regulators and apoptosis (22).
Annexin V is a protein that exhibits specific affinity for PS. In non-apoptotic cells, most PS molecules are localized on the inner leaflet of the plasma membrane, but shortly after the onset of apoptosis, PS redistributes to the outer layer of the membrane (23). Cells in the early stages of apoptosis usually bind AV-FITC in the absence of PI uptake (lower right quadrant), while those in the late stages of apoptosis bind AV-FITC and in the presence of PI uptake (upper quadrant).
Caspases are a family of intracellular cysteine proteases with specificity for aspartic acid residues and play important roles in drug-inducing apoptosis in a large variety of cancer cells (24,25). Two members of this group of enzymes, known as ‘initiator’ and ‘effector’ caspases, also play a significant role in the apoptotic process (25,26). Caspase-3 is the common effector for most apoptotic pathways (19) and appears to play a special role as a key ‘executioner’ in that its active form is responsible for the cleavage and breakdown of several cellular components related to DNA repair and regulation. Once activated, caspase-3 is able to cleave a number of important cellular substrates and causes membrane blebbing, disassembly of the cell structure and DNA fragmentation, which eventually lead to cell death. Some initiator caspases, such as caspase-9, activate pro-caspase-3, which then cleaves the cellular substrates needed for the orchestration of apoptosis and forms a ‘wheel of death’ (19,25–27). Findings of studies have shown that apoptosis, especially caspase-mediated cell death, plays an important role in the etiology, pathogenesis and therapy of a variety of human malignancies, such as human hepatocellular carcinoma. Additionally, the cytotoxic effects of most anti-hepatocellular carcinoma drugs are based on apoptosis induction (28). These studies indicate that induction of apoptosis may be an index for new anti-tumor drug selection and an important method of assessing the clinical efficacy of many anti-carcinoma drugs (17).
Moreover, we found that the increase in caspase-3 activation is synchronized with the increase in Bax expression and the decrease in Bcl-2, which is in agreement with other studies (29–31). The Bcl-2 family of proteins plays a crucial role in the regulation of apoptosis in many cellular systems, by either inhibiting (Bcl-2, Bcl-XL, Bcl-W, Bfl-1 and Mcl-1) or promoting apoptosis (Bax, Bak, Bad, Bcl-Xs, Bid and Hrk) (32,33). Heterodimerization between pro- and anti-apoptotic members of this family and relative levels of the two types of proteins may determine the susceptibility to a given apoptotic stimulus and the cell fate (34,35). Moreover, these genes are known to be crucial regulators of apoptosis in colon cancer cell lines (36,37).
In conclusion, our study has shown that GLAI inhibits the growth of SW620 cells by inducing apoptosis via the activation of caspase-3. These findings provide a basis for further investigation of triterpenes from G. lucidum in the treatment and prevention of colorectal adenocarcinoma.
Acknowledgements
The authors thank Mr. Hengbing Shi for the technical assistance and Dr John Buswell for the linguistic revision of the manuscript. This study was supported by the National Science Foundation of China (30801031 to Z. Ji) and China Postdoctoral Science Foundation (to Z. Ji).
References
- 1.Wasson RG. Soma-Divine Mushroom of Immortality. Harcourt, Brace & World; New York: 1968. p. 381. [Google Scholar]
- 2.Chang ST, Buswell JA. Ganoderma lucidum (Curt.:Fr) P. Karst. (Aphyllophoromycetideae) – a mushrooming medicinal mushroom. International Journal of Medicinal Mushrooms. 1999;1:139–146. [Google Scholar]
- 3.Wachtel-Galor S, Benzie IFF, Tomlinson B, Buswell JA. Lingzhi (Ganoderma lucidum): molecular aspects of health effects. In: Packer L, Halliwell B, Ong CN, editors. Herbal Medicines. Marcel Dekker Inc; New York: 2004. pp. 179–228. [Google Scholar]
- 4.Furusawa E, Chou SC, Furusawa S, Hirazumi A, Dang Y. Antitumor activity of Ganoderma lucidum, an edible mushroom, on intraperitoneal implanted Lewis lung carcinoma in syngeneic mice. Phytotherapy Research. 1992;6:300–304. [Google Scholar]
- 5.Lieu CW, Lee SS, Wang SY. The effect of Ganoderma lucidum on induction of differentiation in leukemic U937 cells. Anticancer Res. 1992;12:1211–1215. [PubMed] [Google Scholar]
- 6.Wang SY, Hsu ML, Hsu HC, et al. The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int J Cancer. 1997;70:699–705. doi: 10.1002/(sici)1097-0215(19970317)70:6<699::aid-ijc12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Zhu M, Chang Q, Wong LK, Chong FS, Li RC. Triterpene antioxidants from ganoderma lucidum. Phytother Res. 1999;13:529–531. doi: 10.1002/(sici)1099-1573(199909)13:6<529::aid-ptr481>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Shim SB, Kim NJ, Jang IS. Beta-glucuronidase-inhibitory activity and hepatoprotective effect of Ganoderma lucidum. Biol Pharm Bull. 1999;22:162–164. doi: 10.1248/bpb.22.162. [DOI] [PubMed] [Google Scholar]
- 9.Komoda Y, Shimizu M, Sonoda Y, Sato Y. Ganoderic acid and its derivatives as cholesterol synthesis inhibitors. Chem Pharma Bull. 1989;37:531–533. doi: 10.1248/cpb.37.531. [DOI] [PubMed] [Google Scholar]
- 10.Kabir Y, Kimura S, Tamura T. Dietary effect of Ganoderma lucidum mushroom on blood pressure and lipid levels in spontaneously hypertensive rats (SHR) J Nutr Sci Vitaminol. 1988;34:433–438. doi: 10.3177/jnsv.34.433. [DOI] [PubMed] [Google Scholar]
- 11.Lee SY, Rhee HM. Cardiovascular effects of mycelium extract of Ganoderma lucidum: inhibition of sympathetic outflow as a mechanism of its hypotensive action. Chem Pharma Bull. 1990;38:1359–1364. doi: 10.1248/cpb.38.1359. [DOI] [PubMed] [Google Scholar]
- 12.Su CY, Shiao MS, Wang CT. Differential effects of ganodermic acid S on the thromboxane A2-signaling pathways in human platelets. Biochem Pharmacol. 1999;58:587–595. doi: 10.1016/s0006-2952(99)00136-7. [DOI] [PubMed] [Google Scholar]
- 13.Gan KH, Fann YF, Hsu SH, Kuo KW, Lin CN. Mediation of the cytotoxicity of lanostanoids and steroids of Ganoderma tsugae through apoptosis and cell cycle. J Nat Prod. 1998;61:485–487. doi: 10.1021/np9704664. [DOI] [PubMed] [Google Scholar]
- 14.Min BS, Gao JJ, Nakamura N, Hattori M. Triterpenes from the spores of Ganoderma lucidum and their cytotoxicity against meth-A and LLC tumor cells. Chem Pharma Bull. 2000;48:1026–1033. doi: 10.1248/cpb.48.1026. [DOI] [PubMed] [Google Scholar]
- 15.Noda Y, Kaiya T, Kohda K, Kawazoe Y. Enhanced cytotoxicity of some triterpenes toward leukemia L1210 cells cultured in low pH media: possibility of a new mode of cell killing. Chem Pharma Bull. 1997;45:1665–1670. doi: 10.1248/cpb.45.1665. [DOI] [PubMed] [Google Scholar]
- 16.Wu TS, Shi LS, Kuo SC. Cytotoxicity of Ganoderma lucidum triterpenes. J Nat Prod. 2001;64:1121–1122. doi: 10.1021/np010115w. [DOI] [PubMed] [Google Scholar]
- 17.Beauparlant P, Shore GC. Therapeutic activation of caspases in cancer: a question of selectivity. Curr Opin Drug Discov Devel. 2003;6:179–187. [PubMed] [Google Scholar]
- 18.Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Qi SN, Yoshida A, Wang ZR, Ueda T. GP7 can induce apoptotic DNA fragmentation of human leukemia cells through caspase-3-dependent and -independent pathways. Int J Mol Med. 2004;13:163–167. [PubMed] [Google Scholar]
- 20.Zhang JF, Liu JJ, Liu PQ, Lin DJ, Li XD, Chen GH. Oridonin inhibits cell growth by induction of apoptosis on human hepatocelluar carcinoma BEL-7402 cells. Hepatol Res. 2006;35:104–110. doi: 10.1016/j.hepres.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 22.Subramaniam V, Vincent IR, Jothy S. Upregulation and dephosphorylation of cofilin: modulation by CD44 variant isoform in human colon cancer cells. Exp Mol Pathol. 2005;79:187–193. doi: 10.1016/j.yexmp.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Martin SJ, Reutelingsperger CP, McGahon AJ, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donepudi M, Grutter MG. Structure and zymogen activation of caspases. Biophys Chem. 2002;101–102:145–153. doi: 10.1016/s0301-4622(02)00151-5. [DOI] [PubMed] [Google Scholar]
- 25.Denault JB, Salvesen GS. Caspases: keys in the ignition of cell death. Chem Rev. 2002;102:4489–4500. doi: 10.1021/cr010183n. [DOI] [PubMed] [Google Scholar]
- 26.Boatright KM, Salvesen GS. Caspase activation. Biochem Soc Symp. 2003:233–242. doi: 10.1042/bss0700233. [DOI] [PubMed] [Google Scholar]
- 27.Philchenkov AA. Caspases as regulators of apoptosis and other cell functions. Biochemistry. 2003;68:365–376. doi: 10.1023/a:1023635510363. [DOI] [PubMed] [Google Scholar]
- 28.Huether A, Hopfner M, Sutter AP, Schuppan D, Scherubl H. Erlotinib induces cell cycle arrest and apoptosis in hepatocellular cancer cells and enhances chemosensitivity towards cytostatics. J Hepatol. 2005;43:661–669. doi: 10.1016/j.jhep.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 29.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan A, Roth KA, Sayers RO, et al. In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death Differ. 1998;5:1004–1016. doi: 10.1038/sj.cdd.4400449. [DOI] [PubMed] [Google Scholar]
- 31.Tanabe H, Eguchi Y, Shimizu S, Martinou JC, Tsujimoto Y. Death-signalling cascade in mouse cerebellar granule neurons. Eur J Neurosci. 1998;10:1403–1411. doi: 10.1046/j.1460-9568.1998.00148.x. [DOI] [PubMed] [Google Scholar]
- 32.Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002;76:1191–1201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- 33.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 34.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 35.Choi YH, Kong KR, Kim YA, et al. Induction of Bax and activation of caspases during β-sitosterol-mediated apoptosis in human colon cancer cells. Int J Oncol. 2003;23:1657–1662. [PubMed] [Google Scholar]
- 36.Levy P, Robin H, Bertrand F, Kornprobst M, Capeau J. Butyrate-treated colonic Caco-2 cells exhibit defective integrin-mediated signaling together with increased apoptosis and differentiation. J Cell Physiol. 2003;197:336–347. doi: 10.1002/jcp.10345. [DOI] [PubMed] [Google Scholar]
- 37.White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]